Cardiotoxicity is one of the most significant adverse effects of cancer treatment, and is responsible for considerable morbidity and mortality. Among the effects of chemotherapeutic agents on the cardiovascular system, the most frequent and serious is heart failure with ventricular systolic dysfunction. Other toxic effects include hypertension, thromboembolic disease, pericardial disease, arrhythmias and myocardial ischemia. For several decades, cancer therapy-induced cardiomyopathy was almost exclusively associated with the use of cumulative doses of anthracyclines, which cause permanent damage at the cellular level. However, new therapeutic agents, such as the monoclonal antibody trastuzumab, induce transient reversible myocyte dysfunction which is unrelated to the dose used. Early identification of potential cardiovascular injury, accurate diagnosis of cardiotoxic events and implementation of appropriate monitoring plans are essential in patients with cancer. Close cooperation between cardiologists and oncologists is thus crucial, in order to balance the risks and benefits of cardiotoxic anticancer therapy. In this article we review the various responses to cardiotoxic cancer treatments and their relationship with the main antineoplastic drugs used in clinical practice. In addition, we discuss the main guidelines on detection and monitoring of cardiotoxicity in patients with cancer.
A cardiotoxicidade é um dos efeitos adversos mais significativos do tratamento oncológico, responsável por uma considerável morbimortalidade. Entre os eventos lesivos dos agentes/fármacos quimioterápicos no sistema cardiovascular, destaca-se, pela sua maior frequência e gravidade, a ocorrência de insuficiência cardíaca com disfunção ventricular sistólica. Outros efeitos tóxicos cardiovasculares incluem hipertensão arterial, doença tromboembólica, doenças pericárdicas, arritmias e isquemia miocárdica. Durante várias décadas, a cardiomiopatia induzida por terapêutica oncológica era quase exclusivamente associada ao uso de doses cumulativas de antraciclinas, que promovem lesões permanentes a nível celular. No entanto, o uso de novos agentes terapêuticos, como o anticorpo monoclonal trastuzumab, induz uma disfunção transitória reversível dos miócitos sem que haja relação com a dose utilizada. Atualmente, é essencial para os doentes com cancro a identificação precoce da lesão cardiovascular, o diagnóstico preciso de eventos cardiotóxicos e a implementação de planos de monitorização adequados. Neste contexto, é fulcral na prática clínica uma cooperação estreita entre cardiologistas e oncologistas, de forma a equilibrar os riscos cardiotóxicos com os benefícios da terapia antineoplásica em doentes oncológicos. Neste artigo revimos as diversas respostas cardiotóxicas ao uso de tratamentos oncológicos e a sua relação com os principais fármacos antineoplásicos usados na prática clínica. Além disso, serão abordadas as principais linhas de orientação no que respeita às estratégias de deteção/monitorização da cardiotoxicidade em indivíduos com cancro.
Cancer treatment has made dramatic advances in recent years; the development of intensive antineoplastic therapies has substantially improved the prognosis of cancer patients.1 However, despite its unquestionable benefits, the safety aspects of cancer therapy cannot be ignored, not least because these drugs’ mechanisms of action can have harmful effects on the cardiovascular system.2,3
Cancer is currently the second leading cause of death in Portugal after cardiovascular disease,4 and colorectal cancer is the leading cause of cancer-related mortality.5
For several decades, cancer therapy-induced cardiotoxic reactions were almost exclusively associated with the use of anthracyclines, but a new dimension appeared when it was recognized that drugs targeting the action of certain tyrosine kinase receptors or tumor (estrogen) receptors can have undesirable clinical effects on the cardiovascular system.3,6
A large part of the literature on the cardiotoxicity of cancer therapy deals solely with cardiomyopathy, but this is only one of several forms of cardiac dysfunction.7 Others may result in ischemia or changes in blood pressure,8 while antineoplastic agents can lead to thickening of the pericardium or disrupt pericardial fluid balance, resulting in effusion.9 Such treatment can also increase the risk of arrhythmias in patients predisposed to ventricular ectopic beats.10
Protection of cardiac function is a constant challenge for the pharmaceutical industry, regulatory authorities and the physicians who have to deal with adverse reactions to various anticancer agents.3 Assessment of patients exposed to such drugs, analysis of the risks they pose to individuals and patient groups with cancer, prevention and mitigation of cardiac injury, monitoring of cardiac function during and after therapy, and treatment of chemotherapy-related cardiotoxicity, have all generated a vast quantity of knowledge and data that is collectively known as “cardio-oncology”.7,11
In this article we review the various responses to cardiotoxic cancer treatments, and their relationship with the main antineoplastic drugs used in clinical practice. In addition, we analyze the latest guidelines on detection and monitoring of cardiotoxicity in patients with cancer.
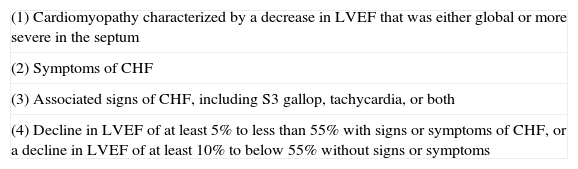
Definition of cardiotoxicityA standard definition of cardiotoxicity is essential for both clinicians and researchers.3 One of the most precise definitions was proposed by the Cardiac Review and Evaluation Committee, as part of its oversight of clinical trials of the monoclonal antibody trastuzumab (Table 1).12 The criteria do not include subclinical cardiovascular lesions which can occur as an initial response to some chemotherapeutic agents.13
Criteria to confirm or revise a preliminary diagnosis of cardiac dysfunction.
| (1) Cardiomyopathy characterized by a decrease in LVEF that was either global or more severe in the septum |
| (2) Symptoms of CHF |
| (3) Associated signs of CHF, including S3 gallop, tachycardia, or both |
| (4) Decline in LVEF of at least 5% to less than 55% with signs or symptoms of CHF, or a decline in LVEF of at least 10% to below 55% without signs or symptoms |
CHF: congestive heart failure; LVEF: left ventricular ejection fraction.
The most common form of cardiotoxicity in cancer treatment is anthracycline-related cardiomyopathy.7 Initial evidence indicated that left ventricular (LV) systolic dysfunction was closely related to cumulative doses of anthracyclines. According to Lefrak et al., repeated administration can result in permanent cellular and interstitial damage, frequently associated with refractory heart failure (HF),14 but new therapeutic agents such as trastuzumab, while they can also cause cardiomyopathy, induce transient and reversible myocyte dysfunction which is unrelated to the dose used, resulting in better prognosis.12,15
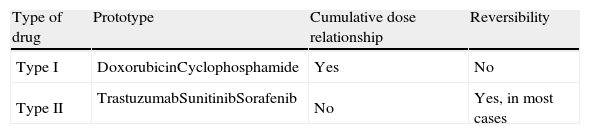
Antineoplastic drugs are classified as type I agents, which cause irreversible damage, or type II, which do not (Table 2).15 Type II agents can be used for years before signs of cardiac injury appear and may be reintroduced after cardiac recovery with an acceptable level of risk.16 However, some clinical trials have cast doubt on whether the cardiotoxic effects of these drugs are in fact reversible, and combined anticancer therapy with class I and II agents is associated with a higher than expected incidence of cardiac dysfunction.17
Anthracyclines, including doxorubicin (DOX), epirubicin (EPI) and daunorubicin (DNR), are still among the most frequently used antineoplastic agents in the treatment of a wide variety of solid tumors and blood cancers.3 Unfortunately, concerns among the medical community about their cardiotoxicity limit their use. Despite over 40 years of research, the mechanisms responsible for the cardiotoxicity of cumulative doses of anthracyclines have not been fully clarified.3,7
Short- and long-term cardiotoxic effectsCardiotoxicity can be acute or chronic, and can appear years after the end of treatment. Acute anthracycline-induced cardiotoxicity is rare, transient and dose-independent. It is characterized by sudden ventricular repolarization alterations, changes in QT interval, ventricular and supraventricular arrhythmias, acute coronary syndromes, pericarditis and myocarditis. These effects are usually observed from the beginning of treatment and up to 14 days after it ends.
Chronic toxicity is dose-dependent. It can be divided into two forms, according to the time of symptom onset. The first type occurs at the beginning of treatment and continues for a year after the end of chemotherapy, while the second type occurs later, more than a year after treatment. The most characteristic manifestation of chronic cardiotoxicity is ventricular systolic or diastolic dysfunction, which can lead to severe cardiomyopathy and even death.3,13
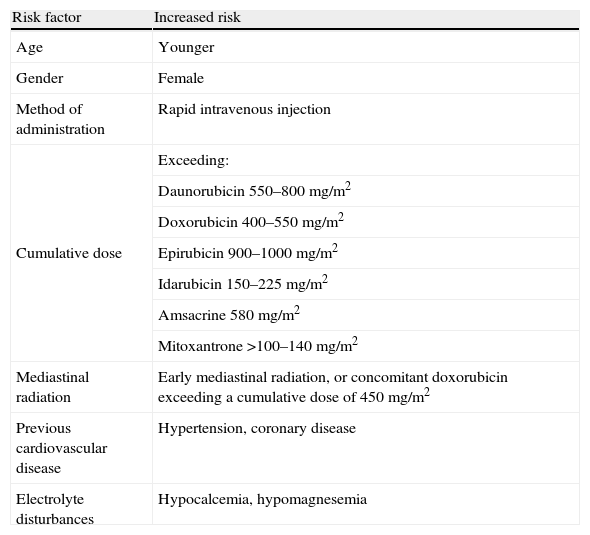
Risk factorsThe progression and degree of cardiotoxicity arising from the use of these drugs varies between individuals due to the influence of various risk factors (Table 3) and genetic predisposition.3
Risk factors associated with anthracycline-induced cardiotoxicity.
| Risk factor | Increased risk |
| Age | Younger |
| Gender | Female |
| Method of administration | Rapid intravenous injection |
| Cumulative dose | Exceeding: |
| Daunorubicin 550–800 mg/m2 | |
| Doxorubicin 400–550 mg/m2 | |
| Epirubicin 900–1000 mg/m2 | |
| Idarubicin 150–225 mg/m2 | |
| Amsacrine 580 mg/m2 | |
| Mitoxantrone >100–140 mg/m2 | |
| Mediastinal radiation | Early mediastinal radiation, or concomitant doxorubicin exceeding a cumulative dose of 450 mg/m2 |
| Previous cardiovascular disease | Hypertension, coronary disease |
| Electrolyte disturbances | Hypocalcemia, hypomagnesemia |
The total cumulative dose is the main risk factor for anthracycline-related congestive HF.18 However, there is no completely safe dose, and so the cardiotoxicity of any given dosage must always be weighed against antineoplastic efficacy.19 Treatment duration may also influence the risk of cardiotoxicity during or after therapy; prolonged administration has been known to reduce the severity of cardiac injury,20 while young patients appear to be more vulnerable to the cardiotoxic effects of these drugs.21
All risk factors are closely linked to early and late cardiotoxicity but not with acute forms.3
Pathophysiological mechanismsVarious mechanisms have been put forward to explain the pathophysiology of anthracycline-related cardiotoxicity, although it is still unclear why these drugs preferentially affect cardiomyocytes. The main hypotheses involve lipid peroxidation and oxidative stress in cardiomyocytes.22 The mechanism of action of anthracyclines suppresses the synthesis of DNA, RNA and proteins,23 as well as of important transcription factors that regulate cardiospecific genes.24,25 Reduced protein expression, together with degradation of myofilaments, results in disruption of sarcomeric proteins such as titin in cardiac cells, leading to cardiac sarcopenia.26 At the same time, destruction of myofilaments may be worsened by combined therapies such as anthracyclines with trastuzumab,16 which can also affect bioenergetics27 and damage DNA.28 Anthracyclines also disrupt the dynamic regulation of cardiac function, altering adrenergic and adenylyl cyclase activity29 and calcium homeostasis.27,30
Clinical and experimental evidence suggests that another possible mechanism for cardiotoxicity is cardiac cell death by apoptosis or necrosis after each exposure to anthracyclines. Given the limited regenerative capacity of the heart, the number of cardiomyocytes progressively falls, leading to ventricular remodeling.22 De Angelis et al. have demonstrated that DOX-induced cardiomyopathy can also be mediated by loss of cardiac stem cells and reversed by restoration of progenitor cell function.31
Strategies to limit cardiotoxicityAlthough there is no generally accepted way to limit or prevent anthracycline cardiotoxicity, various strategies have been adopted, most notably the synthesis of analogues of the natural compounds, the development of tumor-specific formulations, and the use of cardioprotective agents.32
Synthetic analogues of natural compoundsChanges in the structure of anthracyclines have produced compounds with low levels of cardiotoxicity, allowing the administration of higher doses.22 EPI33 and idarubicin (IDA)34 are viable alternatives to DOX and DNR, respectively. Other anthracyclines such as pirarubicin and aclarubicin, although registered in some countries, do not yet play a significant role in global terms.35
Epirubicin. EPI is a semi-synthetic epimer of DOX with a similar oncological spectrum.36 Although its mechanism of action is similar to that of DOX, some of its physical, chemical and pharmacokinetic properties are different,32 and it is significantly less cardiotoxic than DOX when administered in doses that result in the same level of myelosuppression.36
Idarubicin. IDA is a structural analogue of DNR that intercalates into DNA, interacting with topoisomerase II and inhibiting nucleic acid synthesis.32 It is highly lipophilic and thus is easily absorbed into cells, and can be administered intravenously or orally. However, doubts have been expressed as to whether it is in fact any less cardiotoxic.35,37
Tumor-specific formulationsNew techniques have been developed to deliver anthracyclines in ways that reduce their absorption in cardiac tissue.38 The current method for passive delivery of DOX and DNR to the target tumor is by incorporating them into liposomes,39 which offers a good degree of cardioprotection by increasing the drug's molecular size through encapsulation and extending its elimination time. This enables the drug to remain in the organism for longer and with fewer adverse effects, and also keeps it away from organs that have normal capillary junctions, while easily penetrating areas with immature vascular systems, such as tumors.38
By contrast, anthracycline prodrugs, unlike liposomal compounds, can reach the tumor by an active pathway. Various prodrugs have been developed by conjugating anticancer agents with peptides, carbohydrates, antibodies, serum proteins and synthetic polymers.35 These conjugates cannot penetrate normal cells and are activated specifically by cancer cells.35,40
Cardioprotective agentsIt is a priority to develop pharmacological therapies that protect the cardiovascular system without interfering with the antineoplastic effects of anthracyclines.32,41 Given the importance of reactive oxygen species (ROS) and oxidative stress in anthracycline-induced cardiotoxicity, research has focused on drugs and natural compounds that improve the antioxidant defenses of cardiomyocytes.41 Various types of drug, including antioxidants, iron-chelating agents and lipid-lowering drugs, have been tested in animal models and in humans,41–43 but the cardioprotective effect of some is highly questionable.42,43 Dexrazoxane (Cardioxane®) is the only drug approved for clinical use to prevent anthracycline-induced cardiotoxicity,44 but controversy surrounds its use due to possible compromise of antineoplastic efficacy and increase in secondary tumors, and it has been restricted to adult patients by the US Food and Drug Aministration.45
Type II agents: trastuzumabMonoclonal antibodies are among the best-known examples of targeted cancer therapy and are widely used. The HER2 gene is overexpressed in 15–25% of breast cancers, resulting in overproduction of human epidermal growth factor receptor 2 (HER2). Trastuzumab binds to the extracellular domain of HER2, thereby inhibiting its signal transduction.7 Chemotherapeutic regimens that do not include anthracyclines result in lower rates of cardiac dysfunction than combined trastuzumab and anthracyclines, but trastuzumab associated with vinorelbine, gemcitabine or liposomal DOX do not present significant risk of cardiotoxicity.46
Risk factorsOne of the main risk factors for cardiotoxicity associated with trastuzumab is the use of high cumulative doses of anthracyclines (>300 mg/m2).47 Other important risk factors include LV dysfunction, irrespective of anthracycline use, pre-existing systemic hypertension, body mass index >25 and advanced age. However, chest radiotherapy concomitantly with trastuzumab is clinically viable.48
Recent evidence shows that elderly cancer patients (aged over 70) with a history of heart disease and/or diabetes present a higher incidence of trastuzumab-related cardiotoxic effects in breast cancer treatment. According to Serrano et al., this population should be closely and continuously monitored.49
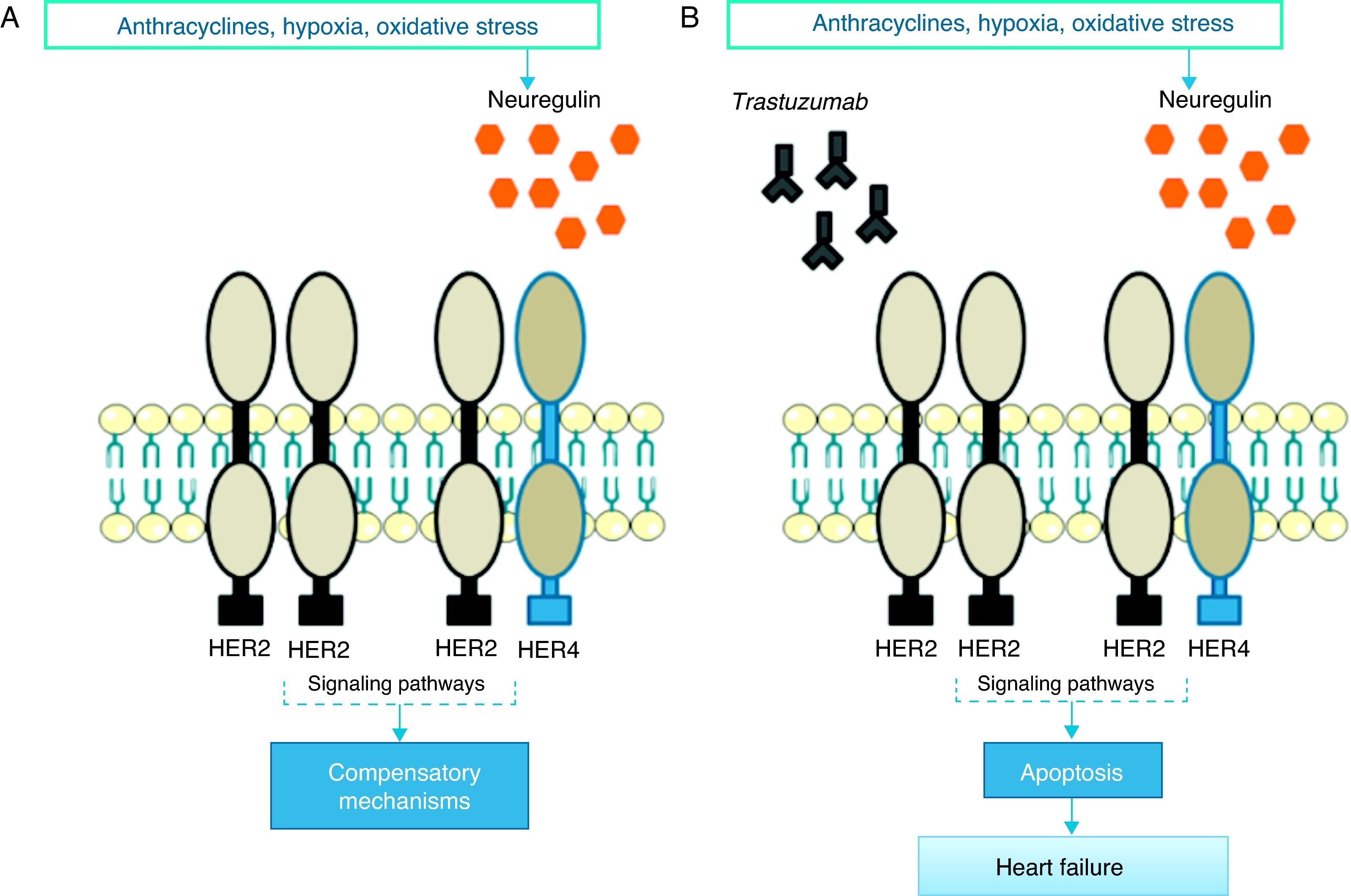
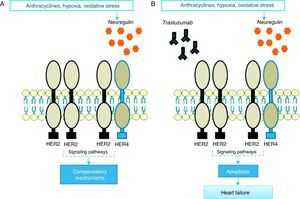
Pathophysiological mechanismsThe precise pathophysiological mechanisms by which trastuzumab acts within cells are not known, but they are thought to be closely linked to inhibition of HER2 cardiac signaling. Studies have demonstrated the important role of HER2 in cardiomyocyte survival and development50,51; genetically modified mice with reduced HER2 levels develop dilated cardiomyopathy, reduced adaptation to pressure overload and greater sensitivity to anthracycline toxicity,52,53 while at the cellular level overexpression of HER2 and/or neuregulin (NRG)-mediated activation of the HER2/HER4 signaling pathway confers greater protection against oxidative stress and prevents apoptosis.54 High serum levels of HER2 have been detected in individuals with chronic HF,55 and clinical trials have shown that administration of recombinant human NRG-1 improves cardiac function in chronic HF and is well tolerated.56 Thus, while cardiac stress leads to increased HER2 expression and HER2/HER4 activation by NRG, inhibition of HER2 by trastuzumab induces ventricular dysfunction (Figure 1).57
In response to oxidative stress, neuregulin activates compensatory mechanisms via HER2 receptors (A); in the presence of trastuzumab, HER2/HER2 and HER2/HER4 dimers are blocked and the compensatory mechanisms are inactivated, leading to apoptosis and heart failure (B).
However, trastuzumab's pathophysiological mechanism is probably more complex and does not only involve HER2 inhibition. Studies have revealed low toxicity after the use of lapatinib, a tyrosine kinase inhibitor with dual action against HER2 and epidermal growth factor (EGF).58 There may be various reasons for the differences in toxicity between these two drugs. Studies analyzing the effect on cardiomyocytes of cytotoxic immune reactions triggered by the IgG1 domain of trastuzumab show that antibody-dependent cell-mediated cytotoxicity in tumor cells is closely linked to cardiotoxicity.59 Another suggested mechanism involves a single intracellular response after activation of HER2 in cardiomyocytes. After binding to HER2, trastuzumab modulates mitochondrial integrity via the BCL-X family of proteins, depleting ATP and leading to contractile dysfunction. Curiously, some studies indicate that lapatinib has the opposite effect, reducing trastuzumab's cytotoxicity when the two drugs are administered together.60,61 This is due to their different effect on adenosine monophosphate-activated protein kinase (AMPK): while this is reduced by trastuzumab, reducing intracellular ATP, it is enhanced by lapatinib, leading to increased ATP production by oxidative pathways.61
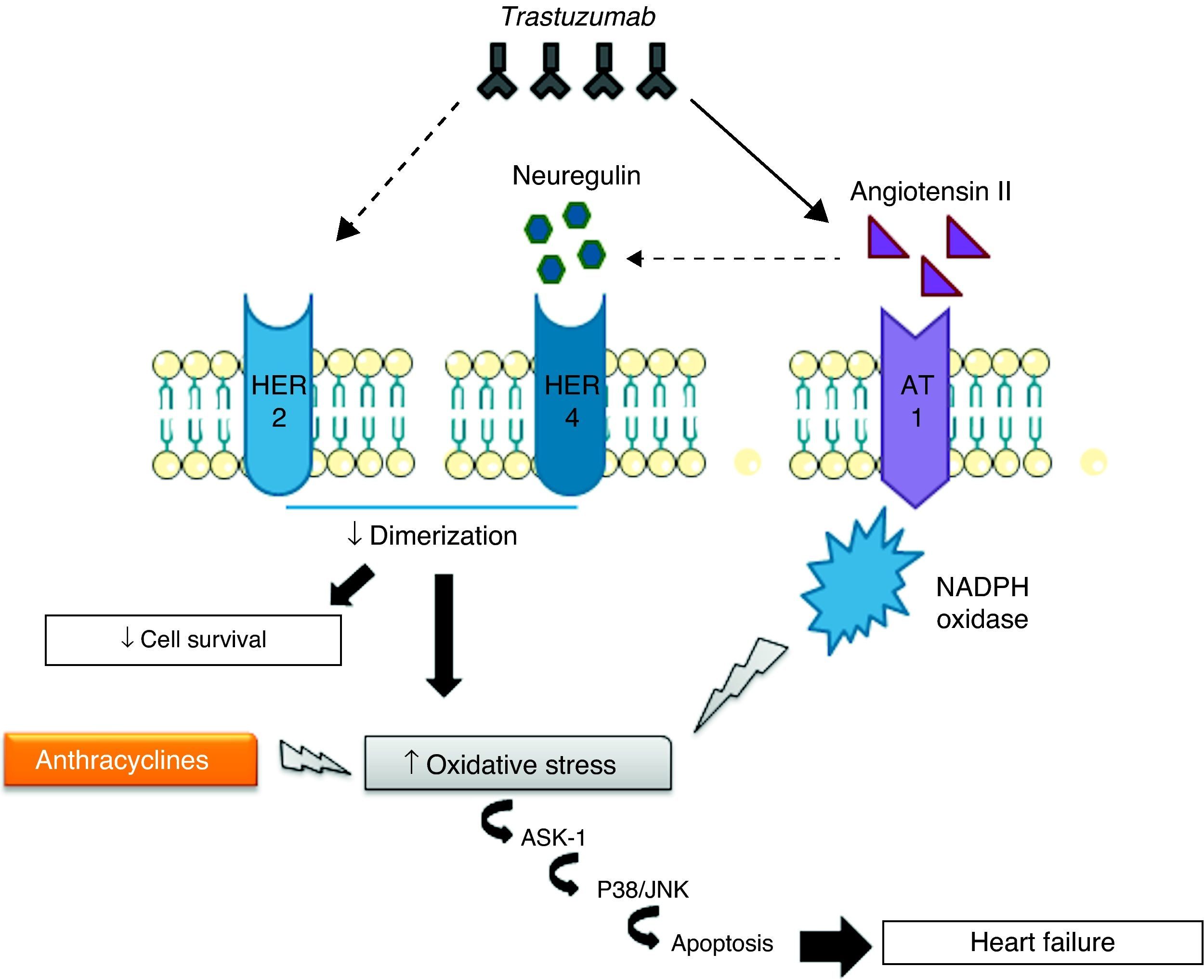
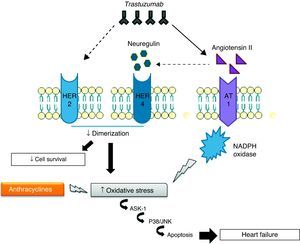
Cardiotoxicity of concomitant trastuzumab and anthracyclinesThe mechanisms of cardiotoxicity associated with concomitant use of trastuzumab and anthracyclines are of particular importance in view of the frequency with which combined therapy is used to treat breast cancer.62 Such therapy leads to increased ROS levels and reductions in antioxidant compounds, promoting oxidative stress that results in cardiac dysfunction and overexpression of angiotensin II (Ang II), high levels of which inhibit the action of NRG, preventing it from binding to HER receptors and thereby blocking antiapoptotic signaling pathways. Inhibition of these pathways can also increase ROS production, while Ang II also affects the activation and upregulation of NADPH oxidase63,64 by interacting with the G protein-coupled AT1 receptor, which activates NADPH oxidase via protein kinase C.63 NADPH oxidase in turn produces superoxide anion radicals, which are potent ROS. Finally, AT1 receptor signaling is linked to the activation of apoptosis signaling kinase 1 (ASK1), a member of the MAPK family, and thus leads to cell death and cardiac dysfunction.65
To summarize, trastuzumab directly inhibits anti-apoptotic signaling pathways and causes overexpression of Ang II, which stimulates ROS production and inhibits the action of NRG (Figure 2).66
Strategies to limit cardiotoxicityVarious strategies have been developed to reduce the cardiotoxicity of trastuzumab without significantly compromising its therapeutic efficacy, including optimization of chemotherapeutic combinations, shortening of treatment duration and careful monitoring of patients.57
Trastuzumab without anthracyclinesThe Breast Cancer International Research Group 006 trial was the first to test an adjuvant chemotherapeutic regimen with trastuzumab and without anthracyclines to treat breast cancer, which was shown to have equivalent antineoplastic efficacy with a lower incidence of cardiotoxic events than combined therapy with trastuzumab and anthracyclines.67 Although other trials have confirmed the efficacy of this approach,68 there are conflicting opinions regarding the role of anthracyclines in combined cancer treatments.69
Trastuzumab-toxin conjugatesMonoclonal antibodies that are tumor-specific but insufficiently cytotoxic in themselves can be chemically bound to cytotoxic agents to direct them to specific antigens on target tumors, which confers more control over apoptosis in tumor cells and greater selectivity in their action.70 Phillips et al. demonstrated that trastuzumab conjugated with DM1, derived from the fungal toxin maytansine, is associated with low cardiotoxicity,71 and clinical trials have confirmed the efficacy and tolerability of the trastuzumab-DM1 conjugate to treat breast cancer.72
Short-term treatment regimensThere is considerable interest in short-term treatment regimens due to their greater safety and better cost-benefit ratio.3 The Finland Herceptin (FinHER) trial compared adjuvant docetaxel and vinorelbine with or without trastuzumab for early breast cancer. Trastuzumab was administered before other cardiotoxic drugs and in combination with docetaxel and vinorelbine for only nine weeks, in order to test the hypothesis that this would reduce cardiotoxicity while maintaining antineoplastic efficacy. There was a strong association between low cardiotoxicity and short trastuzumab treatment regimens.73
Individualized anthracycline therapyTopoisomerase II-α (TOP2A) is a target for anthracyclines, and is considered a possible predictor of response to these agents. The TOP2A gene, which is located close to the HER2 gene, is more often overexpressed in HER2-positive tumors (34–90%) than in other cancers (5–10%). Several reports and retrospective clinical analyses have found that treatment for HER2-positive breast cancer that included anthracyclines has greater efficacy, suggesting that anthracycline use should be reduced in patients with HER2-negative tumors.74–76 However, clinical trials have drawn different conclusions,77 and the use of adjuvant anthracyclines to treat patients with overexpression of both HER2 and TOP2A remains the subject of debate.3
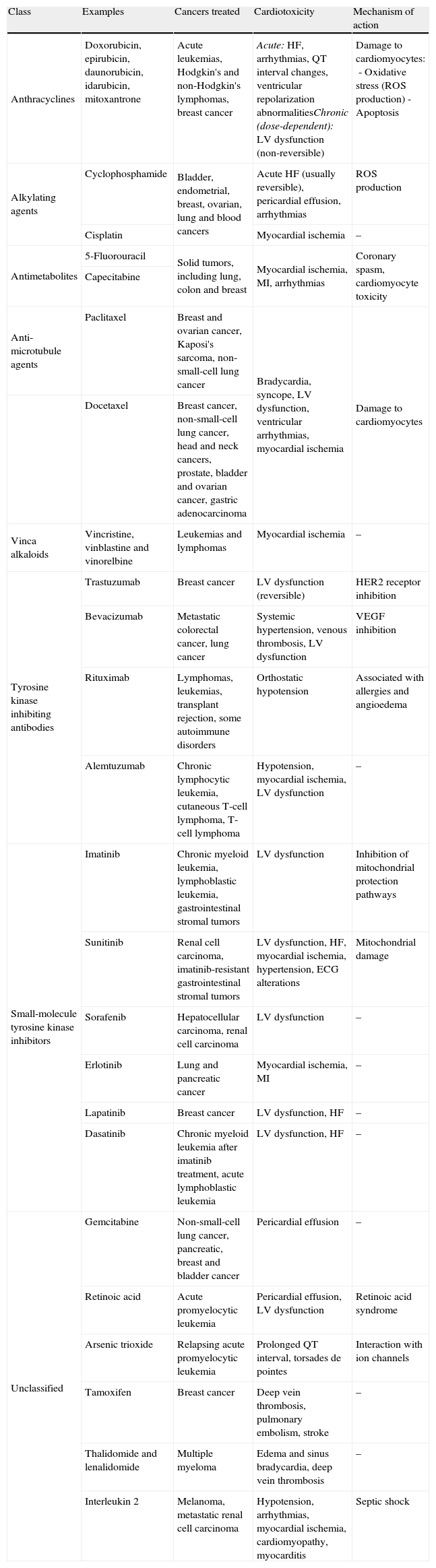
Other cardiotoxic effectsMyocardial ischemiaIndividuals with cancer survive longer than they used to, which means that they are more exposed to the risk of atherosclerosis.78 Certain antineoplastic drugs, particularly capecitabine, 5-fluorouracil (5-FU) and bevacizumab, are associated with coronary disease in cancer patients, while anti-microtubule agents (paclitaxel and docetaxel), tyrosine kinase inhibitors (sorafenib and sunitinib) and the Vinca alkaloids vincristine and vinorelbine have been linked to ischemic events.2,79
The ischemic event most often associated with 5-FU or its fluoropyrimidine prodrug capecitabine is coronary vasospasm; many affected individuals have previous coronary disease, which may increase 5-FU's ischemic effect. Once the ischemic event is under control, treatment should resume, taking care to avoid recurrence.80
Bevacizumab is a monoclonal antibody targeted at vascular endothelial growth factor (VEGF) that has demonstrated anti-tumor activity when combined with chemotherapy. This agent has occasionally been associated with arterial thromboembolic events, including myocardial infarction in 0.6–1.5% of patients.7,81 The mechanisms behind its cardiotoxicity are unclear.82
ArrhythmiasThe overall incidence of arrhythmias in cancer patients is unknown and varies with different treatments.83 Risk factors include advanced age, radiotherapy of the heart, the presence of amyloid infiltration, and any underlying conduction system disturbance.2 At the same time, cancer itself creates an arrhythmogenic environment independently of other risk factors.83 Atrial fibrillation is the most prevalent arrhythmia in cancer patients, and according to Onaitis et al. is responsible for significant morbidity following cancer surgery, with an incidence of up to 12.6%.84
It is difficult to establish a causal relationship between arrhythmic events and use of a particular drug. The chemotherapeutic agents known to cause arrhythmias are anthracyclines (DOX and EPI), anti-microtubule agents (paclitaxel and docetaxel), antimetabolites (capecitabine, 5-FU and gemcitabine), alkylating agents (cisplatin and cyclophosphamide), tyrosine kinase inhibitors (trastuzumab and cetuximab), arsenic trioxide, thalidomide and interleukin 2.83
Systemic hypertensionSome antiangiogenic cancer drugs, including bevacizumab, sunitinib, sorafenib, vatalanib, pazopanib, motesanib, axitinib and aflibercept, induce or exacerbate systemic hypertension.85,86 Before the introduction of these drugs, the prevalence of hypertension in cancer patients was similar to the adult population in general, but the improved survival of these patients and wider use of chemotherapeutic agents that affect systemic blood pressure mean that it is now diagnosed more frequently.85,86
Antiangiogenic cancer drugs reduce the activity of the tyrosine kinase of VEGF receptors, which produce nitric oxide, increasing capillary permeability and endothelial cell proliferation.85,86 Pre-existing hypertension is an important risk factor for severe hypertensive sequelae in cancer patients, and aggressive antihypertensive medication before and during treatment with anti-angiogenic drugs is essential.2
ThromboembolismVenous thromboembolism is a leading cause of death in cancer patients and is associated with the use of antiangiogenic drugs, thalidomide, lenalidomide, bevacizumab and hormone therapy such as tamoxifen.87
The antineoplastic agent most often linked to thromboembolic events (5% incidence in monotherapy88) is thalidomide.2 Lenalidomide, a thalidomide analogue, is generally less toxic, but the risk of thromboembolism is still high.89 The thrombogenic mechanism of these drugs involves direct action on endothelial cells and increased platelet aggregation.2
Tamoxifen, an estrogen receptor antagonist, is associated with increased thromboembolic complications,90 while aromatase inhibitors such as anastrozole and letrozole, which block conversion of androgens to estrogen in post-menopausal women, have been linked to harmful cardiac effects (Table 4).91
Cardiotoxicity of the main classes of cancer drugs used in clinical practice.
| Class | Examples | Cancers treated | Cardiotoxicity | Mechanism of action |
| Anthracyclines | Doxorubicin, epirubicin, daunorubicin, idarubicin, mitoxantrone | Acute leukemias, Hodgkin's and non-Hodgkin's lymphomas, breast cancer | Acute: HF, arrhythmias, QT interval changes, ventricular repolarization abnormalitiesChronic (dose-dependent): LV dysfunction (non-reversible) | Damage to cardiomyocytes:- Oxidative stress (ROS production)- Apoptosis |
| Alkylating agents | Cyclophosphamide | Bladder, endometrial, breast, ovarian, lung and blood cancers | Acute HF (usually reversible), pericardial effusion, arrhythmias | ROS production |
| Cisplatin | Myocardial ischemia | – | ||
| Antimetabolites | 5-Fluorouracil | Solid tumors, including lung, colon and breast | Myocardial ischemia, MI, arrhythmias | Coronary spasm, cardiomyocyte toxicity |
| Capecitabine | ||||
| Anti-microtubule agents | Paclitaxel | Breast and ovarian cancer, Kaposi's sarcoma, non-small-cell lung cancer | Bradycardia, syncope, LV dysfunction, ventricular arrhythmias, myocardial ischemia | Damage to cardiomyocytes |
| Docetaxel | Breast cancer, non-small-cell lung cancer, head and neck cancers, prostate, bladder and ovarian cancer, gastric adenocarcinoma | |||
| Vinca alkaloids | Vincristine, vinblastine and vinorelbine | Leukemias and lymphomas | Myocardial ischemia | – |
| Tyrosine kinase inhibiting antibodies | Trastuzumab | Breast cancer | LV dysfunction (reversible) | HER2 receptor inhibition |
| Bevacizumab | Metastatic colorectal cancer, lung cancer | Systemic hypertension, venous thrombosis, LV dysfunction | VEGF inhibition | |
| Rituximab | Lymphomas, leukemias, transplant rejection, some autoimmune disorders | Orthostatic hypotension | Associated with allergies and angioedema | |
| Alemtuzumab | Chronic lymphocytic leukemia, cutaneous T-cell lymphoma, T-cell lymphoma | Hypotension, myocardial ischemia, LV dysfunction | – | |
| Small-molecule tyrosine kinase inhibitors | Imatinib | Chronic myeloid leukemia, lymphoblastic leukemia, gastrointestinal stromal tumors | LV dysfunction | Inhibition of mitochondrial protection pathways |
| Sunitinib | Renal cell carcinoma, imatinib-resistant gastrointestinal stromal tumors | LV dysfunction, HF, myocardial ischemia, hypertension, ECG alterations | Mitochondrial damage | |
| Sorafenib | Hepatocellular carcinoma, renal cell carcinoma | LV dysfunction | – | |
| Erlotinib | Lung and pancreatic cancer | Myocardial ischemia, MI | – | |
| Lapatinib | Breast cancer | LV dysfunction, HF | – | |
| Dasatinib | Chronic myeloid leukemia after imatinib treatment, acute lymphoblastic leukemia | LV dysfunction, HF | – | |
| Unclassified | Gemcitabine | Non-small-cell lung cancer, pancreatic, breast and bladder cancer | Pericardial effusion | – |
| Retinoic acid | Acute promyelocytic leukemia | Pericardial effusion, LV dysfunction | Retinoic acid syndrome | |
| Arsenic trioxide | Relapsing acute promyelocytic leukemia | Prolonged QT interval, torsades de pointes | Interaction with ion channels | |
| Tamoxifen | Breast cancer | Deep vein thrombosis, pulmonary embolism, stroke | – | |
| Thalidomide and lenalidomide | Multiple myeloma | Edema and sinus bradycardia, deep vein thrombosis | – | |
| Interleukin 2 | Melanoma, metastatic renal cell carcinoma | Hypotension, arrhythmias, myocardial ischemia, cardiomyopathy, myocarditis | Septic shock |
ECG: electrocardiographic; HER2: human epidermal growth factor receptor 2; HF: heart failure; LV: left ventricular; MI: myocardial infarction; ROS: reactive oxygen species.
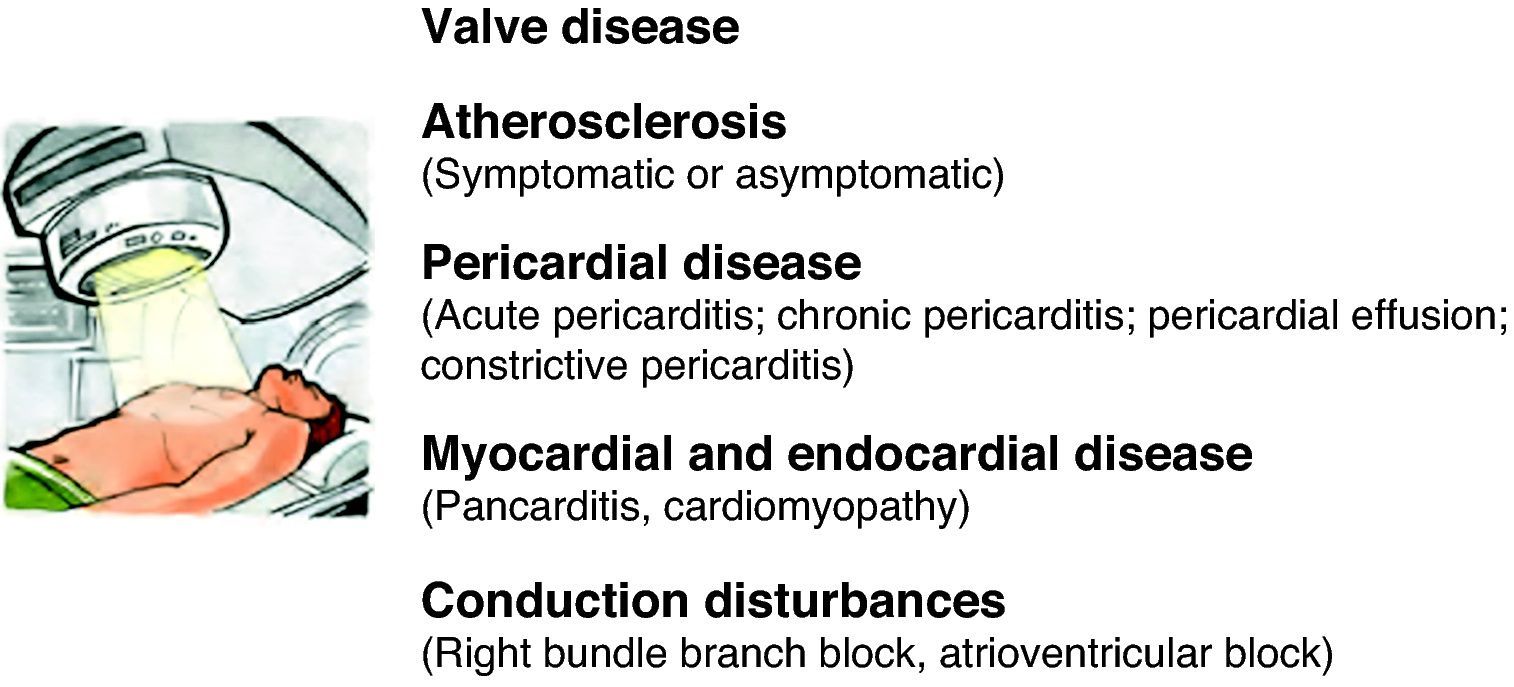
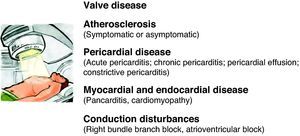
An estimated 50% of cancer patients receive radiotherapy, which, together with the development of new chemotherapeutic agents, has dramatically improved the prognosis of various types of cancer. However, late cardiovascular effects are frequently found following radiotherapy.92 Most clinical data on radiation-induced heart disease come from studies of individuals with breast cancer or Hodgkin's disease who developed symptomatic disease during or after treatment.93 Pericardial disease is frequently associated with radiotherapy, but other disorders can appear months or years after treatment, including myocardial fibrosis and cardiomyopathy, accelerated coronary disease, conduction disturbances and valve dysfunction (Figure 3). The extent of cardiotoxicity depends mainly on radiation dose, the area of the heart exposed, and the particular technique applied. Other risk factors such as the patient's age at the time of exposure are also important, with younger patients (aged under 20) being more susceptible to cardiac injury.94
Testing and monitoringPreclinical testingIt is generally accepted that before any anti-cancer compound is approved for commercial use, data from preclinical trials should guide cardiac monitoring during subsequent human trials.3 However, the precise molecular mechanisms behind the cardiotoxicity of some drugs are unknown, which makes prediction and early detection of such effects difficult at the pre-commercialization stage. Primary cardiac cell cultures are often used as models in toxicological testing, but embryonic stem cell-derived cardiomyocytes are also promising models that could increase available resources and improve the predictive ability of non-clinical tests.95 Although a variety of animal models have been developed, they can only help to clarify the molecular mechanisms behind drug-induced cardiac dysfunction and cannot predict clinical outcomes.96
Monitoring and early detectionThe need to monitor patients treated with anthracyclines, even after the end of therapy, depends on specific factors such as the patient's age, radiation dose, and cumulative anthracycline dose.97 Individual susceptibility, for example genetic background, should also be assessed to identify vulnerable patients and to personalize treatment. Identification of high-risk subgroups and their incorporation into monitoring systems is strongly recommended.3,98
Little is known of the long-term cardiac effects of drugs such as trastuzumab and the extent to which their cardiotoxic effects are reversible. This is mainly because medium-term (2–3 years in the case of trastuzumab) follow-up data do not indicate whether heart failure treatment results in permanent recovery or reduces the risk of late cardiac dysfunction. There has also been no systematic investigation of risk factors associated with cardiotoxicity in these compounds. Careful assessment of cardiac function is thus recommended in all eligible patients, especially those with reduced myocardial reserve.3
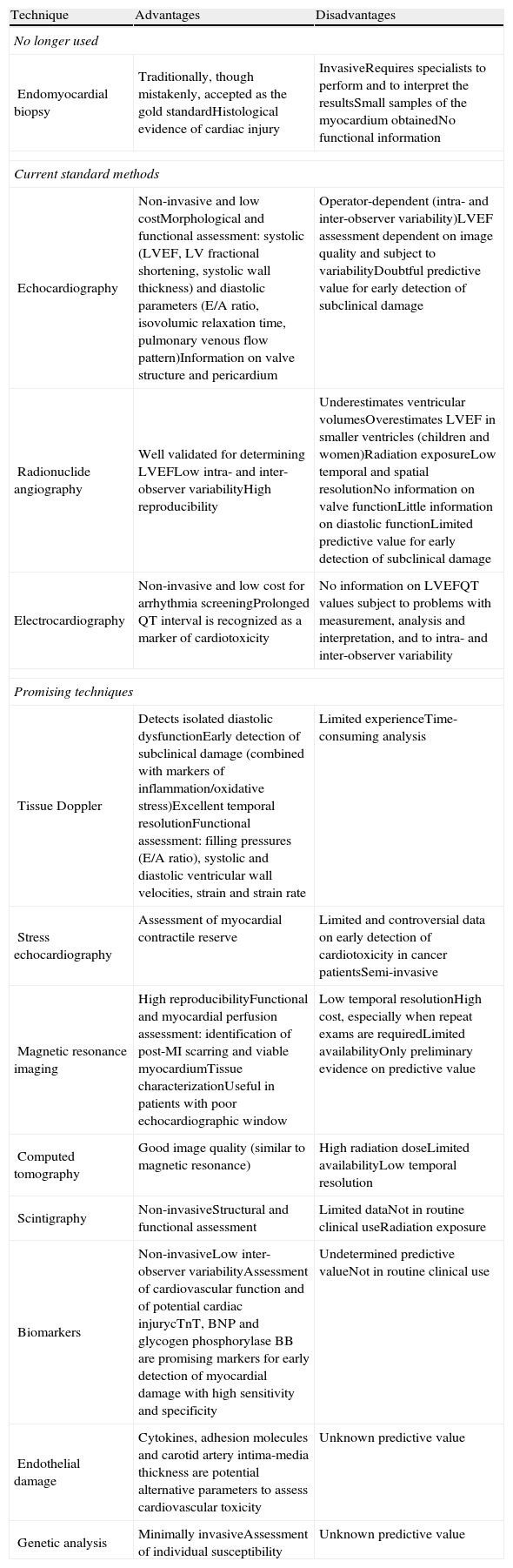
Left ventricular ejection fraction (LVEF) assessed by radionuclide angiography or echocardiography is the index most often used to monitor cardiac function during cancer treatment,99 but it can underestimate cardiac damage due to the adaptive capacity of the myocardium, which can keep LVEF within normal limits even in the presence of myocardial dysfunction.100 Other methods for early detection of cardiotoxicity have therefore been explored, including analysis of individual susceptibility, detection of temporary events such as release of natriuretic peptides, and identification of subclinical changes, such as variations in diastolic parameters (Table 5).99
Advantages and disadvantages of the principal techniques used for monitoring cardiotoxicity in clinical practice.
| Technique | Advantages | Disadvantages |
| No longer used | ||
| Endomyocardial biopsy | Traditionally, though mistakenly, accepted as the gold standardHistological evidence of cardiac injury | InvasiveRequires specialists to perform and to interpret the resultsSmall samples of the myocardium obtainedNo functional information |
| Current standard methods | ||
| Echocardiography | Non-invasive and low costMorphological and functional assessment: systolic (LVEF, LV fractional shortening, systolic wall thickness) and diastolic parameters (E/A ratio, isovolumic relaxation time, pulmonary venous flow pattern)Information on valve structure and pericardium | Operator-dependent (intra- and inter-observer variability)LVEF assessment dependent on image quality and subject to variabilityDoubtful predictive value for early detection of subclinical damage |
| Radionuclide angiography | Well validated for determining LVEFLow intra- and inter-observer variabilityHigh reproducibility | Underestimates ventricular volumesOverestimates LVEF in smaller ventricles (children and women)Radiation exposureLow temporal and spatial resolutionNo information on valve functionLittle information on diastolic functionLimited predictive value for early detection of subclinical damage |
| Electrocardiography | Non-invasive and low cost for arrhythmia screeningProlonged QT interval is recognized as a marker of cardiotoxicity | No information on LVEFQT values subject to problems with measurement, analysis and interpretation, and to intra- and inter-observer variability |
| Promising techniques | ||
| Tissue Doppler | Detects isolated diastolic dysfunctionEarly detection of subclinical damage (combined with markers of inflammation/oxidative stress)Excellent temporal resolutionFunctional assessment: filling pressures (E/A ratio), systolic and diastolic ventricular wall velocities, strain and strain rate | Limited experienceTime-consuming analysis |
| Stress echocardiography | Assessment of myocardial contractile reserve | Limited and controversial data on early detection of cardiotoxicity in cancer patientsSemi-invasive |
| Magnetic resonance imaging | High reproducibilityFunctional and myocardial perfusion assessment: identification of post-MI scarring and viable myocardiumTissue characterizationUseful in patients with poor echocardiographic window | Low temporal resolutionHigh cost, especially when repeat exams are requiredLimited availabilityOnly preliminary evidence on predictive value |
| Computed tomography | Good image quality (similar to magnetic resonance) | High radiation doseLimited availabilityLow temporal resolution |
| Scintigraphy | Non-invasiveStructural and functional assessment | Limited dataNot in routine clinical useRadiation exposure |
| Biomarkers | Non-invasiveLow inter-observer variabilityAssessment of cardiovascular function and of potential cardiac injurycTnT, BNP and glycogen phosphorylase BB are promising markers for early detection of myocardial damage with high sensitivity and specificity | Undetermined predictive valueNot in routine clinical use |
| Endothelial damage | Cytokines, adhesion molecules and carotid artery intima-media thickness are potential alternative parameters to assess cardiovascular toxicity | Unknown predictive value |
| Genetic analysis | Minimally invasiveAssessment of individual susceptibility | Unknown predictive value |
BNP: brain natriuretic peptide; cTnT: cardiac troponin T; LV: left ventricular; LVEF: left ventricular ejection fraction; MI: myocardial infarction.
Although there are guidelines on monitoring for cardiotoxic effects, it is not known how long monitoring should continue after treatment ends.99 Cardiovascular surveillance is thus essential in cancer patients in order to detect the cardiotoxic effects of antineoplastic therapy as early as possible.
ConclusionThe extensive use of chemotherapy and radiotherapy in clinical practice has led to considerable controversy due to their potential adverse cardiovascular effects in surviving cancer patients, including cardiomyopathy, ischemia, arrhythmias, hypertension, pericardial disease and thromboembolism. There is no consensus on standardizing the monitoring of cardiac function in these patients, and no reliable models able to predict the risk of cardiotoxicity.
With regard to cancer drugs, specialists in the area recognize the need to understand the mechanisms responsible for cardiotoxicity. Careful management and assessment of cardiac risk is essential in these patients, and the long-term effects of different treatments require further study. To this end, it is increasingly important to establish dynamic partnerships between oncologists and cardiologists in order to reduce mortality and improve patients’ quality of life, while maintaining the efficacy of the treatment.
To summarize, cardiotoxicity induced by cancer treatment should be considered a multidisciplinary problem, the approach to which should include basic science, oncology and cardiovascular medicine.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was funded by the Portuguese Foundation for Science and Technology (project no. FCOMP-01-0124-FEDER-011051, FEDER, COMPETE, Ref. FCT-PTDC/SAU-FCF/100442/2008), the João Porto 2008 Study Grant, and by the University of Porto/Santander Totta (Projectos pluridisciplinares IJUP 2009 and 2010). Carmen Brás-Silva is a researcher for Programa Ciência 2008.
Please cite this article as: Adão R, et al. Cardiotoxicidade associada à terapêutica oncológica: mecanismos fisiopatológicos e estratégias de prevenção. Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.11.002.