Higher values of red blood cell distribution width (RDW) have recently been associated with worse outcome in patients with cardiovascular disease. However, its relation to bleeding events in patients with non-ST elevation acute coronary syndromes has not been established.
AimTo determine the prognostic value of RDW in patients with non-ST segment elevation acute coronary syndromes, particularly regarding the risk of major bleeding.
MethodsWe analyzed 513 consecutive patients admitted with non-ST elevation acute coronary syndromes. The population was divided into tertiles of baseline RDW and clinical, laboratory characteristics and adverse events were analyzed for each group. The primary outcome was defined as the occurrence of major bleeding (according to the Crusade bleeding score). The predictive value of RDW for risk of major bleeding was determined.
ResultsThe mean RDW was 15.13%±1.62%. Patients in the third tertile were older and more frequently had renal dysfunction or previous coronary revascularization. Higher values of RDW were associated with greater risk of major bleeding and in-hospital death. RDW >15.7% was an independent predictor of bleeding events (odds ratio 3.1, 95% CI 1.4-6.9).
ConclusionsIn a population of patients with non-ST elevation acute coronary syndromes, RDW was associated with higher in-hospital mortality and was an independent predictor of in-hospital major bleeding.
Níveis elevados de índice de dispersão eritrocitária (RDW) têm sido recentemente associados a um pior prognóstico em doentes com doença cardiovascular. A sua relação com o risco de eventos hemorrágicos em doentes com síndrome coronária aguda sem supradesnivelamento do segmento ST não está, no entanto, estabelecida.
ObjetivoDeterminar o valor prognóstico do RDW em doentes com síndrome coronária aguda sem supradesnivelamento do segmento ST em particular sobre o risco de hemorragia major.
MétodosForam estudados 513 doentes consecutivos internados com o diagnóstico de síndrome coronária aguda sem supradesnivelamento do segmento ST. A população foi agrupada segundo os tercis da distribuição do RDW e caracterizada de acordo com as características clínicas, laboratoriais e eventos adversos. O evento primário foi definido como a presença de hemorragia major durante o internamento (definida pela classificação do registo Crusade). Determinou-se o valor preditor do RDW sobre o risco de hemorragia major.
ResultadosO RDW médio foi de 15,13 ± 1,62%. Os doentes incluídos no terceiro tercil apresentaram uma idade mais avançada e mais frequentemente disfunção renal ou antecedentes de revascularização coronária. Valores crescentes de RDW associaram-se a um aumento do risco de hemorragia major e de mortalidade hospitalar. Um valor de RDW > 15,7% foi um preditor independente de eventos hemorrágicos (odds ratio ajustado 3,1, IC 95% 1,4-6,9).
ConclusõesNuma população de doentes com síndrome coronária aguda sem supradesnivelamento do segmento ST, o RDW esteve associado a uma maior mortalidade intra-hospitalar, tendo constituído um fator de risco independente para hemorragia major intra-hospitalar.
Red blood cell distribution width (RDW) is a routine laboratory parameter that shows variation in red blood cell size on a standard hemogram and is often used in assessing blood diseases. Higher RDW has been associated with worse outcome in the elderly, in patients with heart failure or ischemic heart disease and in those undergoing percutaneous coronary revascularization, irrespective of baseline hematocrit.1–9 An association with a higher adverse event rate in patients with acute coronary syndrome (ACS) has also been reported in the literature.3,10
The aim of this study was to assess the relationship between RDW values and risk for bleeding events in patients with non-ST elevation ACS (NSTE-ACS).
MethodsStudy populationWe analyzed 513 consecutive patients admitted to a coronary care unit with a diagnosis of NSTE-ACS between January 2007 and December 2008. NSTE-ACS includes non-ST elevation myocardial infarction (NSTEMI), defined as chest pain associated with elevated biomarkers of myocardial necrosis with or without alterations in ventricular repolarization suggestive of ischemia, and unstable angina, which is characterized by precordial pain with no rise in necrosis markers. Patients who did not undergo blood tests on admission were excluded.
Patient characteristicsDemographic characteristics, body mass index, diastolic and systolic blood pressure (SBP), and heart rate (HR) at admission were determined for each patient. Cardiovascular risk factors (dyslipidemia, hypertension and diabetes) and history of coronary artery disease (previous myocardial infarction and percutaneous or surgical revascularization), cerebrovascular or peripheral arterial disease were also determined, as well as medication (aspirin, angiotensin-converting enzyme inhibitors, beta-blockers or statins) taken as outpatients or begun during hospitalization. The patients were stratified according to the Crusade bleeding score.
Laboratory characteristicsRDW values were obtained from baseline laboratory tests performed in a central laboratory using a Cell-Dyn 3700 analyzer. Reference values were between 11.6% and 14%. In addition, admission values for hemoglobin (Hb), serum creatinine and creatinine clearance (estimated by the Cockcroft-Gault formula), platelet count, prothrombin time, activated partial thromboplastin time and international normalized ratio were recorded.
OutcomeThe primary outcome was defined as the occurrence of major bleeding during hospitalization according to the Crusade scoring system: >12% fall in baseline hematocrit; intracranial bleeding; documented retroperitoneal bleeding; need for red cell concentrate (RCC) transfusion with baseline hematocrit >28%; need for RCC transfusion with baseline hematocrit <28% and documented bleeding.
Secondary outcomes were in-hospital death and combined in-hospital death and major bleeding.
Statistical analysisThe population was divided into tertiles according to RDW values. The three groups were compared in terms of demographic, clinical and laboratory characteristics and adverse event rate. Continuous variables were expressed as means ± standard deviation and compared using the one-way ANOVA test and the Student's t test. Categorical variables were expressed as frequencies and percentages and compared using the chi-square test. Results with p<0.05 were considered statistically significant. A logistic regression model was used to assess the correlation between RDW values and bleeding events and to determine predictors of major bleeding, which included the variables that presented statistically significant differences on univariate analysis, and Hb. RDW was included as a categorical variable. The statistical program used was SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
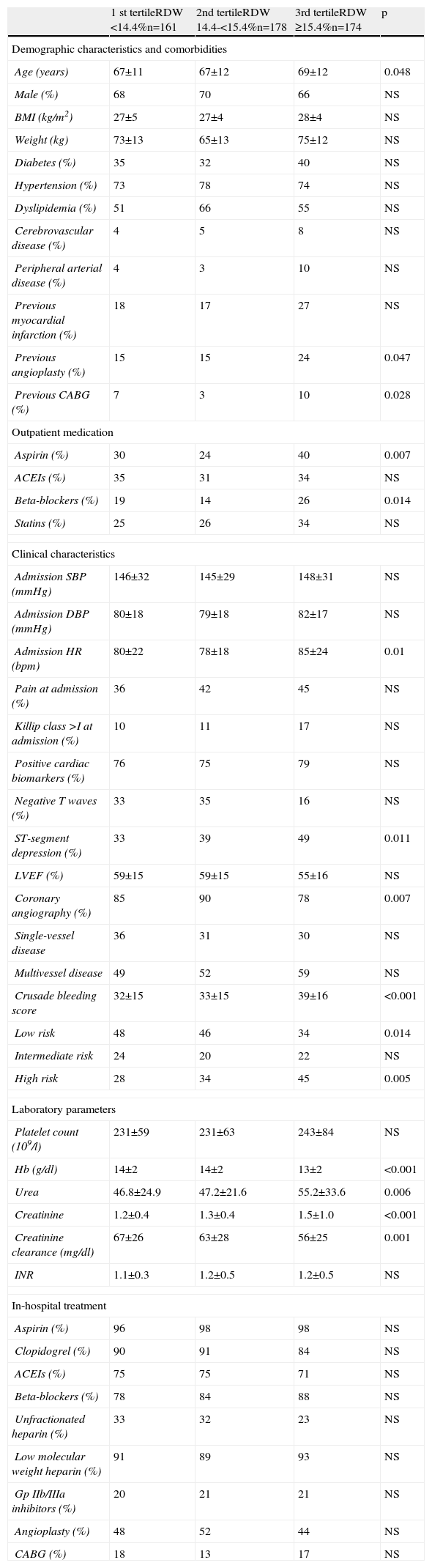
ResultsMean RDW was 15.1%±1.6% (median 14.9%). Of the 76.6% of patients presenting with NSTEMI, 83% underwent coronary angiography, all via femoral access. Patients with higher RDW values were older and were more often previously medicated with aspirin and beta-blockers, and more had a history of coronary revascularization. Those in the third tertile more frequently had ST-segment depression, higher HR and lower Hb at admission. There were no significant differences between the three groups in antithrombotic therapy begun during hospitalization, or in the number of patients undergoing angioplasty. Those in the third tertile had higher Crusade bleeding scores. The other variables assessed are compared in Table 1.
Demographic, clinical and laboratory characteristics according to tertile of red blood cell distribution width.
| 1 st tertileRDW <14.4%n=161 | 2nd tertileRDW 14.4-<15.4%n=178 | 3rd tertileRDW ≥15.4%n=174 | p | |
| Demographic characteristics and comorbidities | ||||
| Age (years) | 67±11 | 67±12 | 69±12 | 0.048 |
| Male (%) | 68 | 70 | 66 | NS |
| BMI (kg/m2) | 27±5 | 27±4 | 28±4 | NS |
| Weight (kg) | 73±13 | 65±13 | 75±12 | NS |
| Diabetes (%) | 35 | 32 | 40 | NS |
| Hypertension (%) | 73 | 78 | 74 | NS |
| Dyslipidemia (%) | 51 | 66 | 55 | NS |
| Cerebrovascular disease (%) | 4 | 5 | 8 | NS |
| Peripheral arterial disease (%) | 4 | 3 | 10 | NS |
| Previous myocardial infarction (%) | 18 | 17 | 27 | NS |
| Previous angioplasty (%) | 15 | 15 | 24 | 0.047 |
| Previous CABG (%) | 7 | 3 | 10 | 0.028 |
| Outpatient medication | ||||
| Aspirin (%) | 30 | 24 | 40 | 0.007 |
| ACEIs (%) | 35 | 31 | 34 | NS |
| Beta-blockers (%) | 19 | 14 | 26 | 0.014 |
| Statins (%) | 25 | 26 | 34 | NS |
| Clinical characteristics | ||||
| Admission SBP (mmHg) | 146±32 | 145±29 | 148±31 | NS |
| Admission DBP (mmHg) | 80±18 | 79±18 | 82±17 | NS |
| Admission HR (bpm) | 80±22 | 78±18 | 85±24 | 0.01 |
| Pain at admission (%) | 36 | 42 | 45 | NS |
| Killip class >I at admission (%) | 10 | 11 | 17 | NS |
| Positive cardiac biomarkers (%) | 76 | 75 | 79 | NS |
| Negative T waves (%) | 33 | 35 | 16 | NS |
| ST-segment depression (%) | 33 | 39 | 49 | 0.011 |
| LVEF (%) | 59±15 | 59±15 | 55±16 | NS |
| Coronary angiography (%) | 85 | 90 | 78 | 0.007 |
| Single-vessel disease | 36 | 31 | 30 | NS |
| Multivessel disease | 49 | 52 | 59 | NS |
| Crusade bleeding score | 32±15 | 33±15 | 39±16 | <0.001 |
| Low risk | 48 | 46 | 34 | 0.014 |
| Intermediate risk | 24 | 20 | 22 | NS |
| High risk | 28 | 34 | 45 | 0.005 |
| Laboratory parameters | ||||
| Platelet count (109/l) | 231±59 | 231±63 | 243±84 | NS |
| Hb (g/dl) | 14±2 | 14±2 | 13±2 | <0.001 |
| Urea | 46.8±24.9 | 47.2±21.6 | 55.2±33.6 | 0.006 |
| Creatinine | 1.2±0.4 | 1.3±0.4 | 1.5±1.0 | <0.001 |
| Creatinine clearance (mg/dl) | 67±26 | 63±28 | 56±25 | 0.001 |
| INR | 1.1±0.3 | 1.2±0.5 | 1.2±0.5 | NS |
| In-hospital treatment | ||||
| Aspirin (%) | 96 | 98 | 98 | NS |
| Clopidogrel (%) | 90 | 91 | 84 | NS |
| ACEIs (%) | 75 | 75 | 71 | NS |
| Beta-blockers (%) | 78 | 84 | 88 | NS |
| Unfractionated heparin (%) | 33 | 32 | 23 | NS |
| Low molecular weight heparin (%) | 91 | 89 | 93 | NS |
| Gp IIb/IIIa inhibitors (%) | 20 | 21 | 21 | NS |
| Angioplasty (%) | 48 | 52 | 44 | NS |
| CABG (%) | 18 | 13 | 17 | NS |
ACEIs: angiotensin-converting enzyme inhibitors; BMI: body mass index; CABG: coronary artery bypass grafting; DBP: diastolic blood pressure; Gp: glycoprotein; Hb: hemoglobin; HR: heart rate; INR: international normalized ratio; LVEF: left ventricular ejection fraction; SBP: systolic blood pressure.
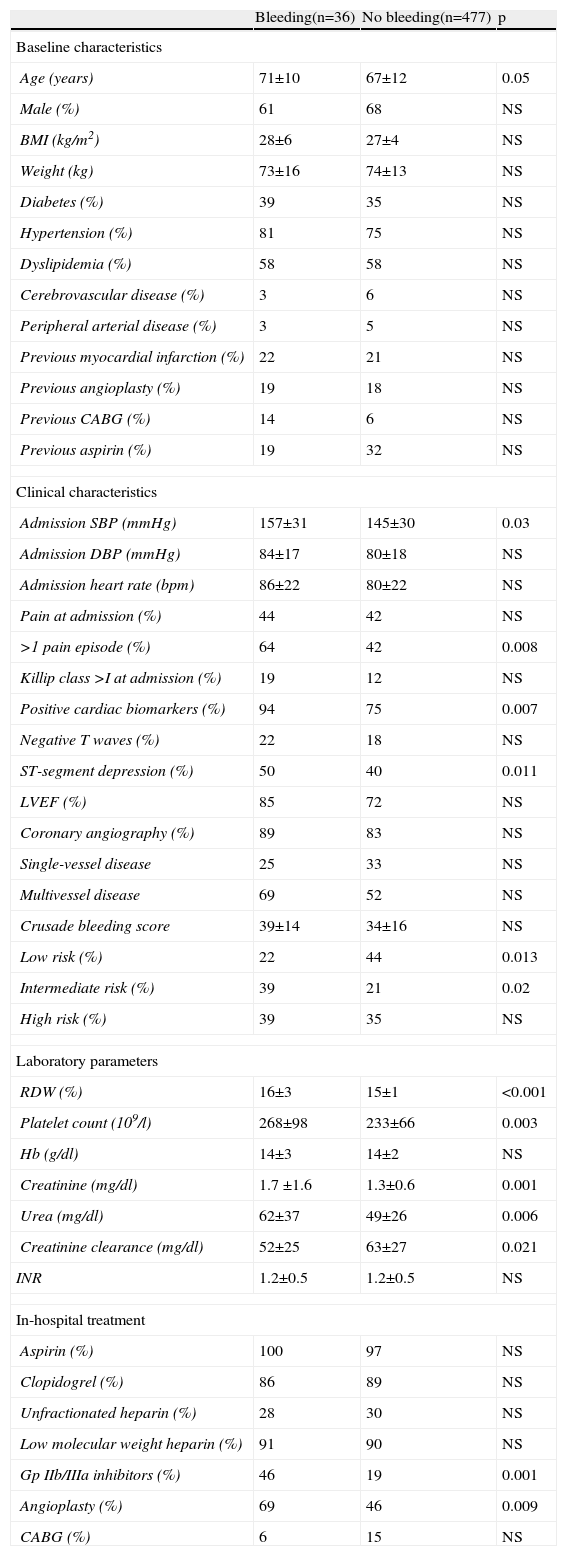
The rate of major bleeding was 7% (36 events). Of these patients, 32 (89%) had undergone coronary angiography. In 44% of cases (n=16), the bleeding was associated with the arterial puncture site (14 groin hematomas and two cases of retroperitoneal bleeding). The site of bleeding was not identified in 33% of patients (n=12). There was also one case of alveolar hemorrhage, one of subarachnoid hemorrhage, two cases of genitourinary bleeding, three of gastrointestinal bleeding and one associated with recent orthopedic surgery. On univariate analysis (Table 2), bleeding was associated with older age, higher SBP and renal dysfunction. These patients were more often medicated with glycoprotein (Gp) IIb/IIIa inhibitors and more had undergone coronary angioplasty.
Demographic, clinical and laboratory characteristics according to occurrence of bleeding.
| Bleeding(n=36) | No bleeding(n=477) | p | |
| Baseline characteristics | |||
| Age (years) | 71±10 | 67±12 | 0.05 |
| Male (%) | 61 | 68 | NS |
| BMI (kg/m2) | 28±6 | 27±4 | NS |
| Weight (kg) | 73±16 | 74±13 | NS |
| Diabetes (%) | 39 | 35 | NS |
| Hypertension (%) | 81 | 75 | NS |
| Dyslipidemia (%) | 58 | 58 | NS |
| Cerebrovascular disease (%) | 3 | 6 | NS |
| Peripheral arterial disease (%) | 3 | 5 | NS |
| Previous myocardial infarction (%) | 22 | 21 | NS |
| Previous angioplasty (%) | 19 | 18 | NS |
| Previous CABG (%) | 14 | 6 | NS |
| Previous aspirin (%) | 19 | 32 | NS |
| Clinical characteristics | |||
| Admission SBP (mmHg) | 157±31 | 145±30 | 0.03 |
| Admission DBP (mmHg) | 84±17 | 80±18 | NS |
| Admission heart rate (bpm) | 86±22 | 80±22 | NS |
| Pain at admission (%) | 44 | 42 | NS |
| >1 pain episode (%) | 64 | 42 | 0.008 |
| Killip class >I at admission (%) | 19 | 12 | NS |
| Positive cardiac biomarkers (%) | 94 | 75 | 0.007 |
| Negative T waves (%) | 22 | 18 | NS |
| ST-segment depression (%) | 50 | 40 | 0.011 |
| LVEF (%) | 85 | 72 | NS |
| Coronary angiography (%) | 89 | 83 | NS |
| Single-vessel disease | 25 | 33 | NS |
| Multivessel disease | 69 | 52 | NS |
| Crusade bleeding score | 39±14 | 34±16 | NS |
| Low risk (%) | 22 | 44 | 0.013 |
| Intermediate risk (%) | 39 | 21 | 0.02 |
| High risk (%) | 39 | 35 | NS |
| Laboratory parameters | |||
| RDW (%) | 16±3 | 15±1 | <0.001 |
| Platelet count (109/l) | 268±98 | 233±66 | 0.003 |
| Hb (g/dl) | 14±3 | 14±2 | NS |
| Creatinine (mg/dl) | 1.7 ±1.6 | 1.3±0.6 | 0.001 |
| Urea (mg/dl) | 62±37 | 49±26 | 0.006 |
| Creatinine clearance (mg/dl) | 52±25 | 63±27 | 0.021 |
| INR | 1.2±0.5 | 1.2±0.5 | NS |
| In-hospital treatment | |||
| Aspirin (%) | 100 | 97 | NS |
| Clopidogrel (%) | 86 | 89 | NS |
| Unfractionated heparin (%) | 28 | 30 | NS |
| Low molecular weight heparin (%) | 91 | 90 | NS |
| Gp IIb/IIIa inhibitors (%) | 46 | 19 | 0.001 |
| Angioplasty (%) | 69 | 46 | 0.009 |
| CABG (%) | 6 | 15 | NS |
BMI: body mass index; CABG: coronary artery bypass grafting; DBP: diastolic blood pressure; Gp: glycoprotein; Hb: hemoglobin; INR: international normalized ratio; RDW: red blood cell distribution width; SBP: systolic blood pressure.
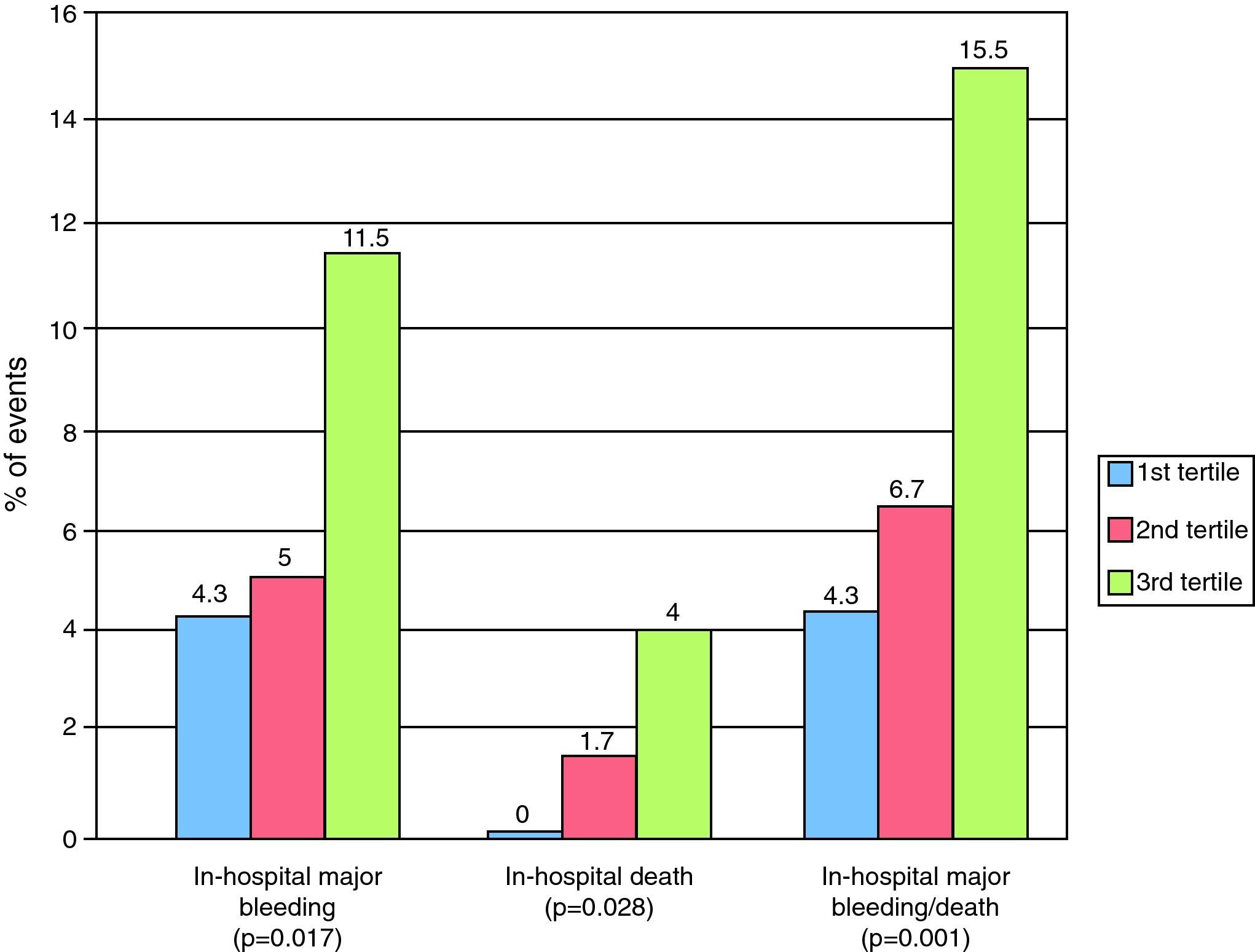
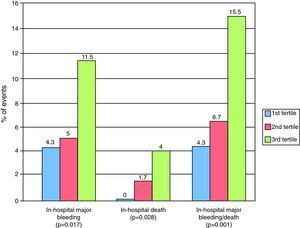
Higher RDW values were associated with a higher Crusade score and a higher percentage of in-hospital bleeding, death and combined in-hospital death and major bleeding (Figure 1). The c-statistic for the Crusade bleeding score in our population was 0.597.
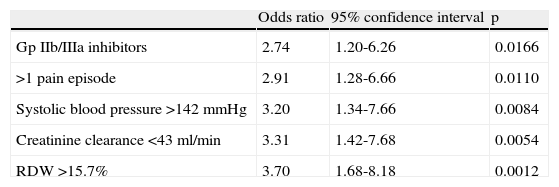
Independent predictors of bleeding events were use of Gp IIb/IIIa inhibitors, more than one pain episode, SBP >142mmHg, creatinine clearance <43ml/min, and RDW >15.7% (Table 3).
Independent predictors of major bleeding.
| Odds ratio | 95% confidence interval | p | |
| Gp IIb/IIIa inhibitors | 2.74 | 1.20-6.26 | 0.0166 |
| >1 pain episode | 2.91 | 1.28-6.66 | 0.0110 |
| Systolic blood pressure >142 mmHg | 3.20 | 1.34-7.66 | 0.0084 |
| Creatinine clearance <43 ml/min | 3.31 | 1.42-7.68 | 0.0054 |
| RDW >15.7% | 3.70 | 1.68-8.18 | 0.0012 |
Gp: glycoprotein; RDW: red blood cell distribution width.
The association between RDW and increased ischemic events in patients with cardiovascular disease is well known, but there have been few studies on its relation to bleeding events. In our study population, RDW was an independent predictor of major bleeding in patients with NSTEMI.
As reported in other studies, patients in the highest tertile of RDW presented characteristics associated with more severe coronary artery disease, particularly older age and a greater frequency of previous myocardial revascularization.2,3 They more frequently presented higher HR, ST-segment depression, anemia and renal dysfunction at admission. However, this patient group was treated more conservatively, less often undergoing coronary angiography. As in other populations with acute coronary syndrome, high RDW values were associated with worse outcome and greater in-hospital mortality in our study.2,3,5–7,10
As in the literature, the Crusade score showed poor discriminatory ability to predict bleeding events in our population.12,13 It is thus important to identify new risk factors that will lead to better stratification of risk for major bleeding in patients with NSTE-ACS.
The presence of renal failure and use of Gp IIb/IIIa inhibitors were also independent predictors of bleeding, as noted in previous studies.11 However, no correlation was found in our population with other conventional risk factors, such as female gender or heart failure. Bleeding was associated with the arterial puncture site in a significant proportion of cases, which highlights the importance of using radial access to reduce adverse events.
Several studies have reported that increased ischemic risk is often associated with a higher bleeding event rate, which makes management of these patients more difficult. As with other factors associated with more severe cardiovascular disease and worse prognosis in patients with NSTE-ACS, RDW is also a predictor of bleeding. The fact that it is an easily available laboratory parameter in routine blood tests that does not entail additional costs may encourage its wider use in the future.
The mechanisms behind its association with worse outcome in patients with cardiovascular disease have yet to be fully clarified but are probably multifactorial. High RDW values may indicate accelerated red blood cell destruction, nutritional deficiency (folic acid or vitamin B12), a proinflammatory state, or renal dysfunction that impairs erythropoiesis and hence red blood cell maturation.1,2,5 Further studies will be required for a better understanding of the relation of RDW to increased adverse events in patients with cardiovascular disease.
Study limitationsThe present study has the limitations of all single-center retrospective, non-randomized analyses. Although baseline Hb and other parameters were included in the multivariate analysis, the presence of confounding factors, known or unknown, such as comorbidities and levels of erythropoietin, iron, folic acid and vitamin B12, cannot be excluded. It is also important to assess the prognostic value of RDW in patients with ST-elevation ACS, who were not included in this analysis, as well as its ability to predict events in the long term.
ConclusionsIn our population, higher RDW values were associated with greater in-hospital mortality and were an independent predictor of in-hospital major bleeding in patients with NSTE-ACS. The ready availability of this parameter at no additional cost may encourage its wider use in clinical practice in the future.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gonçalves S, et al. Implicações do índice de dispersão eritrocitária no risco de eventos hemorrágicos em doentes com síndrome coronária aguda sem supra-desnivelamento do segmento ST. Rev Port Cardiol. 2012. doi:10.1016/j.repc.2012.05.018.