Myocardial ischemia can be assessed with cardiac magnetic resonance perfusion imaging (MRPI). This study aimed to analyze the clinical utility of MRPI in the diagnosis of significant coronary artery disease.
MethodsFifty-five patients were examined with a 1.5 T MR scanner (Siemens Symphony), with a first pass of 0.10 mmol/kg gadolinium chelate, at rest and during adenosine vasodilatation (140 μg/kg/min for 4 min) using an inversion recovery steady-state free precession sequence. The results were compared with coronary angiography and with SPECT myocardial perfusion images. Agreement for qualitative diagnosis was measured by the kappa coefficient, taking statistical significance as 95%. Minimum clinical follow-up was 12 months.
ResultsIn 19 patients (34.5%) MRPI was negative for myocardial ischemia and necrosis, in 17 (30.9%) it was negative for ischemia but positive for necrosis, in 7 (12.7%) only ischemia was present and in 12 (21.8%) the ischemic area was larger than the necrotic area. The correlation between MRPI and coronary angiography for ischemia detection by coronary artery territory was very good: left anterior descending and right coronary – k=0.8571 (0.59-1), circumflex – k=0.8108 (0.59-1). By contrast, there was no correlation in terms of myocardial ischemia detection between MRPI and SPECT.
ConclusionsMRPI is able to diagnose significant coronary disease in a high risk population, by detection of myocardial ischemia.
Introdução e objectivos: A isquémia do miocárdio pode ser avaliada através da ressonância magnética de perfusão (RMp). O objetivo do trabalho foi avaliar o uso clínico da RMp no estudo da doença cardíaca isquémica (DCI).
MétodosForam estudados 55 doentes através de RMp, num aparelho de 1,5 T (Siemens Symphony), através da injeção de quelato de gadolínio (0,10 mmol/kg), utilizando uma sequência IR-SSFP. O stress foi induzido pela adenosina (140 μg/kg/min durante 4 min). Os resultados foram comparados com os das coronariografias e das cintigrafias de perfusão do miocárdio. A concordância entre as variáveis qualitativas foi a avaliada através do coeficiente kappa. Foi considerada significância estatística a 95%. Efetuou-se um follow-up clínico mínimo de 12 meses.
ResultadosDas 55 ressonâncias magnéticas (RM) efetuadas, em 19 (34,5%) não se detetou isquemia ou necrose do miocárdio, em 17 (30,9%) foi diagnosticada área de necrose sem isquemia, em 7 (12,7%) isquemia isolada e em 12 (21,8%) isquemia em doente com enfarte prévio. A comparação específica em função do território de irrigação coronária entre a RMp e a coronariografia foi muito boa: descendente anterior e coronária direita – k = 0,8571; intervalo 0,59-1, circunflexa – k = 0,8108; intervalo 0,59-1. Não se conseguiu obter qualquer razão de concordância entre as RMp e as cintigrafias realizadas.
ConclusõesNuma população com elevada prevalência de doença coronária, a RMp mostrou ser um exame capaz de diagnosticar doença coronária significativa, através da identificação de isquemia do miocárdio.
Magnetic resonance imaging (MRI) was first used to obtain ECG-gated images of the heart 30 years ago. At first it was used only for morphological and functional cardiac studies, but over the last 12 years other techniques have been developed, including late enhancement and myocardial perfusion imaging, which greatly increases the utility of MRI in the diagnosis of coronary artery disease (CAD).1 Since then, the diagnostic performance of cardiac magnetic resonance perfusion imaging (MRPI) in significant coronary stenosis has been investigated in many studies comparing it with coronary angiography and other imaging modalities, which found a sensitivity of 70-93% and specificity of 71-97%.2–15
There is greater awareness of MRI studies of myocardial viability16–20 by late enhancement techniques than of myocardial perfusion imaging.
Impaired myocardial perfusion is directly related to myocardial ischemia. In clinical practice myocardial perfusion is more commonly assessed by scintigraphy, particularly single-photon emission computed tomography (SPECT).
Myocardial perfusion is preserved at rest until narrowing of the coronary lumen exceeds 90%,3 so to detect ischemia by imaging techniques, the oxygen requirements of the cardiomyocytes must be increased by physical exercise or pharmacological agents. With MRI, increased coronary blood flow is achieved through the use of vasodilators such as adenosine.
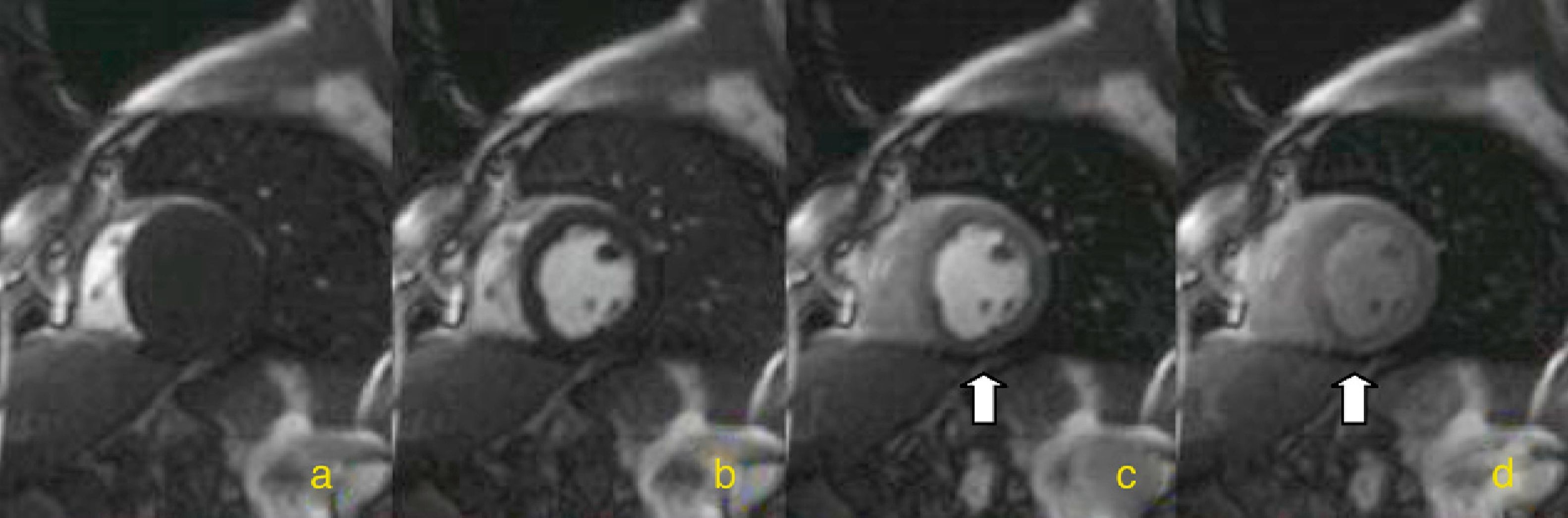
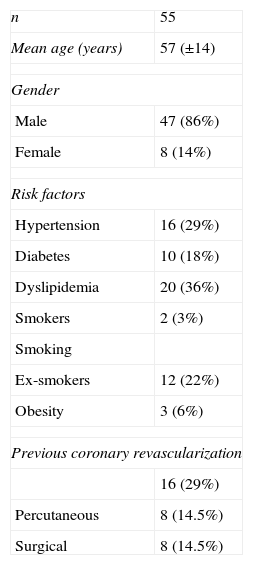
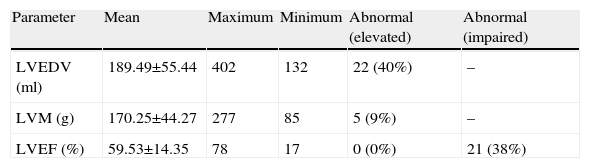
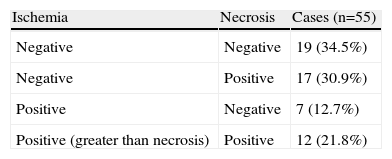
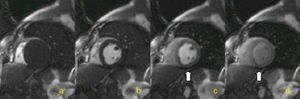
Myocardial perfusion is assessed by MRI in dynamic T1-weighted sequences after rapid intravenous administration of gadolinium chelate (Figure 1).
This study aimed to analyze the clinical utility of MRPI in the diagnosis of significant CAD.
MethodsFifty-five consecutive patients referred for MRPI at Coimbra University Hospitals between January 2004 and November 2010 were included in the study. Patients were selected by cardiologists at hospital consultations for CAD. After exclusion of those with contraindications for MRI (defibrillators, pacemakers, cochlear implants, foreign bodies of ferromagnetic material or disabling claustrophobia), contraindications for administration of gadolinium (history of gadolinium allergy or renal dysfunction with glomerular filtration rate <30 ml/min/1.73 m2) or of adenosine (history of bronchospasm or asthma, persistent hypotension with systolic blood pressure <90 mmHg, unstable angina, myocardial infarction (MI) less than two days previously, high-degree atrioventricular block, uncontrolled arrhythmias, or critical aortic stenosis), no patients were excluded once selected for MRPI.
The purpose of referral for MRPI was for diagnosis of suspected CAD or for risk stratification and/or assessment of myocardial viability in patients with known disease. Of the 55 patients who underwent MRPI, 28 (51%) had a known history of CAD, 25 (45%) with previous MI and 16 (29%) with previous coronary revascularization. Three patients who had undergone revascularization had no history of MI. The 27 patients (49%) with no history of CAD had an intermediate or high pretest probability of disease. Patient characteristics including age, date of MRPI, gender and cardiovascular risk factors were recorded, as were the coronary territories that had been revascularized, either surgically or percutaneously, for subsequent analysis of correlations with MRPI findings.
MRPI was considered positive for ischemia in the presence of a perfusion defect that was larger than the necrotic area, whether at the same site or elsewhere.
The MRPI findings on the affected myocardial territories were compared to those from SPECT imaging performed in the previous six months and from coronary angiography performed in the following six months, unless there had been a coronary event (unstable angina or MI) during those periods. Comparison with coronary angiography was not performed in two patients with hypertrophic cardiomyopathy. All affected territories were analyzed, both ischemic and necrotic. In the comparison between MRPI and coronary angiography, the territory of the left anterior descending coronary artery was considered to be the anterior wall, septal and apical segments, the territory of the left circumflex to be the left ventricular lateral wall segments, and the territory of the right coronary artery to be the left ventricular inferior wall segments.21
Coronary angiography was performed in two views in accordance with standard practice in the cardiology department of Coimbra University Hospitals. Significant stenosis was defined as >50% reduction in lumen diameter of a major epicardial artery or one of its main branches (caliber of ≥2 mm).
In the SPECT studies, myocardial perfusion was imaged after two injections of technetium-99m-tetrofosmin in a peripheral vein following a stress-rest protocol. ECG-gated tomographic image acquisition was performed 30-45 min after injection using a dual-detector gamma camera equipped with a low-energy, high-resolution collimator, with a 64 × 64 pixel matrix and acquiring 32 projections in 30 s through a trajectory of 180°. Perfusion defects were analyzed visually and left ventricular ejection fraction was quantified.
The MRI exams were performed on a Siemens Symphony Maestro Class 1.5 T scanner, using two passes (rest and stress) followed by assessment of late myocardial enhancement. Perfusion studies used T1-weighted inversion recovery steady-state free precession sequences with inversion pulse (flip angle 15°, inversion time 100 ms, echo time 1.11 ms, repetition time 182 ms, with a 78 × 128 pixel matrix) to obtain four images (three short cardiac axis and one long axis) per cardiac cycle or every other cardiac cycle, depending on heart rate.
A 4-min adenosine infusion was administered at 140 μg/kg/min. Perfusion imaging was performed after intravenous injection of 0.10 mmol/kg gadolinium at a flow rate of 4 ml/s. Left ventricular function was assessed in all patients.
Mean clinical follow-up was 33 months (minimum 12 months after MRPI), by consultation of hospital records and/or telephone contact with patients or relatives. Only major events were recorded (new MI or unstable angina or need for coronary revascularization).
Statistical analysisIn the statistical analysis, measures of central tendency (means) and of dispersion (standard deviation and 95% confidence intervals of the mean) were determined for quantitative variables. Qualitative (nominal) variables were expressed as frequencies (n) and percentages. Agreement for qualitative diagnosis was measured by the kappa coefficient, taking statistical significance as 95%.
ResultsDemographic characteristics and risk factors of the 55 patients who underwent MRPI are shown in Table 1.
Demographic and clinical characteristics of the study population.
| n | 55 |
| Mean age (years) | 57 (±14) |
| Gender | |
| Male | 47 (86%) |
| Female | 8 (14%) |
| Risk factors | |
| Hypertension | 16 (29%) |
| Diabetes | 10 (18%) |
| Dyslipidemia | 20 (36%) |
| Smokers | 2 (3%) |
| Smoking | |
| Ex-smokers | 12 (22%) |
| Obesity | 3 (6%) |
| Previous coronary revascularization | |
| 16 (29%) | |
| Percutaneous | 8 (14.5%) |
| Surgical | 8 (14.5%) |
Data presented as mean ± standard deviation or n (%).
Left ventricular morphology and function were assessed by MRI [in the 55 patients]. Mean left ventricular end-diastolic volume (LVEDV) was 189.49±55.44 with LV dilatation in 22 patients (22%); mean left ventricular mass was 170.25±44.27 g, and was elevated in five patients (9%) (including two with diagnosed hypertrophic cardiomyopathy). Mean left ventricular ejection fraction was 59.53±14.35%, impaired in 21 cases (38%) (Table 2).
Data from magnetic resonance perfusion imaging.
| Parameter | Mean | Maximum | Minimum | Abnormal (elevated) | Abnormal (impaired) |
| LVEDV (ml) | 189.49±55.44 | 402 | 132 | 22 (40%) | – |
| LVM (g) | 170.25±44.27 | 277 | 85 | 5 (9%) | – |
| LVEF (%) | 59.53±14.35 | 78 | 17 | 0 (0%) | 21 (38%) |
Means are presented as specified or as mean ± standard deviation; abnormal values as n (%).
LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVM: left ventricular mass.
Additional findings of the MRI studies included six cases of aortic stenosis (10.9%), seven of mitral regurgitation (12.7%), four of aortic regurgitation (7.3%), two of mitral stenosis (3.6%) and one of lesions from previous myocarditis (1.8%).
In 19 patients (34.5%) MRPI was negative for myocardial ischemia and necrosis, in 17 (30.9%) it was negative for ischemia (the ischemic area under stress coinciding with the necrotic area), in 7 (12.7%) it was positive for ischemia (perfusion defects only appearing under stress) and in 12 (21.8%) the ischemic area was larger than the necrotic area (positive for ischemia in patients with previous MI) (Table 3).
None of the 36 patients with MRPI negative for ischemia suffered major clinical events related to CAD during follow-up; only three underwent coronary angiography, which was negative for significant coronary lesions in all cases.
In two of the 19 patients with positive MRPI no comparison was made with the results of coronary angiography due to the presence of hypertrophic cardiomyopathy and hence of a cause of ischemia other than CAD. Of the other 17 patients, 11 underwent coronary angiography, which revealed significant coronary lesions in nine.
Of the six patients with MRPI positive for ischemia who did not undergo coronary angiography in the following six months, none suffered an acute coronary event, while in the 14 patients who did undergo coronary angiography in the following six months, no revascularization or MI was recorded between the exams.
In two patients with positive MRPI the result of coronary angiography was negative; in one, although a coronary lesion responsible for an infarcted area was also identified by MRI, the lesion did not coincide with the suspected ischemic area.
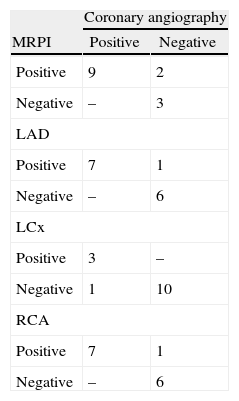
The overall correlation between MRPI and coronary angiography for ischemia detection was good (k=0.6585; interval 0.2467-1). The correlation between the techniques by coronary artery territory was very good: left anterior descending and right coronary – k=0.8571 (0.59-1), circumflex – k=0.8108 (0.59-1) (Table 4).
Comparison of results of magnetic resonance perfusion imaging and coronary angiography by coronary artery territory (n=14).
| Coronary angiography | ||
| MRPI | Positive | Negative |
| Positive | 9 | 2 |
| Negative | – | 3 |
| LAD | ||
| Positive | 7 | 1 |
| Negative | – | 6 |
| LCx | ||
| Positive | 3 | – |
| Negative | 1 | 10 |
| RCA | ||
| Positive | 7 | 1 |
| Negative | – | 6 |
LAD: left anterior descending coronary artery; LCx: left circumflex coronary artery; MRPI: magnetic resonance perfusion imaging; RCA: right coronary artery.
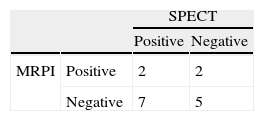
Of the 55 patients who underwent MRPI, 16 had undergone SPECT imaging in the previous six months; no revascularization or MI was recorded between the exams.
There was no correlation between the MRPI and SPECT results; the agreement between the exams was no better than random (Table 5).
Only two patients underwent all three exams, one with positive SPECT, negative MRPI and no coronary lesions on angiography, and one with negative SPECT and positive MRPI and angiography. No major coronary event or revascularization was recorded between the exams.
During follow-up, none of the patients with negative MRPI (n=36), and none of those with positive MRPI who did not undergo repeat coronary angiography, suffered a major coronary event. There was one death from cancer.
No patient required suspension of adenosine during the 4-min infusion period. All reactions to adenosine (flushing, chest discomfort, dizziness, atrioventricular block) were transitory and ended immediately after administration.
DiscussionThe ability of MRPI to detect significant coronary stenosis compared to coronary angiography has been investigated in various studies, most of them in single centers and in patients referred for coronary angiography.3–15
In 2007 Nandalur et al. published a meta-analysis of the diagnostic performance of stress cardiac MRI in the detection of CAD, based on 14 single-center studies with a total of 1183 patients. The technique had good specificity (81%) and sensitivity (91%).2
Wolff et al. conducted a multicenter dose-ranging study of gadolinium with 99 patients scheduled for coronary angiography and found an area under the ROC curve of 0.90±0.04 and good sensitivity (93%) and specificity (75%).9
The population of the present study consisted of patients referred for hospital cardiology consultations with suspected or diagnosed ischemic heart disease; there was a high prevalence of CAD, 16 patients (29%) having undergone coronary revascularization prior to MRPI. This high prevalence undoubtedly explains the high proportion of patients with increased LVEDV (n=22; 40%) and with impaired systolic function (n=21; 38%).
Late enhancement MRI revealed images compatible with previous MI in 29 patients (53%), of whom 25 had a clinical history of infarction; this was thus a high risk population.
According to the literature, the performance of MRPI in diagnosing significant CAD appears to be similar to that of SPECT, with figures for sensitivity and specificity of 83-95% and 53-95% for SPECT and 70-93% and 71-97% for MRPI, respectively.1,3–15,22
However, there was no correlation between the two techniques in the present study, which may be due to bias in the selection of patients referred for MRPI and the small sample size. In some cases MRPI was requested because clinical suspicion was not in agreement with the SPECT findings; 16 patients had undergone SPECT in the 12 months before MRPI, which the attending cardiologist requested because of doubts concerning the SPECT result. In most cases in which SPECT was positive and subsequent MRPI was negative, there were no further imaging studies, since coronary angiography was not considered the gold standard.
Only two patients underwent SPECT, MRPI and coronary angiography; no major coronary event or revascularization was recorded between the exams. Considering coronary angiography as the gold standard, these patients represented one false positive and one false negative for CAD by SPECT imaging, while they were shown to be a true positive and a true negative by MRPI.
It is notoriously difficult to evaluate SPECT images when vessels of more than one coronary territory are affected. In our study, of the 11 patients with positive MRPI who also underwent coronary angiography, seven had significant lesions in two or all three territories. The existence of significant lesions in more than one territory did not hinder identification of perfusion defects by MRI, probably due to its higher resolution.
Unlike SPECT, MRPI does not involve ionizing radiation, which is particularly advantageous in revascularized patients who may require multiple follow-up exams. Other advantages compared to SPECT include the absence of attenuation artifacts and its greater anatomical resolution, enabling visualization of subendocardial defects, which may have contributed to the lack of correlation between these exams in a population that had already been thoroughly “triaged” by scintigraphic imaging.
MRI also provides precise assessment of systolic function and calculation of LVEDV, and late enhancement images identify non-viable myocardium with great accuracy.16–19 It can also detect other alterations including valve disease (17 cases in our population), while the additional morphological characterization provided by late enhancement identified a scar in one patient that was compatible with previous myopericarditis.
Adenosine stress MRI is a safe diagnostic method, and there were no significant complications in our exams.
The good agreement between MRPI and coronary angiography, even in our small sample with a high prevalence of CAD, highlights the diagnostic power of MRPI. Its high spatial resolution was the reason for the excellent correlation observed when the techniques were compared by coronary territory.
The advantages of MRPI – no ionizing radiation, good spatial resolution, sufficiently rapid sequences to acquire images during a single breath-hold, and the large amount of information obtained in a short exam (around 30 min) – together with its ability to assess myocardial morphology and function, perfusion and viability, mean that it is likely to be increasingly used in the diagnosis and assessment of CAD.16–19
The main obstacle to the wider use of MRPI is its limited availability in an extremely large patient population who require prompt assessment.
In this high risk population, no patient with MRPI negative for myocardial ischemia presented lesions on coronary angiography or suffered a major coronary event during follow-up. However, it should be noted that six patients with positive MRPI did not subsequently undergo angiography, and also did not suffer major coronary events. The prognostic value of MRPI is thus difficult to evaluate in this small population.
The fact that coronary angiography was not performed in all patients makes it impossible to establish a gold standard exam, which is one of the main limitations of the study. The small sample and the low number of SPECT exams available for comparison also limit the ability of this study to validate the technique.
ConclusionMRPI is able to diagnose significant coronary disease in a high risk population, by detection of myocardial ischemia.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Donato P, et al. Ressonância Magnética Cardíaca de Perfusão em Stress: a experiência de um centro nacional. Rev Port Cardiol 2012. http://dx.doi.org/10.1016/j.repc.2012.05.016