Functional class is an important predictor of prognosis in chronic heart failure (CHF). However, it is often subjective and poorly reproducible.
ObjectiveWe sought to identify diagnostic markers of high functional class.
MethodsWe prospectively studied 37 patients with symptomatic CHF and ejection fraction <40%. The study protocol included clinical evaluation, echocardiography (M-mode, 2D, Doppler and tissue Doppler) and laboratory tests including copeptin, vasopressin and NT-proBNP. We compared patients in NYHA class II with those in NYHA class >II. Overall mortality was assessed at 18 months.
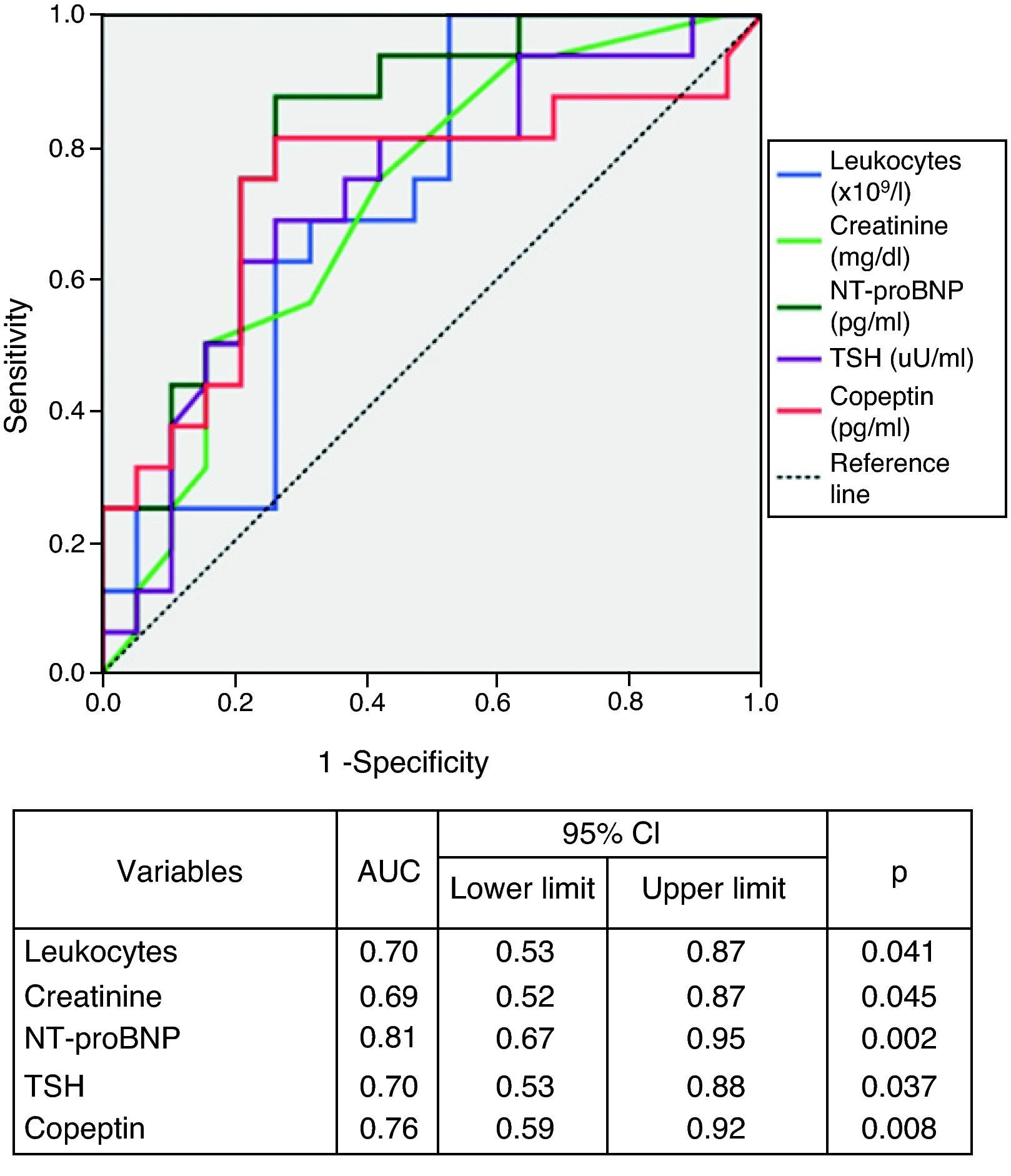
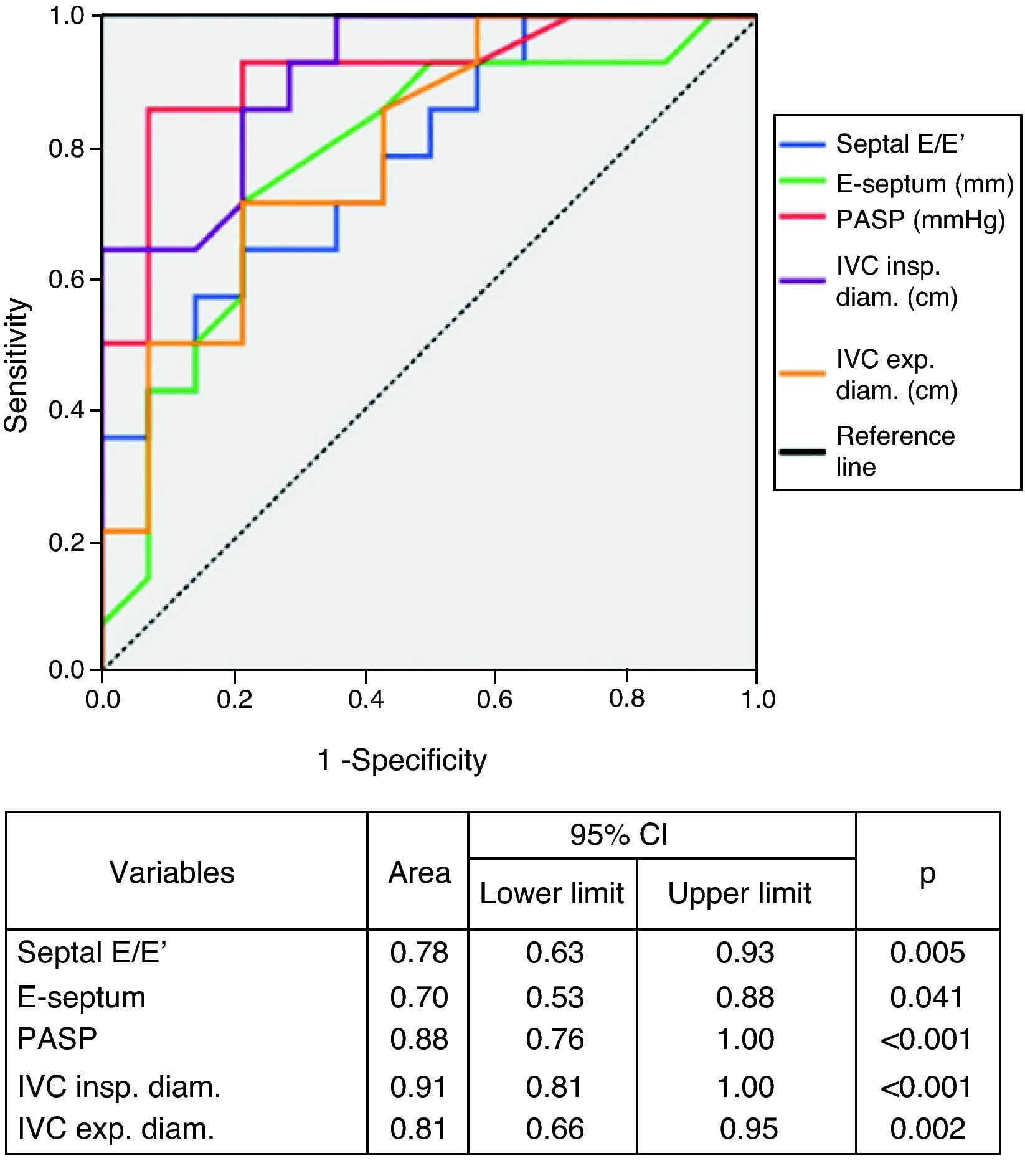
ResultsMortality was higher in the more advanced symptomatic stages (p<0.05). Patients in NYHA class >II had higher creatinine, copeptin and NT-proBNP levels (p<0.05). E/E′, E-septum distance, pulmonary artery systolic pressure (PASP) and inferior vena cava (IVC) dimensions were also significantly greater (p<0.05). The biomarkers copeptin (area under the curve [AUC]=0.76, p<0.01) and NT-proBNP (AUC=0.81, p<0.01) and the echocardiographic parameters PASP (AUC=0.88, p<0.01) and IVC inspiratory diameter (AUC=0.91, p<0.01) showed the best performance for diagnosis of functional class >II. In multivariate regression analysis only PASP and serum creatinine were independent predictors of NYHA functional class >II.
ConclusionCopeptin and NT-proBNP have high sensitivity and specificity in the diagnosis of functional classes with prognostic impact and may be useful in defining a standardized functional classification.
The structural and hemodynamic echocardiographic changes associated with NYHA class >II were left ventricular filling pressure, PASP and central venous pressure.
A classificação apropriada da gravidade da insuficiência cardíaca crónica (ICC) é um dos preditores de prognóstico mais importantes. No entanto, a avaliação clínica e diagnóstica de sinais e sintomas sugestivos de ICC pode ser difícil, subjetiva e pouco reprodutível.
ObjetivoProcurámos identificar marcadores diagnósticos de estadio funcional elevado.
População e métodosEstudo prospetivo em 37 doentes com antecedentes de ICC sintomática, estável e com fração de ejeção ventricular esquerda<40%. O protocolo incluiu avaliação clínica, ecocardiográfica (MM, 2D, Doppler convencional e tecidular) e avaliação analítica incluindo doseamento de copeptina, vasopressina, aldosterona, NT-proBNP. Comparou-se o grupo de doentes em classe ii da NYHA com os doentes em classe funcional superior a ii. Foi determinada a mortalidade global aos 18 meses.
ResultadosA mortalidade é maior nos estadios sintomáticos mais avançados (p<0,05). Nesses estadios, os doentes, apresentam maior concentração de leucócitos, creatinina, TSH, copeptina e NT-proBNP (p<0,05). A relação E/E’, distância E-septo, pressão sistólica na artéria pulmonar (PSAP) e dimensões da veia cava inferior (VCI) são também significativamente superiores (p<0,05). Os biomarcadores copeptina (AUC 0,76; p<0,01) e NT-proBNP (AUC 0,81; p<0,01) e os parâmetros ecocardiográficos PSAP (AUC 0,88; p<0,01) e diâmetro inspiratório da VCI (AUC 0,91; p<0,01) apresentaram o melhor desempenho no diagnóstico de estadio sintomático da NYHA superior a ii. Na avaliação multivariada, apenas a PSAP e a creatininémia foram preditores independentes de classe funcional da NYHA>ii (p<0,05).
ConclusãoA copeptina e NT-proBNP apresentam elevada sensibilidade e especificidade no diagnóstico dos estadios funcionais com relevância prognóstica, pelo que poderão ser úteis na definição de classes funcionais padronizadas.
As alterações estruturais e hemodinâmicas ecocardiográficas relacionadas com classes funcionais superiores a ii foram a pressão de enchimento ventricular esquerda, a PSAP e a pressão venosa central.
Chronic heart failure (CHF) has high incidence and prevalence, which are rising with ageing populations. Accurate classification of its severity has become increasingly important when deciding on drug therapies and implantable devices that have been shown to reduce morbidity and mortality, including renin–angiotensin–aldosterone1,2 and adrenergic3 system blockade and use of resynchronization and implantable defibrillation systems.4 Despite these advances, palliation and treatment of symptoms are often difficult.
The most widely used classification of CHF symptom severity is the New York Heart Association (NYHA) system, consisting of four functional classes (I–IV), which has been shown to be valuable in predicting clinical events in CHF.5 However, mild symptoms are not synonymous with mild cardiac dysfunction, and there may be little correlation between severity of symptoms and degree of ventricular dysfunction.6
Clinical evaluation of patients with signs and symptoms suggestive of CHF can be difficult and has poor reproducibility; studies assessing patients referred for echocardiography show a high rate of false positives.7 Population-based studies have also demonstrated that up to 60% of patients with left ventricular (LV) dysfunction are asymptomatic, making diagnosis more difficult.8 It is likely that these patients would also benefit from various therapies if identified and treated earlier.9
Ejection fraction (EF) is widely used as a prognostic variable in CHF patients, but the disease's course in those with systolic dysfunction, particularly with EF between 20% and 30%, is highly variable and some studies have stressed the role of hemodynamic changes such as raised LV filling pressures in determining prognosis.10,11
The aim of this study was to assess the correlation between clinical, laboratory and echocardiographic variables and symptom severity in patients with CHF, particularly the ability of the biomarkers NT-proBNP and copeptin to diagnose high functional class. We also tested the hypothesis that clinical presentation correlates more strongly with other laboratory and echocardiographic variables than with EF in patients with known LV dysfunction. Mortality was assessed at 18 months.
MethodsThis was a comparative cohort study with prospective assessment of the prognosis of 37 patients with stable symptomatic CHF (NYHA class II–IV) with LV systolic dysfunction. Patients with primary valve disease, a history of chronic renal failure or serum creatinine >3mg/dl were excluded. Outpatients (n=14) were included, as well as those admitted for decompensated CHF as long as they returned to their usual functional class once discharged after clinical stabilization (n=23).
Patients were selected in our center's echocardiography laboratory following an echocardiogram showing LVEF ≤40% together with signs and symptoms of CHF. At this time, the images were recorded in digital format in accordance with the echocardiographic protocol, a clinical history was taken by interview and peripheral venous blood samples were collected. Blood pressure and heart rate were measured after five minutes in decubitus.
Clinical follow-up was the responsibility of the attending physician, the investigators being blinded to the laboratory results and interventions. The principal end-point was all-cause mortality at 18 months (548 days). Survival was assessed by telephone interview and consultation of hospital records at the end of the predefined follow-up period.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of our institution. All participants gave their informed consent.
Laboratory protocolAt the initial evaluation, blood samples were taken from an antecubital vein after five minutes in dorsal decubitus. The hemogram and data on creatinine, sodium, thyroid stimulating hormone (TSH) and NT-proBNP levels were processed and analyzed in the hospital laboratory.
NT-proBNP levels were determined by solid-phase electrochemiluminescence immunoassay with two binding sites, using the IMMULITE 2000 system (Siemens Healthcare Diagnostics, Breda, Holland), commercially available and validated in clinical practice.
Some of the blood was collected into tubes containing EDTA, to which aprotinin (500kIU/ml) was added, and then centrifuged at 1600g for 15min at 4°C. The plasma was removed, divided into aliquots and stored at −80°C. At the end of follow-up, copeptin levels were determined by radioimmunoassay in accordance with the manufacturer's protocol (Phoenix Pharmaceutical Inc., CA, US). Briefly, the test is based on competitive binding to specific iodine-125 labeled copeptin (125I-copeptin) antibodies and to copeptin in the sample and a standard solution. The greater the quantity of copeptin in the sample, the less 125I-copeptin binds to the antibody. A standard curve was generated according to the scintillation count of solutions with known copeptin concentrations (standard and control solutions), thus enabling the sample's copeptin concentration to be calculated.
Echocardiographic protocolEchocardiographic assessment was performed on ATL HDI 5500 (ATL Philips Medical Systems, Bothell, Washington, US) or Aloka ProSound Alpha 10 (Aloka, Tokyo, Japan) systems. The protocol consisted of conventional study by 2D, M-mode and Doppler echocardiography, and tissue Doppler study of the mitral annulus.
LV global systolic function was assessed by E-septum distance, fractional shortening and EF by Simpson's biplane method. In cases of significant mitral regurgitation (grade >II/IV), dP/dT was also determined.
LV diastolic function was assessed by Doppler analysis of LV filling flow and diastolic septal mitral annular velocities, and the E/E′ ratio was calculated. Nagueh's formula12 was used to estimate LV filling pressures; an E/E′ ratio <8 is highly specific for LV filling pressures <15mmHg, while E/E′ >15 indicates pressures >15mmHg.
Relative wall thickness and LV mass index13,14 were used to characterize echocardiographic patterns of LV remodeling.
Right ventricular (RV) function was assessed by measuring diastolic RV outflow tract diameter, calculating pulmonary artery systolic pressure (PASP) in the presence of significant tricuspid regurgitation (grade >II/IV), and measuring inferior vena cava (IVC) expiratory and inspiratory diameters.
Estimation of PASP was based on the peak velocity of the jet of tricuspid regurgitation. The simplified Bernoulli equation15 describes the relationship of peak tricuspid regurgitation velocity and the peak pressure gradient between the right ventricle and right atrium (P=4×(v)2). This equation allows for estimation of PASP taking into account right atrial (RA) pressure (PASP=tricuspid regurgitation pressure gradient+estimated right atrial pressure). Atrial pressure was estimated based on IVC expiratory diameter and respiratory variation. Respiratory variation of >50% was taken to indicate an RA pressure of 5mmHg when IVC diameter was <20mm and of 10mmHg with IVC diameter >20mm. The absence of significant variation in IVC diameters with inspiration was taken to indicate an RA pressure of 15mmHg for IVC diameters <20mm and of 20mmHg for diameters >20mm.
Statistical analysisContinuous variables are presented as medians and interquartile range (IR). The Mann–Whitney U test was used to compare clinical, echocardiographic and laboratory parameters between the two groups.
Categorical variables are presented as frequencies and percentages, and were compared using the chi-square test.
Receiver operating characteristic (ROC) curves were constructed and the area under the curve (AUC) was calculated to determine the capacity of the various clinical, laboratory and echocardiographic parameters for functional stratification.
A forward stepwise logistic regression model was applied to identify independent predictors of NYHA functional class >II, with p=0.05 for inclusion and p=0.1 for exclusion.
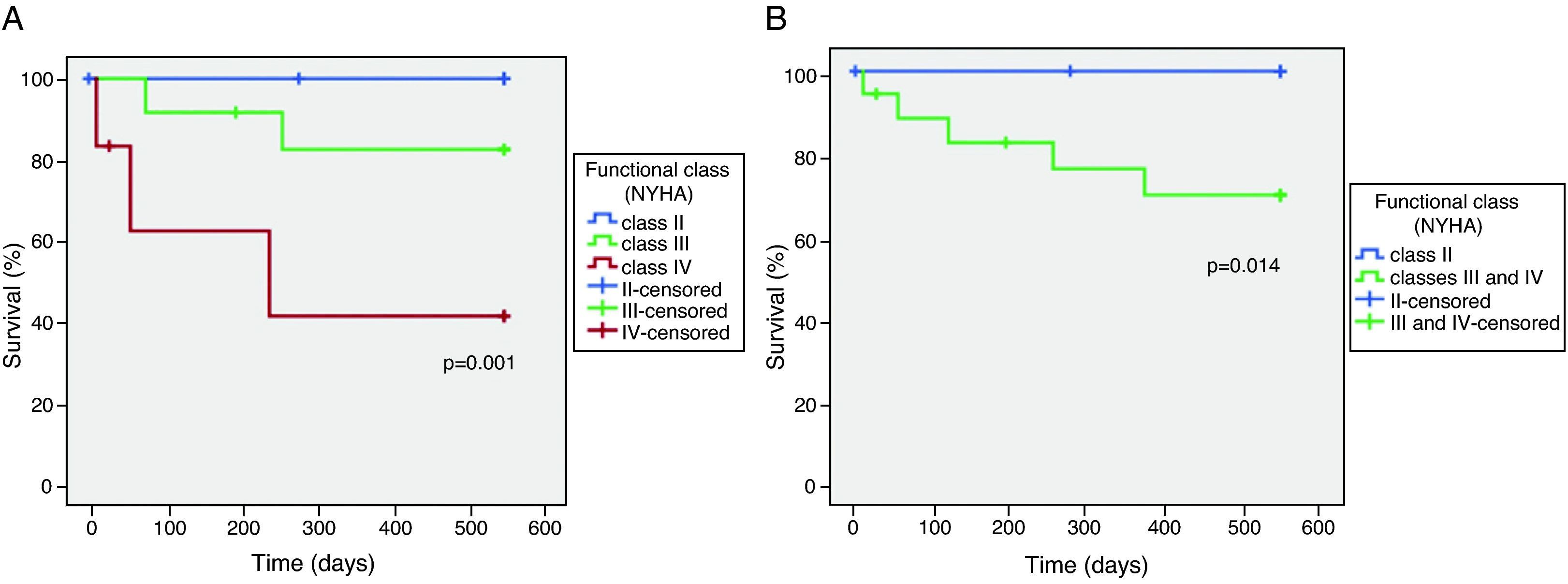
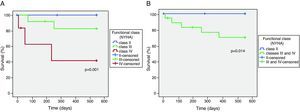
Survival was assessed at 18 months according to functional class on inclusion in the study and compared using the Kaplan–Meier method.
A value of p<0.05 was considered significant in all analyses. SPSS version 17.0 (SPSS Inc., Chicago, Illinois) was used for the statistical analysis.
ResultsPopulation characteristicsThirty-seven patients were included, 78% male (n=29), with a median age of 72 years (IR 63–75). Median LVEF at admission was 31.5% (IR 25–34). On the NYHA classification, 51% (n=19) were in class II, 33% (n=12) in class III and 16% (n=6) in class IV.
Survival and NYHA functional classThe relationship between NYHA functional class at the time of inclusion in the study and overall mortality at 18 months was tested by Kaplan–Meier analysis (Figure 1A). Three patients were lost to follow-up.
Mortality was higher in the more symptomatic groups, with two deaths among patients in NYHA class III and three among the group in class IV. There were no deaths at 18 months among those in class II. Functional class >II at inclusion distinguished patients at risk of death (Figure 1B).
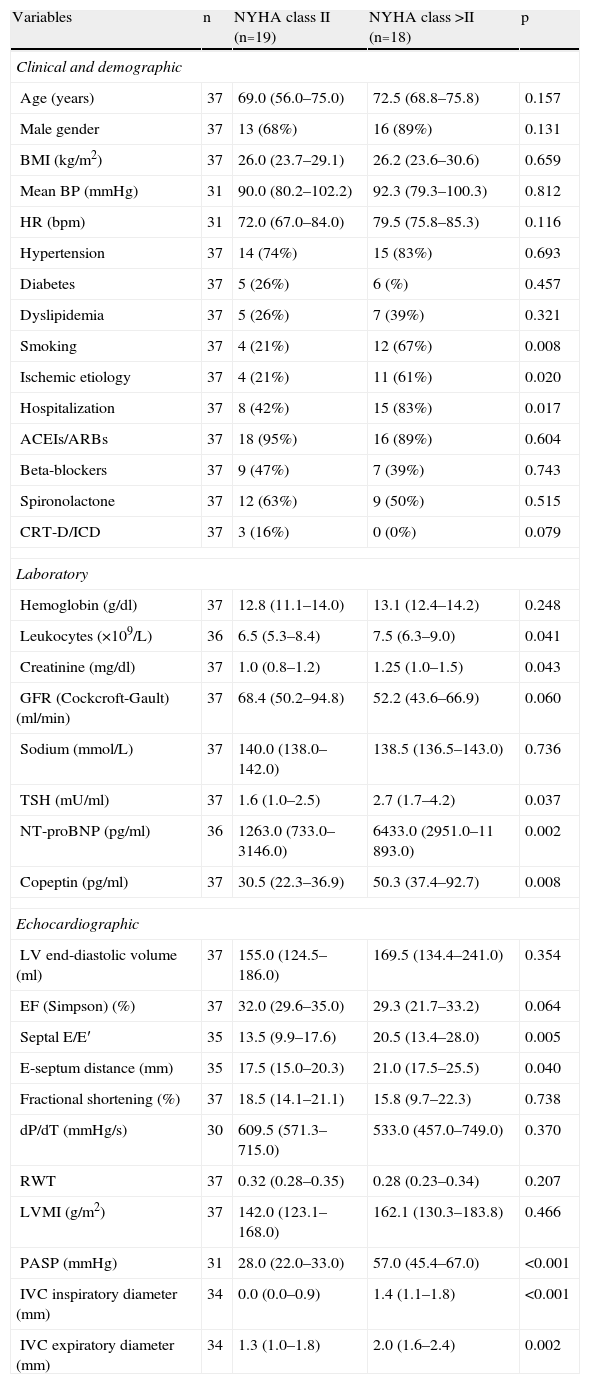
Clinical, laboratory and echocardiographic characteristics according to severity of symptomsThe patients were divided into two groups according to NYHA functional class: those in NYHA class II and those in NYHA class >II (III and IV). The clinical, laboratory and echocardiographic characteristics of the study population according to severity of symptoms are summarized in Table 1.
Clinical, laboratory and echocardiographic characteristics of the study population.
| Variables | n | NYHA class II (n=19) | NYHA class >II (n=18) | p |
| Clinical and demographic | ||||
| Age (years) | 37 | 69.0 (56.0–75.0) | 72.5 (68.8–75.8) | 0.157 |
| Male gender | 37 | 13 (68%) | 16 (89%) | 0.131 |
| BMI (kg/m2) | 37 | 26.0 (23.7–29.1) | 26.2 (23.6–30.6) | 0.659 |
| Mean BP (mmHg) | 31 | 90.0 (80.2–102.2) | 92.3 (79.3–100.3) | 0.812 |
| HR (bpm) | 31 | 72.0 (67.0–84.0) | 79.5 (75.8–85.3) | 0.116 |
| Hypertension | 37 | 14 (74%) | 15 (83%) | 0.693 |
| Diabetes | 37 | 5 (26%) | 6 (%) | 0.457 |
| Dyslipidemia | 37 | 5 (26%) | 7 (39%) | 0.321 |
| Smoking | 37 | 4 (21%) | 12 (67%) | 0.008 |
| Ischemic etiology | 37 | 4 (21%) | 11 (61%) | 0.020 |
| Hospitalization | 37 | 8 (42%) | 15 (83%) | 0.017 |
| ACEIs/ARBs | 37 | 18 (95%) | 16 (89%) | 0.604 |
| Beta-blockers | 37 | 9 (47%) | 7 (39%) | 0.743 |
| Spironolactone | 37 | 12 (63%) | 9 (50%) | 0.515 |
| CRT-D/ICD | 37 | 3 (16%) | 0 (0%) | 0.079 |
| Laboratory | ||||
| Hemoglobin (g/dl) | 37 | 12.8 (11.1–14.0) | 13.1 (12.4–14.2) | 0.248 |
| Leukocytes (×109/L) | 36 | 6.5 (5.3–8.4) | 7.5 (6.3–9.0) | 0.041 |
| Creatinine (mg/dl) | 37 | 1.0 (0.8–1.2) | 1.25 (1.0–1.5) | 0.043 |
| GFR (Cockcroft-Gault) (ml/min) | 37 | 68.4 (50.2–94.8) | 52.2 (43.6–66.9) | 0.060 |
| Sodium (mmol/L) | 37 | 140.0 (138.0–142.0) | 138.5 (136.5–143.0) | 0.736 |
| TSH (mU/ml) | 37 | 1.6 (1.0–2.5) | 2.7 (1.7–4.2) | 0.037 |
| NT-proBNP (pg/ml) | 36 | 1263.0 (733.0–3146.0) | 6433.0 (2951.0–11 893.0) | 0.002 |
| Copeptin (pg/ml) | 37 | 30.5 (22.3–36.9) | 50.3 (37.4–92.7) | 0.008 |
| Echocardiographic | ||||
| LV end-diastolic volume (ml) | 37 | 155.0 (124.5–186.0) | 169.5 (134.4–241.0) | 0.354 |
| EF (Simpson) (%) | 37 | 32.0 (29.6–35.0) | 29.3 (21.7–33.2) | 0.064 |
| Septal E/E′ | 35 | 13.5 (9.9–17.6) | 20.5 (13.4–28.0) | 0.005 |
| E-septum distance (mm) | 35 | 17.5 (15.0–20.3) | 21.0 (17.5–25.5) | 0.040 |
| Fractional shortening (%) | 37 | 18.5 (14.1–21.1) | 15.8 (9.7–22.3) | 0.738 |
| dP/dT (mmHg/s) | 30 | 609.5 (571.3–715.0) | 533.0 (457.0–749.0) | 0.370 |
| RWT | 37 | 0.32 (0.28–0.35) | 0.28 (0.23–0.34) | 0.207 |
| LVMI (g/m2) | 37 | 142.0 (123.1–168.0) | 162.1 (130.3–183.8) | 0.466 |
| PASP (mmHg) | 31 | 28.0 (22.0–33.0) | 57.0 (45.4–67.0) | <0.001 |
| IVC inspiratory diameter (mm) | 34 | 0.0 (0.0–0.9) | 1.4 (1.1–1.8) | <0.001 |
| IVC expiratory diameter (mm) | 34 | 1.3 (1.0–1.8) | 2.0 (1.6–2.4) | 0.002 |
Data presented as medians (percentile 25–percentile 75) or frequencies (%). ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin-receptor blockers; BMI: body mass index; BP: blood pressure; CRT-D/ICD: cardiac resynchronization therapy-defibrillator/implantable cardioverter-defibrillator; EF: ejection fraction; GFR: glomerular filtration rate; HR: heart rate; IVC: inferior vena cava; LV: left ventricular; LVMI: left ventricular mass index; PASP: pulmonary artery systolic pressure; RWT: relative wall thickness; TSH: thyroid stimulating hormone.
The clinical characteristics that varied significantly between the groups were smoking and ischemic etiology, with higher prevalences among the more symptomatic group. Patients in higher NYHA classes were also more frequently hospitalized, but the median time to inclusion in the study (6 days) was similar between the two groups (p=0.844). Treatment did not differ significantly between the groups. In six patients it was not possible to determine blood pressure and heart rate after five minutes in decubitus, as specified in the protocol, and so Table 1 shows these data for 31 patients only.
The laboratory protocol was unable to determine leukocyte count and NT-proBNP level in one patient due to technical problems in processing the blood sample. Other laboratory parameters were assessed in all patients. Those in class >II had significantly higher levels of leukocytes and creatinine, lower albumin and raised TSH. Concentrations of NT-proBNP and copeptin were significantly higher in the more symptomatic group.
The most difficult echocardiographic parameters to assess were PASP, due to absence of significant tricuspid regurgitation in six patients, and IVC diameters, due to poor subcostal image quality in three patients. Of the parameters of LV systolic function studied, only E-septum distance was significantly greater in more symptomatic patients, with no significant differences in LV end-diastolic volume or EF. LV diastolic function was significantly more compromised in patients with more severe symptoms, as shown by a higher E/E′ ratio. Patients in higher functional classes presented significantly greater PASP and IVC expiratory and inspiratory diameters, the direct consequence of LV systolic dysfunction.
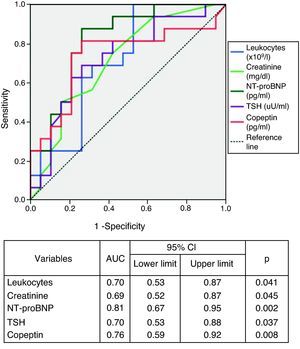
Laboratory parameters and symptom severityThere were significant differences in laboratory parameters between the two groups (Table 1). Those in class >II had higher concentrations of leukocytes (p=0.041), creatinine (p=0.043), TSH (p=0.037), copeptin (p=0.008) and NT-proBNP (p=0.002).
Analysis of the area under the ROC curves revealed that the biomarkers NT-proBNP and copeptin showed the best performance for diagnosis of NYHA class >II (Figure 2). Serum NT-proBNP >1535.5pg/ml predicted functional class >II with 94% sensitivity and 58% specificity, and copeptin >33.75pg/ml had 82% sensitivity and 74% specificity in identifying the more symptomatic group.
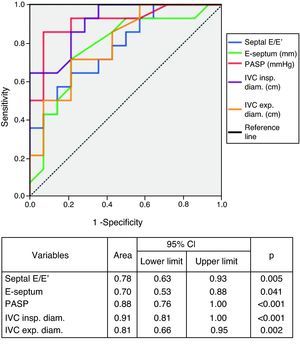
Echocardiographic parameters and symptom severityPatients with more severe symptoms had higher LV filling pressures as assessed by the E/E′ ratio (p=0.005), greater E-septum distance (p=0.040), higher estimated PASP (p<0.001) and greater IVC inspiratory (p<0.001) and expiratory (p=0.002) diameters.
The diagnostic performance of echocardiographic parameters in identifying NYHA class >II is shown in Figure 3. Septal E/E′ ratio, PASP and IVC expiratory and inspiratory diameters showed excellent performance in diagnosing more symptomatic patients. PASP and IVC inspiratory diameter were superior to the biomarkers in this respect. PASP >37.5mmHg showed 93% sensitivity and 79% specificity in identifying more severe symptoms, and IVC inspiratory diameter >9.25mm predicted higher functional class with 93% sensitivity and 71% specificity.
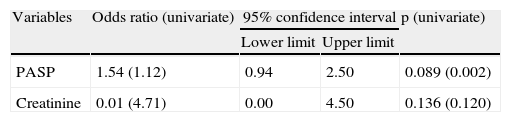
Determinants of symptom severityIn logistic regression analysis, only PASP and serum creatinine were independent predictors of NYHA functional class >II (Table 2).
Forward stepwise logistic regression analysis of predictors of NYHA function class >II.
| Variables | Odds ratio (univariate) | 95% confidence interval | p (univariate) | |
| Lower limit | Upper limit | |||
| PASP | 1.54 (1.12) | 0.94 | 2.50 | 0.089 (0.002) |
| Creatinine | 0.01 (4.71) | 0.00 | 4.50 | 0.136 (0.120) |
PASP: pulmonary artery systolic pressure.
The results of our study show that mortality is higher in patients with CHF and LV dysfunction in the more advanced symptomatic stages, when they present higher levels of leukocytes, creatinine, TSH, copeptin and NT-proBNP, and E/E′ ratio, E-septum distance, PASP and IVC diameters are significantly greater. The biomarkers copeptin and NT-proBNP and the echocardiographic parameters PASP and IVC inspiratory diameter showed the best performance for diagnosis of NYHA functional class >II, but in multivariate analysis only PASP and serum creatinine were independent predictors of NYHA class >II.
Our results are in agreement with previous studies showing a correlation between NYHA functional class and occurrence of clinical events including need for hospitalization and mortality.5,16 A consistent association has been found between NYHA class and prognosis in patients with systolic heart failure17–19 and also in those with diastolic CHF.20 However, there is considerable interobserver variability in categorizing CHF patients by NYHA class.21 There are also discrepancies between the functional class attributed by physicians and that reported by patients.22 The situation is further complicated by the fact that patients’ self-assessed functional status is a prognostic predictor of hospitalizations, quality of life and death.23 Thus, despite its prognostic value, the NYHA classification system may not be applied in CHF in the same way by all health providers; the clinical assessment of a tertiary heart failure unit like ours probably cannot be applied at all levels of medical care. In an attempt to standardize the criteria, laboratory markers have been suggested as a possible way to achieve greater objectivity in clinical evaluation.24
Our study demonstrated a strong correlation between serum levels of the biomarkers NT-proBNP and copeptin and functional class, showing a good ability to distinguish between class II and >II.
BNP and its precursor NT-proBNP are widely used in cardiovascular risk assessment, as reflected in current guidelines.25 High levels of these peptides are associated with advanced age, renal failure, arrhythmias, systolic and diastolic dysfunction, and mortality.26 They reflect individual patient risk and are thus essential tools in stratifying risk.27–29
To our knowledge, this is the first study to assess copeptin in a Portuguese population with CHF. Copeptin is a fragment of pre-provasopressin that is secreted in equimolar quantities to vasopressin, a hormone that, unlike natriuretic peptides, has antidiuretic and vasoconstrictor properties.30,31 There is strong evidence of a correlation between vasopressin and CHF severity, which probably extends to prognosis.32–34 However, it is difficult to measure since it is an unstable peptide, binds strongly to platelets and is rapidly eliminated.35 The main advantages of assessing copeptin are its stability and ease and accuracy of measurement in plasma or serum. Although copeptin correlates with serum levels of BNP and NT-proBNP, its prognostic value appears to be superior in population-based studies.24 The value of copeptin is enhanced by the fact that vasopressin blockade is being investigated as a therapeutic target in CHF. Clinical trials of V2 receptor antagonists have shown improvement in congestive symptoms due to the drugs’ diuretic properties but benefits in survival have yet to be demonstrated.36,37
TSH was significantly higher in more symptomatic patients, and there is a known relation between elevated TSH and prevalence of CHF.38 Hypothyroidism has also been implicated as a cause of anemia, which in turn causes fatigue and decompensation of CHF, as well as being a predictor of mortality.39 This suggests that thyroid function should be assessed in CHF, particularly in more symptomatic patients, since the symptoms of hypothyroidism and heart failure overlap and the presence of one increases the probability of the other also being present.
The origin of symptoms in CHF is poorly understood and continues to be the subject of research.40–42 We used echocardiography to analyze the hemodynamic and structural changes associated with symptom severity in systolic CHF. A weak but statistically significant correlation was found between E-septum distance and NYHA functional class, but most parameters of LV systolic function (EF, fractional shortening and dP/dT) did not correlate with symptoms. Although the small sample size may have contributed to this result, it is not unexpected since there is a known lack of correlation between ventricular dysfunction and severity of symptoms,25 as shown by the results of clinical trials. For example, patients with mild symptoms in the EMPHASIS-HF study43 had a mean EF of 26%, virtually the same as the more symptomatic patients in the RALES trial.44 This highlights the discrepancy between functional class and LV function in CHF patients.
Elevated LV filling pressures are the cause of dyspnea and pulmonary edema in acute heart failure. However, studies performed during exercise in CHF have shown only a weak correlation between pulmonary capillary wedge pressure and functional capacity.40 Nevertheless, we found a correlation between LV filling pressure, as estimated by the E/E′ ratio, and NYHA functional class at rest in patients with stable CHF. This is consistent with the relation found between congestive symptoms and diastolic dysfunction as assessed by pulsed Doppler study of transmitral flow in patients with dilated cardiomyopathy.45
LV dysfunction results in increased end-diastolic ventricular pressure, passive increases in left atrial and pulmonary venous pressure and development of postcapillary pulmonary hypertension.46 This in turn leads to RV dysfunction and systemic venous hypertension, and their clinical and hemodynamic consequences. These changes are highlighted by the association of increased jugular venous pressure and increased LV filling pressures on cardiac catheterization.47,48 This explains our study's finding of increased LV filling pressures, PASP and IVC inspiratory and expiratory diameters, of which only PASP was an independent predictor of more severe symptoms.
Non-cardiac factors that were not assessed in our study may have contributed to CHF symptoms, including changes in muscle structure and function,49,50 endothelial dysfunction51 and ventilatory dysfunction.52
The independent predictors of functional class >II in our population were PASP and creatininemia.
PASP was the strongest independent predictor of functional capacity in our CHF patients. LV dysfunction is a known cause of pulmonary hypertension53 but its development is highly variable and the contributing factors are not fully understood. As mentioned above, the finding of increased E/E′ ratio, PASP and IVC diameters suggests an interrelationship between high ventricular filling pressures and development of pulmonary hypertension and elevated central venous pressure. It has also been demonstrated that there may be a precapillary component to pulmonary hypertension in systolic CHF.54 Our results are in agreement with studies implicating pulmonary hypertension in functional changes and in prognosis.55
One mechanism through which pulmonary hypertension can contribute to CHF symptoms is increased RV afterload,56 and a strong correlation has been found between oxygen consumption and RV function at rest and during exercise.57 There is also a known correlation between pulmonary hypertension and changes in ventilatory efficiency that contribute to the development of hyperpnea and dyspnea.58,59 In addition to functional repercussions, pulmonary hypertension has also been shown to be associated with prognosis,60 probably related to its impact on RV function. The present study found a correlation between functional class and pulmonary hypertension as assessed by PASP, which may explain the poor prognosis recorded in higher NYHA classes. This finding is of clinical importance as it suggests that noninvasive determination of PASP adds valuable information when assessing hemodynamic changes in patients with systolic CHF, particularly those in NYHA class III or IV. Although effective therapies for such pulmonary hypertension have yet to be identified, recent evidence suggests a role for type 5 phosphodiesterase inhibitors.61
While adding only limited value to the logistic regression model, creatininemia, as a marker of renal function, was shown to have predictive value independently of the other variables analyzed. There is a known relationship between elevated serum creatinine and risk for CHF in elderly patients.62 More recent studies using cystatin C as a marker of renal function have demonstrated that the relationship is even closer, beginning in the preclinical phase of renal dysfunction, which serum creatinine levels do not reveal.63,64 Renal failure makes it more difficult to control heart failure and is one of the strongest markers of adverse clinical events in CHF,65 the coexistence of heart and renal disease being termed cardiorenal syndrome.66 However, the presence of symptoms characteristic of heart failure in patients with chronic renal failure but without diagnosed CHF is common,67 which may be due to the congestive symptoms shared by the two conditions. Thus, the association of elevated creatinine and greater functional impairment may arise from a mutual potentiation of symptoms.
The study has certain limitations. Extrapolation of the results to the general population is limited by the use of a convenience sample. Furthermore, the number of patients included in the study is relatively small, but it has the advantage of being a prospective assessment of important clinical variables such as mortality. The differences in clinical characteristics between the two groups could be considered a limitation in terms of the selection method used. However, the fact that patients in the class >II group were more often included during hospitalization may merely reflect the greater severity of their symptoms. Increased hospitalization has been reported in patients in higher functional classes.5 In addition, although PASP was an independent predictor of functional class, it could not be estimated in all patients due to the absence of, or presence of only mild, tricuspid regurgitation.
ConclusionsOur results suggest that mortality is higher in the more advanced symptomatic stages of systolic CHF. The biomarkers copeptin and NT-proBNP showed a good correlation with symptom severity and high sensitivity and specificity for diagnosis of high functional classes. They may thus be useful in defining a standardized functional classification of patients with LV dysfunction.
The main structural and hemodynamic changes associated with higher functional classes were elevated LV filling pressure, PASP and central venous pressure.
PASP and creatinine levels are independent predictors of NYHA functional class >II in patients with systolic CHF, and so their noninvasive measurement provides important information on hemodynamics and should always be assessed in these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Dr. Helena Sequeira and Prof. Rui Pinto for their help in quantifying copeptin levels.
Please cite this article as: Silva Marques J, et al. Biomarcadores da classe funcional na insuficiência cardíaca sistólica. Relevância da copeptina. Rev Port Cardiol 2012. doi:10.1016/j.repc.2012.09.001.