A 49-year-old woman, with no relevant family history, was admitted in 1996 for arrhythmic storm with polymorphic ventricular tachycardia (torsade de pointes) which degenerated into ventricular fibrillation. Iatrogenic causes were excluded, the electrocardiogram (ECG) was normal and there was no structural heart disease. She refused cardioverter-defibrillator implantation. Treatment was begun with amiodarone, which she took irregularly.
She remained asymptomatic until 2014 when she was admitted for a new arrhythmic storm with torsade de pointes, refractory to antiarrhythmic therapy and aggravated by ventricular pacing (65 defibrillations). She had frequent ventricular extrasystoles (with short-coupled period <300 ms) preceding the tachycardia. After administration of isoprenaline infusion electric stability was maintained. In this setting and in the absence of structural heart disease or iatrogenic cause, a diagnosis of short-coupled variant torsade de pointes was established. A cardioverter-defibrillator was implanted and she was treated with verapamil, without recurrence of arrhythmias.
Mulher de 49 anos, sem história familiar de relevo. Em 1996 foi admitida no hospital por tempestade arrítmica: taquicardias ventriculares polimórficas tipo torsade de pointes, que degeneraram em fibrilhação ventricular. No estudo realizado foram excluídas iatrogenias, apresentava eletrocardiograma normal e ausência de cardiopatia estrutural. Recusou implantação de cardiodesfibrilhador. Ficou medicada com amiodarona, com cumprimento irregular. Assintomática até 2014, altura em que teve nova tempestade arrítmica com taquicardias ventriculares polimórficas, refratárias a antiarrítmicos e agravadas por pacing ventricular (65 desfibrilhações). Após introdução de isoprenalina em perfusão manteve estabilidade de ritmo, constatando-se extrassístoles ventriculares com período de acoplamento curto (<300 ms) a preceder as taquicardias. O diagnóstico de short coupled variant torsade de pointes foi estabelecido na ausência de iatrogenia ou cardiopatia estrutural. Implantou cardiodesfibrilhador e ficou medicada com verapamil, sem recorrência de eventos arrítmicos.
A 49-year-old woman, with no relevant family history and not taking any prescribed or illegal drugs, was admitted in 1996 for arrhythmic storm with polymorphic ventricular tachycardia (PVT) (torsade de pointes), with some episodes degenerating into ventricular fibrillation (VF) refractory to lidocaine, procainamide and propranolol and requiring several defibrillations. The 12-lead ECG was normal and electrolyte and metabolic abnormalities were excluded. Stabilization was achieved with amiodarone. During hospitalization she underwent transthoracic echocardiography (TTE) and cardiac catheterization, which were normal. Nonspecific characteristics were observed on endomyocardial biopsy. The electrophysiological study (EPS) performed under amiodarone (400 mg/day) was negative for inducible ventricular tachycardia. An implantable cardioverter-defibrillator (ICD) was proposed, but the patient refused. She was discharged under treatment with amiodarone and referred for an arrhythmia consultation.
During 18 years of follow-up she took amiodarone irregularly and with subtherapeutic doses (100 mg three times a week). Holter monitoring documented isolated premature ventricular contractions (PVCs), duplets and nonsustained PVT (all asymptomatic).
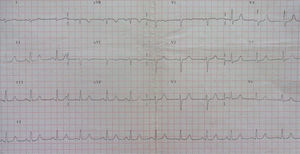
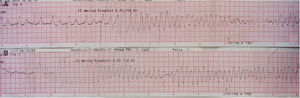
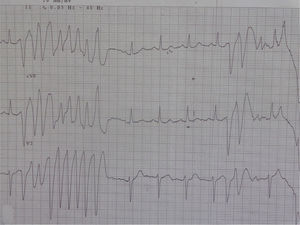
In 2014 she was readmitted for recurrent syncope due to PVT (torsade de pointes). The 12-lead ECG (Figure 1) and TTE were normal. Electrolyte and metabolic abnormalities and iatrogenic toxicological causes were excluded. She began intravenous therapy with magnesium sulfate and amiodarone without rhythm stabilization. Endotracheal intubation with mechanical ventilation was required in the context of multiple defibrillations. Lidocaine was added to amiodarone, also without success. A temporary pacemaker was implanted for ventricular pacing, but it proved to be arrhythmogenic (Figure 2A) and was promptly removed. Coronary angiography was normal. Veno-arterial extracorporeal membrane oxygenation (ECMO) was introduced because of hemodynamic instability, and removed after 24 hours due to lower limb ischemia. At this stage lidocaine was replaced by isoprenaline, maintaining amiodarone, with rhythm stabilization.
In an attempt to optimize therapy, isoprenaline was suspended at 48 hours and replaced by a beta-blocker in combination with amiodarone. This change triggered new episodes of nonsustained PVT. Isoprenaline was then reintroduced, achieving rhythm stabilization again. In the periods when the patient was without isoprenaline therapy, 65 defibrillations were performed. After nine days, isoprenaline was suspended, maintaining only amiodarone 200 mg/day.
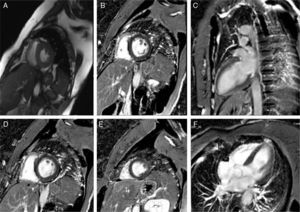
After rhythm stability was achieved, the etiological study was completed by performing cardiac magnetic resonance imaging, which was normal (Figure 3). Reassessment of the ECG tracings showed that all episodes of PVT were preceded by PVCs with no compensatory pause (absence of long R-R interval), followed by a short-coupling PVC (280–290 ms) (Figures 2B and 4). At this point, taking into account the patient's clinical history and complementary tests, a diagnosis of short-coupled variant torsade de pointes was established. Amiodarone was suspended and verapamil was started (120 mg twice daily). A cardioverter-defibrillator was also implanted with permanent atrial pacing. Genetic study was performed.
The hospitalization was prolonged by lower limb ischemia associated with ECMO cannulation requiring surgery (fasciectomy and thromboembolectomy in separate operations).
Six months after hospital discharge the patient was asymptomatic, with Holter monitoring showing sinus rhythm alternating with atrial pacing, without PVC or PVT. Genetic testing to identify mutations responsible for long QT syndrome and catecholaminergic tachycardia was negative.
DiscussionPVT can be associated with several diseases, but mostly appears in the presence of congenital or acquired long QT interval1 or bradycardia.2–4 It is common in patients with sudden cardiac death (SCD) without structural heart disease.1 In torsade de pointes electrolyte and metabolic disturbances should be excluded, since changes in potassium channels create conditions for early afterdepolarizations (due to modification in ventricular repolarization), triggering PVC and PVT (perpetuated by re-entry phenomena).2 In the context of a pharmacological iatrogenic cause, PVT usually occurs when the corrected QT interval (QTc) is >500 ms (90% of cases) or more than 25% of baseline QTc.2,5 It is also important to exclude relevant family history, including SCD, heart disease or rhythm disturbances.
In the case presented, all the above causes were excluded. The 12-lead ECG was normal in terms of heart rhythm, without prolongation of QTc interval and without electrical instability (alternating T, notched T and U waves and ST-segment changes). Therefore, in this context long or short QT syndrome, Brugada syndrome or early repolarization syndrome were unlikely.6
Analysis of periods of torsade de pointes in our patient did not demonstrate short-long-short sequences, which are a common characteristic in arrhythmias triggered by electrolyte, metabolic, or pharmacological alterations.1,2,6 However, it revealed a PVC not followed by a compensatory pause (absence of long R-R) and a short-coupled interval (QTc 280–290 ms), similar to an R-on-T phenomenon. This was first described in 1994 by Leenhardt et al.7 in a series of 14 patients without structural heart disease and a history of syncope related to PVT with normal QTc and short-coupled PVC (<300 ms). As this is a rare condition, there are only short descriptive series and isolated case reports.
In our patient the arrhythmic storm was refractory and perpetuated by ventricular pacing and was only controlled with administration of isoprenaline. As pointed out by Chiladakis et al.8 in a similar case, ventricular pacing appears to be responsible for increasing dispersion in ventricular activation time and the heterogeneity of ventricular repolarization, aggravating the arrhythmia.7,8 Isoprenaline and atrial pacing (used on the same principle) limit repolarization dispersion, potentially controlling the arrhythmia.7,8
Catecholaminergic polymorphic ventricular tachycardia appeared to be unlikely in our patient, since no stress or adrenergic stimulation was identified, and electrical stability was achieved under isoprenaline.6
Structural heart disease was excluded, as well as significant coronary heart disease by cardiac catheterization. The endomyocardial biopsy performed at the time of the first event was nonspecific.
In view of the above facts and taking into account especially the presence of a short-coupled interval preceding PVT, the diagnosis of short-coupled variant torsade de pointes was established. Amiodarone was accordingly replaced by verapamil at maximum tolerated dose, since there is no established optimal target dose, given the small number of series and case reports.
In the series by Leenhardt et al.,7 verapamil was the only useful drug, in contrast to other antiarrhythmic drugs, including beta-blockers and amiodarone, but did not change the risk of SCD. Its value is due to its reduction of the refractory period, increasing the coupling interval and decreasing the number of PVCs.8–11 Eisenberg et al.,12 in a series of 15 patients, showed that a shorter PVC coupling interval increased the risk of SCD.
The fact that our patient remained stable over a long period of time appears to be independent of amiodarone therapy, given her irregular compliance and the subtherapeutic dose. Dual-chamber ICD implantation is the only way to reduce mortality in these patients. In the series by Leenhardt et al.,7 torsade de pointes and VF were documented preceded by short-coupled PVCs. When programming the ICD, atrial pacing was preferred because it appeared to be responsible for better clinical stabilization.
The use of ECMO in these cases is empirical. Indication for ECMO is established in cardiogenic shock related to acute myocardial infarction and myocarditis, but not in cases of arrhythmic storm.13 In an emergency context, its use is described in arrhythmic storms due to Brugada syndrome.13
The utility of EPS and ablation in these patients is controversial.10 In the series by Leenhardt et al.,7 only two patients had inducible PVT, although in some cases the triggering PVCs were monomorphic. EPS is indicated in polymorphic VT without structural heart disease refractory to antiarrhythmic therapy, when successful ablation is expected, with mapping preferably done during the arrhythmic storm.14 Successful ablation does not invalidate the need for an ICD.14
Short-coupled variant torsade de pointes may be associated with genetic disorders of variable penetrance. In the series by Leenhardt et al.,7 four patients had a family history of SCD. However, the management of these patients in terms of genetic and family screening is not well established.6
With this case, we aimed to demonstrate the need for an integrated assessment of clinical data and findings of complementary exams. This integration is crucial for the diagnosis of rare diseases.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.