Aortic intramural hematoma (IMH) is an acute aortic syndrome characterized by bleeding into the media of the aortic wall without intimal disruption or the classic flap formation. Its natural history is variable and still poorly understood, so strategies for therapeutic management are not fully established. In some cases there is partial or complete regression of the hematoma under medical treatment, but most progress to dissection, aneurysmal dilatation or aortic rupture.
The authors present the case of a 44-year-old hypertensive male patient admitted with a diagnosis of IMH of the descending aorta. Despite initial symptom resolution and optimal medical therapy, the IMH evolved to a pseudoaneurysm, which was successfully treated by an endovascular approach.
O hematoma intramural da aorta (IMH) é uma síndrome aórtica aguda caracterizada pela ocorrência de hemorragia a nível da camada média da parede da aorta, sem evidência de ruptura ou flap da íntima. A história natural desta entidade clínica é muito variável e ainda pouco conhecida, pelo que a sua abordagem terapêutica não está completamente estabelecida. Nalguns casos pode ocorrer regressão parcial ou completa do hematoma sob tratamento médico, mas numa proporção significativa existe evolução para dissecção, dilatação aneurismática ou ruptura.
Os autores apresentam o caso de um homem de 44 anos, hipertenso, admitido com o diagnóstico de IMH da aorta descendente. Apesar de a resolução dos sintomas e do adequado controlo da tensão arterial com a terapêutica médica, o IMH evoluiu a curto prazo para a formação de um pseudoaneurisma, que foi tratado eficazmente por via endovascular.
Aortic intramural hematoma (IMH) is considered a variant of classic aortic dissection (AoD) and is characterized by bleeding into the media of the aortic wall without intimal disruption. It accounts for 10–30% of acute aortic syndromes1 and has similar clinical presentation, morbidity and mortality to AoD. However, its natural history and therapeutic management are not as well established as for AoD. Much of the uncertainty is due to its dynamic and unpredictable behavior over time; it can regress or evolve to dissection, aneurysmal dilatation or rupture, and thus requires continuous clinical monitoring and imaging follow-up.
Case reportA 44-year-old man, a smoker (48 pack-years), obese (body mass index 31 kg/m2) and with untreated, uncontrolled hypertension, went to the emergency department for sudden-onset chest pain, which he described as stabbing, radiating to the interscapular region and worsening in dorsal decubitus. At admission he was hypertensive (160/85 mmHg), with no significant difference between the arms, normal heart rate (78 bpm), and strong, symmetrical peripheral pulses, and no other relevant abnormalities on physical examination.
The 12-lead electrocardiogram showed sinus rhythm and voltage criteria for left ventricular hypertrophy (LVH). Laboratory tests indicated mild leukocytosis with neutrophilia and elevated C-reactive protein, but no elevation of myocardial necrosis biomarkers. Transthoracic echocardiography showed moderate left atrial dilatation, left ventricular size at the upper normal limit, moderate concentric LVH, mild dilatation of the aortic root and ascending aorta with no evidence of flap formation or aortic regurgitation, and preserved global systolic function of both ventricles.
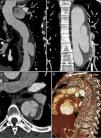
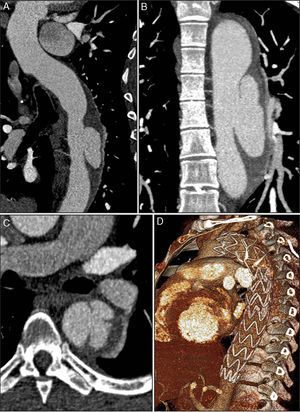
Chest computed tomography angiography (CTA) was performed to investigate the aortic disease, which revealed mild dilatation of the ascending aorta (maximum diameter 42 mm) and circumferential thickening of the aortic wall (approximately 10 mm) consistent with IMH, beginning immediately after the emergence of the left subclavian artery and involving the entire descending aorta and the proximal segment of the abdominal aorta, up to the emergence of the renal arteries (Figure 1A). It also showed a type A patent ductus arteriosus and a partially calcified atherosclerotic plaque (Figure 1B), and two ulcers in the wall of the proximal descending aorta (Figure 1C).
Initial computed tomography angiography (CTA), showing circumferential thickening of the aortic wall (arrows) consistent with intramural hematoma, beginning immediately after the emergence of the left subclavian artery and involving the entire descending aorta and the proximal segment of the abdominal aorta (A); patent ductus arteriosus (arrowhead) and a partially calcified atherosclerotic plaque (arrow) (B); two ulcers in the proximal descending aorta wall (arrows) (C).
A diagnosis of uncomplicated Stanford type B IMH of the descending aorta was assumed; the patient was admitted to the cardiac intensive care unit and therapy was begun with intravenous sodium nitroprusside and labetalol, which resulted in blood pressure (BP) control and complete resolution of symptoms. The case was referred for medical and surgical evaluation and it was decided to maintain medical therapy with clinical and imaging monitoring surveillance, given the patient's stable condition under medical therapy and the absence of complications.
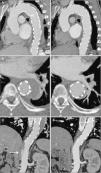
CTA was repeated on the eighth day of hospitalization, and showed a slight increase in hematoma thickness (to around 15 mm), although with no increase in length, and one of the ulcers in the aortic wall appeared deeper and more irregular (Figure 2). The case was again discussed with the cardiothoracic surgical team, and it was decided not to operate and to maintain medical therapy and surveillance. The patient remained clinically stable during hospitalization and was discharged on the 19th day, medicated with four classes of antihypertensive drugs including beta-blockers and referred for outpatient cardiology consultation, and imaging follow-up was scheduled.
Control CTA on the eighth day, showing a slight increase in hematoma thickness to 15 mm and a crescent-shaped formation (C), still extending from the left subclavian artery to the emergence of the renal arteries (A); one of the aortic wall ulcers observed on the initial exam now presenting a deeper and more irregular appearance (arrow) (B).
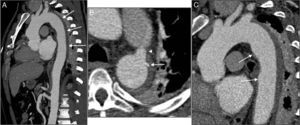
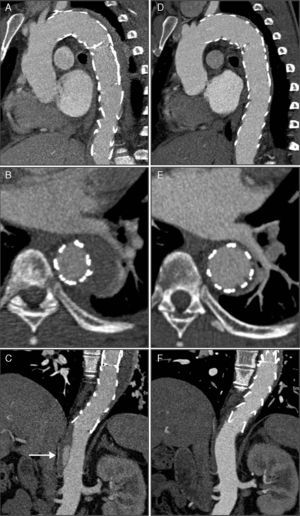
CTA one month after hospital discharge showed intimal rupture of one of the aortic ulcers and evolution to localized dissection, with a pseudoaneurysm of the proximal segment of the descending aorta measuring 27 mm×51 mm (Figure 3). In the light of these findings, the patient was rehospitalized and after discussion with the interventional cardiologist and cardiothoracic surgeon, it was decided to perform thoracic endovascular aneurysm repair (TEVAR). Two endoprostheses (Valiant® 38 mm×150 mm and 34 mm×150 mm) were implanted, the proximal prosthesis adjacent to the emergence of the left subclavian artery without obstructing its flow, and the distal prosthesis extending down to the beginning of the abdominal aorta, thus covering the ulcers, the pseudoaneurysm, the patent ductus arteriosus and most of the IMH. Post-procedural CTA confirmed that the TEVAR had been successful but an image consistent with dissection was observed in the abdominal aorta distally to the distal endoprosthesis, from the celiac trunk to the superior mesenteric artery, both of which emerged from the true lumen, as did the renal arteries (Figure 4A–C). It was not possible to confirm whether this dissection had been present prior to the TEVAR procedure, since the previous CTA had limited acquisition of the thoracic region. It was decided to adopt a conservative approach to this finding.
CTA 30 days after discharge, showing evolution of the hematoma to dissection and a pseudoaneurysm measuring 27 mm×51 mm in diameter with a 23-mm neck (A, B and C). The pseudoaneurysm was located in the mid segment of the descending aorta, and intimal rupture originated in the aortic wall ulcer that had shown signs of progression on the second exam; three-dimensional volume reconstruction following thoracic endovascular aneurysm repair, showing two overlapping endoprostheses, from the left subclavian artery to the beginning of the abdominal aorta (D).
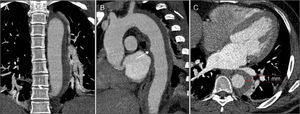
CTA immediately after thoracic endovascular aneurysm repair (A, B and C) and six months after the procedure (D, E and F). The two overlapping aortic endoprostheses can be seen, covering the intramural hematoma and the ductus arteriosus, from the left subclavian artery to the beginning of the abdominal aorta, with no evidence of endoleaks (A); aortic dissection distal to the prosthesis (arrow), originating at the celiac trunk and extending to the superior mesenteric artery (C); compared to the post-procedural exam, at six months almost total regression of the intramural hematoma (D and E) and disappearance of the abdominal aortic dissection (F) can be seen.
The patient was followed in regular outpatient cardiology consultations after hospital discharge. CTA at six months continued to show a good treatment result, with almost total regression of the IMH and disappearance of the abdominal AoD (Figure 4D–F). At present, after 18 months of follow-up, the patient is clinically stable, asymptomatic and with controlled BP (mean 107/73 mmHg on 24-hour ambulatory BP monitoring); CTA at 12 months continued to show a good result.
DiscussionIMH is characterized by bleeding into the media of the aortic wall that usually results from spontaneous rupture of the vasa vasorum or from an atherosclerotic ulcer penetrating the internal elastic lamina.1 Hypertension is the main predisposing factor for IMH, which accounts for 10–30% of acute aortic syndromes.1 It generally affects older patients (mean age 68 years) with more cardiovascular risk factors than AoD, and more frequently involves the descending aorta (60–85% of cases).2,3 As in AoD, the Stanford classification is used to categorize IMH as proximal (type A), involving the ascending aorta, or distal (type B) with no involvement of the ascending aorta.
The clinical presentation and diagnosis of IMH and AoD are similar. The most common symptom is chest pain, anterior pain predominating in proximal IMH, while in cases involving the descending aorta, dorsal (interscapular) or lumbar pain is more common.4 Other manifestations that can occur in AoD, such as poor organ perfusion, weak pulse, myocardial infarction and neurological symptoms, are less frequent in IMH.
Since different acute aortic syndromes are difficult to distinguish by clinical presentation alone, imaging studies are essential for etiological diagnosis. CTA, magnetic resonance and transesophageal echocardiography have similar diagnostic accuracy,5 but CTA is the first-line imaging method for diagnosis and follow-up of IMH. The diagnostic criteria are circumferential or crescent-shaped thickening of the aortic wall of ≥7 mm and/or evidence of accumulation of blood in the media without intimal disruption or flap formation.5 On CTA, the thickening appears denser than the blood and adjacent layers of the aortic wall (approximately 50–70 Hounsfield units) and does not enhance after contrast administration.
The natural history of IMH is variable and still poorly understood. According to the literature, regression or complete reabsorption of the hematoma occurs in 10–34% of cases, intimal disruption and subsequent dissection in 28–47%, and aneurysmal dilatation or rupture in 20–45%.2,6 In-hospital mortality is similar to that of AoD (20.7% and 23.9%, respectively)2 and the clinical behavior of IMH varies according to its location, as in AoD. IMH of the ascending aorta has a higher and earlier risk of death or complications such as dissection, rupture, aortic regurgitation, cardiac tamponade or myocardial infarction, while type B IMH generally has a more benign course. The rate of progression to AoD reported in the literature is 3–14% for type B IMH and 11–88% for IMH involving the ascending aorta.7 In a meta-analysis of 143 patients, cases with type A IMH treated surgically had lower mortality than those treated medically (14% vs. 36%), while cases involving the descending aorta had similar mortality with medical or surgical treatment (14% vs. 20%).3
Although there are no established treatment guidelines for IMH, it is recommended that these patients be treated in the same way as those with AoD, based on current evidence.7 Initial medical therapy to stabilize patients is essential in all acute aortic syndromes, and is based on reducing BP, the first-line agents being intravenous beta-blockers such as metoprolol, propanolol, labetalol or esmolol, for a target systolic BP of 100–120 mm Hg and heart rate of <60 bpm.7,8 If necessary, this can be combined with intravenous vasodilators, such as sodium nitroprusside or nitroglycerin. Early surgery is the treatment of choice for patients with type A IMH, whereas intensive medical therapy is recommended for uncomplicated IMH of the descending aorta (aggressive BP control and alpha- and beta-blocker therapy), as for type B AoD.6–10 Given the unpredictable evolution of IMH, patients treated medically require close clinical and imaging follow-up at 1, 3, 6 and 12 months following diagnosis, and annually thereafter.1 Patients with type B IMH who present persistent or recurrent pain, refractory hypertension, organ or lower limb ischemia, or evidence of progression (dilatation, dissection or rupture) on imaging follow-up studies should undergo endovascular or surgical treatment; the former has become the first-line option in recent years.7,11–13
Although there have been no randomized trials comparing surgery with TEVAR in the treatment of aortic disease, the studies that have been performed show that patients treated with TEVAR have lower rates of periprocedural mortality and neurological, bleeding, cardiac and respiratory complications compared to conventional surgical repair.14 The main complications associated with percutaneous treatment are related to vascular access, since a large caliber artery for insertion of a 22–24F introducer is usually required to implant the endoprosthesis. There is a risk of paraplegia or paraparesis, but this is significantly lower than with surgery.14 Another problem with TEVAR is the risk of endoleaks, which occur in 10–20% of patients7 and necessitate re-intervention. However, the favorable short- and medium-term results reported have led to TEVAR being used in an increasing number of patients with different types of thoracic aortic disease including IMH, although doubts remain as to the long-term efficacy and durability of this procedure. Imaging follow-up by CTA or magnetic resonance is recommended after TEVAR, prior to discharge, at 6 and 12 months, and annually thereafter.13
The natural history of IMH is poorly understood and strategies for therapeutic management are not fully established, but recent studies have set out to identify predictors of disease progression. Proximal location (Stanford type A) is considered an independent predictor of progression to dissection, rupture or aneurysm formation.4,15 Kaji et al. reported that patients with type A IMH and maximum aortic diameter of ≥50 mm on initial CTA have a greater risk of disease progression than those with aortic diameter of <50 mm.16
In cases of type B IMH, Sueyoshi et al.17 demonstrated that a maximum aortic diameter of ≥40 mm and maximum hematoma thickness of ≥10 mm on initial CTA were independent predictors of progression. Another study found that a progressive increase in aortic diameter and wall thickness, age <55 years and absence of beta-blocker therapy were predictors of IMH progression.4
The presence of penetrating ulcers in association with IMH is also a major factor in increased risk of progression in patients under medical therapy.1,18 In a series of 65 patients treated medically, progression to aortic rupture, expansion of the hematoma or dissection occurred significantly more often in those with penetrating aortic ulcers (48% vs. 8%), particularly larger ones (maximum diameter ≥20 mm or maximum depth ≥10 mm).18 Some authors have therefore suggested that patients with IMH of the descending aorta and a penetrating ulcer should be referred for endovascular treatment.
In the case presented of type B IMH with no evidence of complications, it was initially decided to adopt a strategy of medical therapy and surveillance, in accordance with current guidelines. Despite symptom resolution and optimal BP control, CTA on the eighth day of hospital stay showed a slight increase in IMH thickness, and one month after discharge progression to dissection and pseudoaneurysm formation was documented. Although there are no clear treatment indications for these patients, the present case highlights the importance of close imaging follow-up for the early identification of complications or disease progression; it also supports early intervention when there is evidence of increased hematoma thickening.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ponte M, Dias Ferreira N, Bettencourt N, et al. Hematoma intramural da aorta: evolução (im)previsível? Rev Port Cardiol. 2014;33:467.