Brugada syndrome (BrS) is associated with increased risk of ventricular arrhythmias and sudden death. Some drugs can trigger the electrocardiographic and arrhythmic manifestations of this syndrome. Cold medicines for symptom relief are sold without prescription in Brazil and most contain antihistamines and adrenergic agonists. We report a case of BrS probably triggered by the use of such medication.
A síndrome de Brugada (SBr) está associada a um risco elevado de arritmias ventriculares e morte súbita. Alguns fármacos podem propiciar a ocorrência da manifestação eletrocardiográfica e arrítmica dessa síndrome. Os medicamentos antigripais, para sintomas, vendidos sem receita médica no Brasil, são compostos, na sua grande maioria, de anti-histamínicos e agonistas adrenérgicos. Aqui relatamos um caso de SBr provavelmente manifesta pelo uso desse tipo de medicação.
Brugada syndrome (BrS) is characterized by a propensity to develop cardiac arrhythmias in patients who have a typical electrocardiographic pattern of ST-T segment elevation in the right precordial leads (V1–V3) and structurally normal hearts. Such patients are at high risk of sudden cardiac death (SCD) as a result of ventricular fibrillation.1 The syndrome, an ion channel disease, was first described in 1992 in eight patients who presented recurrent episodes of SCD and persistent ST-T segment alterations in V1–V3.2 The mechanism responsible for these alterations is abnormal depolarization, mainly conduction delay in the terminal portion in the right ventricular outflow tract.2 It has been suggested that sodium channel defects lead to reduced ion current during action potentials, probably due to one or more of the following: failure of the sodium channel to express in the cell membrane; a shift in the voltage dependence of channel activation and inactivation; and accelerated inactivation of the sodium channel.3–5
Various medications have been reported as inducing type 1 ECG pattern in patients with the ion channel defect responsible for the syndrome, including drugs that alter sodium channel properties. Over-the-counter cold medicines usually contain a combination of antihistamines, adrenergic agonists and analgesics. We report the case of a patient in whom a diagnosis of BrS was made after use of this type of medication.
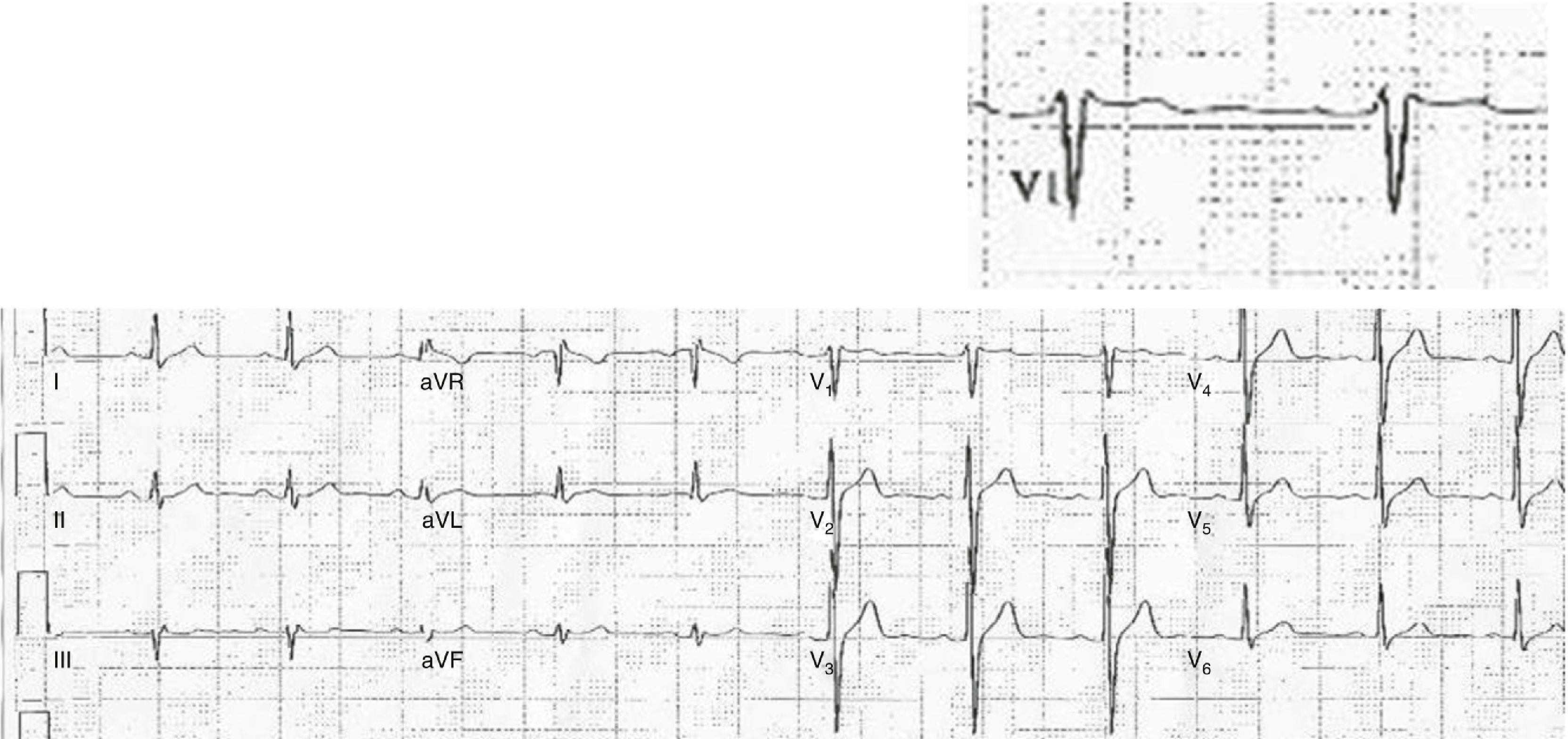
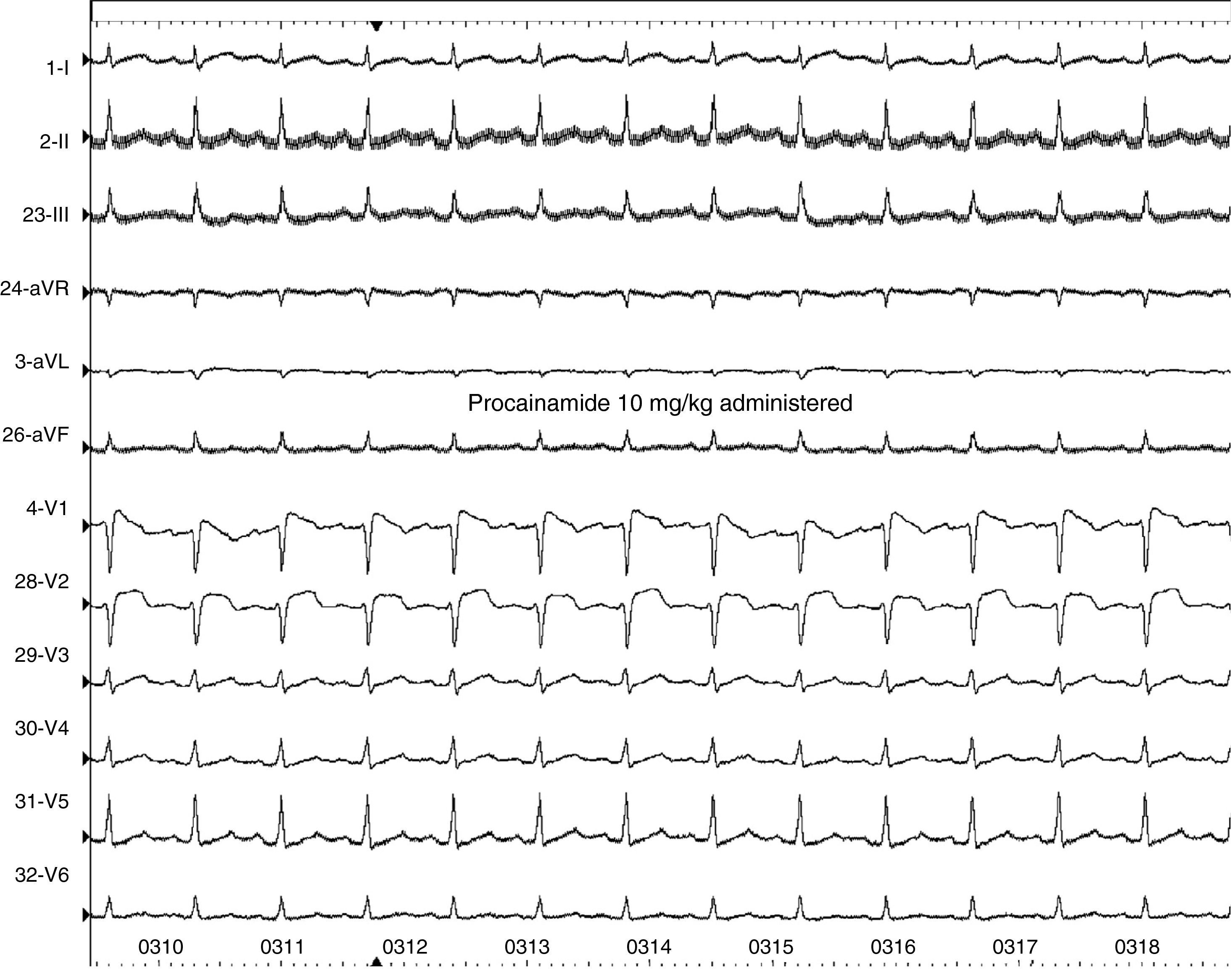
Case reportA 44-year-old man went to the emergency department (ED) due to an episode of syncope after using cold medicine (brompheniramine 12 mg + phenylephrine 15 mg); he reported no fever in the previous days. The patient suffered another transient loss of consciousness during initial observation in the ED. Resuscitation maneuvers were begun and the patient recovered. The ECG on arrival showed slight saddleback-type ST elevation in V1 of >1 mm, but not in V2 (Figure 1). After exclusion of a coronary event or pulmonary embolism, electrophysiological study was performed, which showed ST-T normalization compared to baseline ECG, prolonged HV interval and induction of ventricular fibrillation (VF) by programmed ventricular stimulation; procainamide infusion induced a type 1 Brugada pattern (Figure 2). All these findings are characteristic of BrS, and a cardioverter-defibrillator was implanted; a week after discharge, the patient received an appropriate shock for VF.
DiscussionThe real prevalence of BrS is not known, one reason for which is that the electrocardiographic characteristics of the syndrome can be transient. In the case presented, the patient presented ECG alterations and syncope, probably of arrhythmic origin, after taking commercially available cold medicine containing phenylephrine and brompheniramine. The mechanism by which BrS can be triggered after use of adrenergic agonists is not fully understood, but one hypothesis is a sudden increase in vagal tone once the adrenergic effect has worn off.6–9 This has been demonstrated in studies on BrS patients whose ECG alterations are more evident immediately after exercise or large meals.7,8 However, one study in which phenylephrine was administered to patients with BrS failed to show the typical ECG alterations of the syndrome.6 Brompheniramine appears to directly alter the expression of the SCN5A gene,10 and animal studies have demonstrated reduced sodium ion current following infusion of this drug.10
In the case presented, there are two possible limitations to inferring a causal relationship between use of cold medicine and the event: the fact that fever, while not reported by the patient or observed in the ED, could have occurred and is known to be associated with the appearance of BrS1; and the fact that no test was performed with the medication in question (phenylephrine and brompheniramine) to confirm a direct relation with the appearance of characteristic ECG alterations, since this would have put the patient at risk. However, brompheniramine and other antihistamines should be used with caution in this patient population.
ConclusionThe case presented illustrates the need for appropriate pharmacovigilance. Although the drugs in question were not administered in the electrophysiology laboratory, the sequence of events and previous reports in the literature suggest a strong possibility of a relationship between the use of cold medicine and the arrhythmic event.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Luz Leiria TL, et al. Síndrome de Brugada e uso de antigripais: existe relação? Rev Port Cardiol. 2013. http://dx.doi.org/10.1016/j.repc.2012.11.005.