To characterize the epidemiology and risk factors for acute kidney injury (AKI) after pediatric cardiac surgery in our center, to determine its association with poor short-term outcomes, and to develop a logistic regression model that will predict the risk of AKI for the study population.
MethodsThis single-center, retrospective study included consecutive pediatric patients with congenital heart disease who underwent cardiac surgery between January 2010 and December 2012. Exclusion criteria were a history of renal disease, dialysis or renal transplantation.
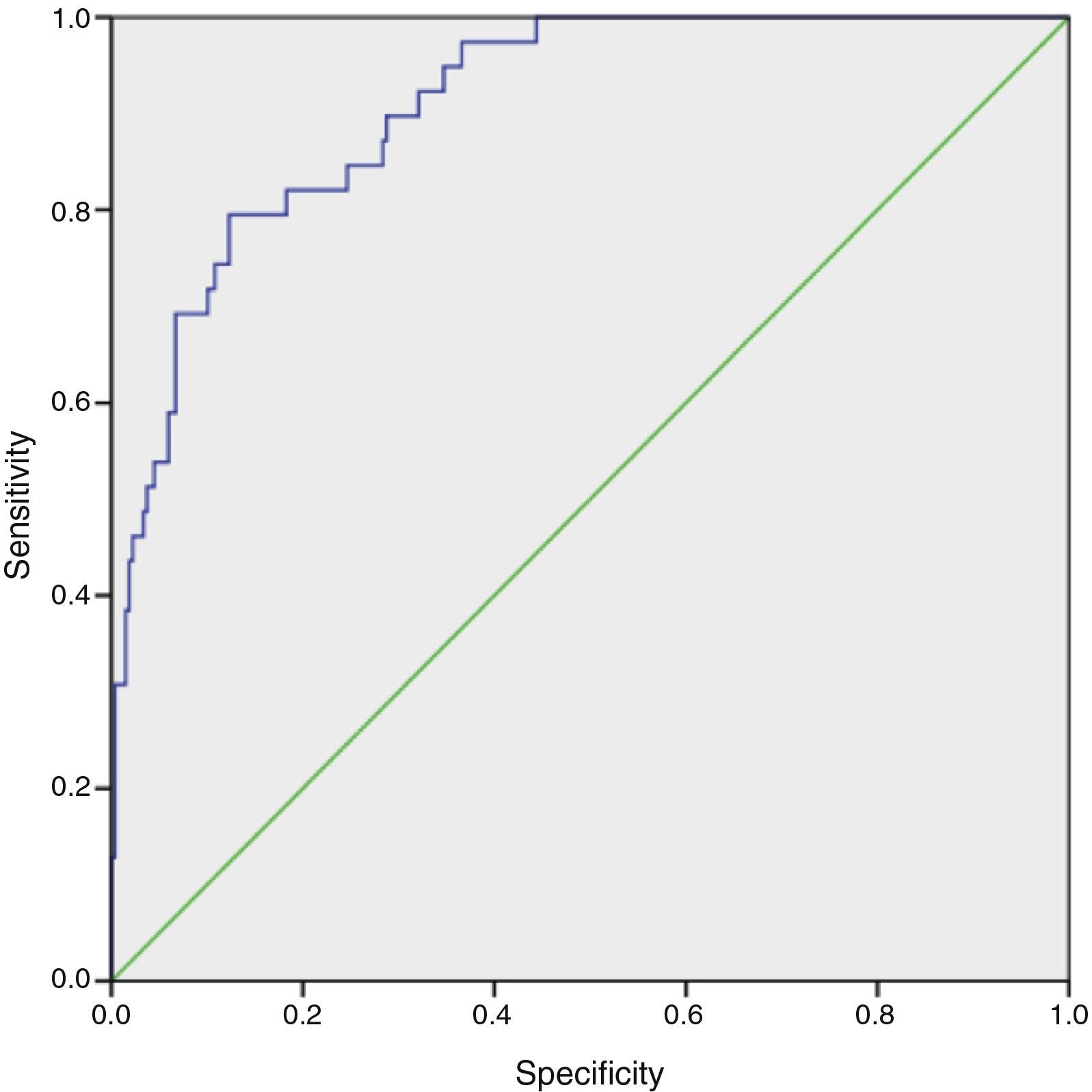
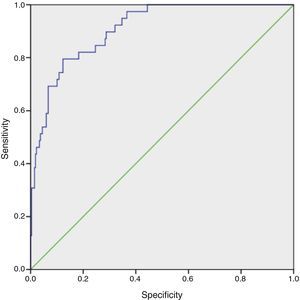
ResultsOf the 325 patients included, median age three years (1 day–18 years), AKI occurred in 40 (12.3%) on the first postoperative day. Overall mortality was 13 (4%), nine of whom were in the AKI group. AKI was significantly associated with length of intensive care unit stay, length of mechanical ventilation and in-hospital death (p<0.01). Patients’ age and postoperative serum creatinine, blood urea nitrogen and lactate levels were included in the logistic regression model as predictor variables. The model accurately predicted AKI in this population, with a maximum combined sensitivity of 82.1% and specificity of 75.4%.
ConclusionsAKI is common and is associated with poor short-term outcomes in this setting. Younger age and higher postoperative serum creatinine, blood urea nitrogen and lactate levels were powerful predictors of renal injury in this population. The proposed model could be a useful tool for risk stratification of these patients.
Caracterizar, no nosso centro, a epidemiologia, fatores de risco e impacto prognóstico da insuficiência renal aguda no pós-operatório cardíaco. Desenvolver um modelo de regressão logística para estimativa do risco de insuficiência renal aguda na população em estudo.
MétodosEstudo retrospetivo e monocêntrico em que foram incluídos doentes pediátricos consecutivos com cardiopatia congénita, submetidos a cirurgia cardíaca entre janeiro de 2010 e dezembro de 2012. Foram excluídos aqueles com doença renal prévia, história de diálise ou transplantação renal.
ResultadosForam incluídos 325 doentes, idade mediana = 3 anos (um dia; 18 anos). Quarenta (12,3%) doentes desenvolveram insuficiência renal aguda no primeiro dia após a cirurgia. Nove (69%) dos 13 doentes falecidos no pós-operatório integravam o grupo com insuficiência renal. A ocorrência de insuficiência renal aguda condicionou um aumento do tempo de internamento na unidade de cuidados intensivos, da duração da ventilação mecânica invasiva e da mortalidade intra-hospitalar (p < 0,01). Foi construído um modelo de regressão logística (variável dependente: insuficiência renal aguda pós-operatória, variáveis preditoras: idade e valores séricos de creatinina, ureia e lactatos registados no primeiro dia de pós-operatório). O modelo previu de forma significativa a ocorrência de insuficiência renal aguda pós-operatória nesta população, com uma sensibilidade e especificidade máximas combinadas de 82,1 e 75,4%.
ConclusõesNo pós-operatório cardíaco a insuficiência renal é comum e determina um mau prognóstico. A idade mais jovem e a elevação precoce da creatinina, ureia e lactatos séricos foram preditores robustos da ocorrência de insuficiência renal nesta população, permitindo a construção de um modelo analítico objetivo que poderá ser útil na estratificação de risco nestes doentes.
Congenital heart disease (CHD) is the most common congenital abnormality and occurs in around 0.8% of live births. Around half of cases will require surgical repair, and some of these may present in critical condition, particularly in the neonatal period.1
Acute kidney injury (AKI) is common after pediatric cardiac surgery, with an estimated prevalence of 5%–33%.2 It is associated with significant morbidity and mortality of 20%–79%, depending on the definition of AKI.2 The pathogenesis of AKI in this context is unknown; it is probably caused by multiple factors including low cardiac output, hypoxemia, inflammation and use of nephrotoxic drugs.3 Retrospective studies suggest that AKI associated with cardiac surgery may affect not only short-term outcomes but also the risk of developing chronic renal failure.4 A thorough understanding of its pathophysiology and associated risk factors is therefore necessary to reduce the incidence of AKI in these patients.
The aims of this study were to characterize the epidemiology and risk factors for AKI after pediatric cardiac surgery in our center and to determine its impact on length of mechanical ventilation, length of intensive care unit (ICU) stay and in-hospital mortality.
We also set out to develop a logistic regression model that will predict the risk of AKI in a consistent and objective manner, based on easily obtained clinical and laboratory parameters.
MethodsStudy design and patient selectionThis was a single-center, retrospective, observational study based on data collected from the medical records of consecutive pediatric patients who underwent cardiac surgery in our center between January 2010 and December 2012.
All patients with CHD aged <18 years were included, except those with a history of renal disease, dialysis or renal transplantation.
Clinical and laboratory variablesPreoperative variables were age at time of surgery, gender, weight, height, type of CHD (classified as cyanotic or acyanotic), and serum creatinine and blood urea nitrogen on preoperative laboratory testing.
Intraoperative variables were cardiopulmonary bypass time, aortic cross-clamp time and circulatory arrest time. The Aristotle score was used to classify the complexity of the procedures on a scale of 1 to 4.5
Postoperative variables included serum creatinine, blood urea nitrogen and lactate levels recorded on the first postoperative day.
The postoperative inotropic score was calculated using the formula proposed by Maarslet et al.6: dopamine (μg/kg/min) (×1) + dobutamine (μg/kg/min) (×1) + milrinone (μg/kg/min) (×10) + adrenaline (μg/kg/min) (×100), and classified as low (<8) or high (≥8).
Other variables were need for mechanical ventilation for >48 h postoperatively, length of ICU stay (days), need for peritoneal dialysis and in-hospital death.
Definition of acute kidney injuryAKI was defined according to the pediatric RIFLE (risk, injury, failure, loss and end-stage renal disease) (pRIFLE) criteria, which are based on postoperative fall in glomerular filtration rate (GFR) compared to baseline GFR.7 This classification was derived from a consensus of specialists and has been validated in children with CHD.8
The modified Schwartz formula was used to calculate GFR.9,10 Baseline serum creatinine was taken to be the value obtained in preoperative laboratory testing (within 48 h before surgery). Postoperative GFR was calculated on the basis of serum creatinine levels recorded on the first postoperative day.
Each patient was assigned a pRIFLE class: R (risk of renal dysfunction), I (injury to the kidney), or F (failure of kidney function), according to postoperative change in creatinine clearance. Classes R, L and F correspond to a fall in GFR of 25%, 50% and 75%, respectively, relative to baseline values. Patients with a postoperative GFR of <35 ml/min/1.73 m2 were assigned class F.
All patients in pRIFLE classes I and F were considered to have developed postoperative AKI.
Urine output was not used as a criterion of renal failure in this cohort, since it is known to be affected by intraoperative and postoperative factors such as diuretic use.
Statistical analysisContinuous variables are presented as means ± standard deviation when following a normal distribution and as medians, minimum and maximum otherwise. Categorical variables are expressed as frequencies and percentages.
The Student's t test or the Mann-Whitney test was used in univariate analysis of continuous variables and the chi-square test or Fisher's exact test was used to analyze categorical variables.
A logistic regression model was constructed for multivariate analysis. Postoperative AKI was defined as the dependent variable. Following analysis of preliminary models, the final model included the following as independent predictor variables: age at time of surgery (years) and serum creatinine, blood urea nitrogen and lactate level, recorded on the first postoperative day. Each predictor variable was selected after confirmation of its independent association on univariate analysis with postoperative AKI. The model's goodness of fit was assessed by the Hosmer-Lemeshow test.
A p value of <0.05 was considered statistically significant. The statistical analysis was performed using SPSS® version 20.0 (IBM SPSS, Chicago, IL).
ResultsEpidemiology of acute kidney injury after cardiac surgeryOf the 325 patients included, median age 3 years (1 day–18 years), 7.7% were newborns. One hundred and seventy-four (53.5%) were male, 104 (32%) had cyanotic CHD, and 283 (87%) underwent surgery under cardiopulmonary bypass. Most (68.9%) had an Aristotle score of ≥2.
Forty (12.3%) patients developed AKI on the first postoperative day. Of these, 31 were classified as class I and nine as class F on the pRIFLE criteria. Most (67%) of the patients who needed peritoneal dialysis were in class F, while nine (70%) of the 13 who died in the postoperative period were in the renal failure group (class I or F).
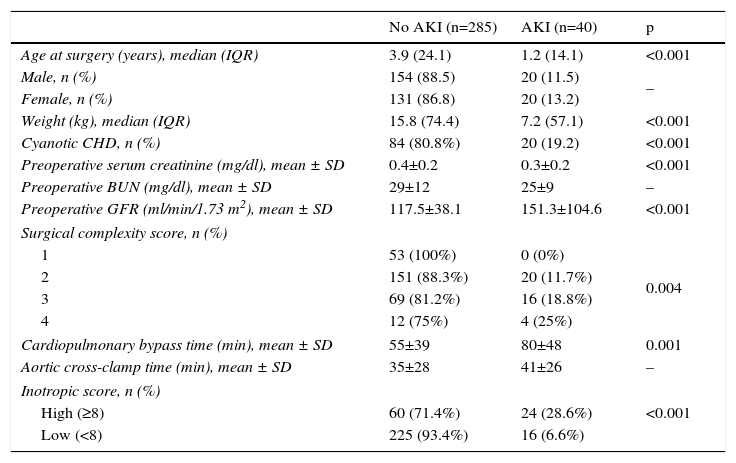
Risk factors for acute kidney injuryPatients who developed postoperative AKI were younger, weighed less, and had lower baseline serum creatinine (p<0.001). Higher Aristotle score and longer cardiopulmonary bypass time (p=0.001) were significantly associated with AKI in this population. However, there was no association with aortic cross-clamp time (p=0.08) (Table 1).
Characteristics of children undergoing cardiac surgery according to the occurrence of acute kidney injury.
| No AKI (n=285) | AKI (n=40) | p | |
|---|---|---|---|
| Age at surgery (years), median (IQR) | 3.9 (24.1) | 1.2 (14.1) | <0.001 |
| Male, n (%) | 154 (88.5) | 20 (11.5) | – |
| Female, n (%) | 131 (86.8) | 20 (13.2) | |
| Weight (kg), median (IQR) | 15.8 (74.4) | 7.2 (57.1) | <0.001 |
| Cyanotic CHD, n (%) | 84 (80.8%) | 20 (19.2) | <0.001 |
| Preoperative serum creatinine (mg/dl), mean ± SD | 0.4±0.2 | 0.3±0.2 | <0.001 |
| Preoperative BUN (mg/dl), mean ± SD | 29±12 | 25±9 | – |
| Preoperative GFR (ml/min/1.73 m2), mean ± SD | 117.5±38.1 | 151.3±104.6 | <0.001 |
| Surgical complexity score, n (%) | |||
| 1 | 53 (100%) | 0 (0%) | 0.004 |
| 2 | 151 (88.3%) | 20 (11.7%) | |
| 3 | 69 (81.2%) | 16 (18.8%) | |
| 4 | 12 (75%) | 4 (25%) | |
| Cardiopulmonary bypass time (min), mean ± SD | 55±39 | 80±48 | 0.001 |
| Aortic cross-clamp time (min), mean ± SD | 35±28 | 41±26 | – |
| Inotropic score, n (%) | |||
| High (≥8) | 60 (71.4%) | 24 (28.6%) | <0.001 |
| Low (<8) | 225 (93.4%) | 16 (6.6%) | |
AKI: acute kidney injury; BUN: blood urea nitrogen; CHD: congenital heart disease; GFR: glomerular filtration rate; IQR: interquartile range; SD: standard deviation.
The risk of developing AKI was 6.6 times higher in newborns than in children aged >13 years (odds ratio [OR] 7.615; 95% confidence interval [CI] 0.977–59.370), double in patients with cyanotic CHD (OR 2.125; 95% CI 1.197–3.774), and 3.3 times higher in those with a high inotropic score (OR 4.304; 95% CI 2.405–7.7).
Prognostic impactThe occurrence of AKI after cardiac surgery was associated significantly with longer ICU stay and longer mechanical ventilation (p<0.01).
In the group that did not develop AKI, median ICU stay was three days (1–36), 55 patients (19.3%) needed mechanical ventilation for more than 48 h, and there were four deaths (1.4%).
In the group with AKI, median ICU stay was 6.5 days (1–30), 21 patients (52.5%) needed mechanical ventilation for more than 48 h, and there were nine deaths (22.5%).
Development of AKI thus resulted in a 13.3-fold increase in mortality risk.
Logistic regression modelA total of 307 cases were included in the logistic regression model, 18 cases (5.5% of the sample) having been excluded due to missing information, which was not permitted in the model design.
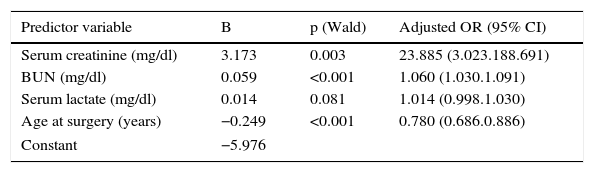
The final model, which included the predictor variables of age at time of surgery and serum creatinine, blood urea nitrogen and lactate levels, recorded on the first postoperative day, predicted the occurrence of AKI after cardiac surgery (omnibus chi-square test: p<0.001) (Table 2).
Predictor variables in the logistic regression model.
| Predictor variable | B | p (Wald) | Adjusted OR (95% CI) |
|---|---|---|---|
| Serum creatinine (mg/dl) | 3.173 | 0.003 | 23.885 (3.023.188.691) |
| BUN (mg/dl) | 0.059 | <0.001 | 1.060 (1.030.1.091) |
| Serum lactate (mg/dl) | 0.014 | 0.081 | 1.014 (0.998.1.030) |
| Age at surgery (years) | −0.249 | <0.001 | 0.780 (0.686.0.886) |
| Constant | −5.976 | ||
BUN: blood urea nitrogen; CI: confidence interval; OR: odds ratio.
The model's goodness of fit was assessed by the Hosmer-Lemeshow test (H=4.25; degrees of freedom=8; p=0.834). The model accurately reflected the actual occurrence of AKI in our population, as the p value in this test was well over 0.05. The area under the receiver operating characteristic curve was 0.909 (95% CI 0.866–0.951), indicating that 90.9% of the model's predictions were correct (Figure 1).
The model is expressed by the equation: logit (AKI)=EXP(−5.976+3.173×creatinine+0.059×blood urea nitrogen+0.014×lactate–0.249×age).
DiscussionPatterns of acute kidney injuryThe findings of this study are in line with current evidence and shows that AKI after cardiac surgery is common, with an incidence of 12.3% in this sample. AKI occurs very early in the course of ICU stay, usually within two days of surgery.
Zapitelli et al., in a cohort of children undergoing cardiac surgery, showed that patients whose serum creatinine did not increase on postoperative days 1 or 2 were unlikely to develop AKI (negative predictive values of 87% and 98% on days 1 and 2, respectively).3
This epidemiological pattern is different from that seen in adults7 and highlights the need to identify susceptible patients as early as possible.
The reason for selecting serum creatinine level on the first postoperative day as a predictor variable in our study was to standardize the analysis and to reduce the effect of confounding factors related to therapy following surgery. It was also our intention to develop a screening tool for early identification of patients at risk of AKI.
Analysis of preoperative risk factors showed that younger children are at greater risk of renal failure. This can be explained by the fact that maximum GFR is not reached until about two years of age, and thus children younger than this may be more susceptible to the ischemic and inflammatory insults caused by cardiac surgery.2
In our population, patients who developed AKI presented lower preoperative serum creatinine, probably due to their younger age, smaller muscle mass and worse nutritional status.
The definition of AKI used in this study was based on variation from baseline of estimated GRF. Although this is a generally accepted definition,7 there are limitations to the use of creatinine as a biomarker of renal failure, particularly in small children. We did not use urine output as a criterion, since it is known to be affected by intraoperative and postoperative factors. Akcan-Arikan et al. demonstrated in a pediatric population that classification of AKI based on variation in GFR was not altered significantly by the addition of urine output as a criterion,7 while Mammen et al. showed that changes in serum creatinine are more sensitive than urine output for identifying AKI.4
The etiology of AKI in the context of cardiopulmonary bypass is related to hypoperfusion, inflammation and loss of pulsatile flow, leading to vasoconstriction and ischemia. The findings of the present study support this line of thinking, revealing a greater incidence of AKI in patients with a higher Aristotle score and longer bypass time. Even so, patients who underwent cardiac surgery without cardiopulmonary bypass had the same increased risk of developing AKI, suggesting alternative mechanisms for injury.11
Development of AKI following cardiac surgery was associated with longer ICU stay and longer mechanical ventilation, which entail greater use of hospital resources and increased costs.
Finally, AKI significantly increased the risk of in-hospital death.
Logistic regression modelRisk stratification for AKI can be based on easily obtainable clinical and laboratory variables and early intervention can reasonably be expected to improved outcome in susceptible patients.
The logistic regression model was shown to be robust in predicting risk of AKI, with a maximum combined sensitivity and specificity of 82.1% and 75.4%, respectively. Although the model has internal validity, it needs to be tested in a different population from the one for which it was developed, to confirm its ecological validity. We plan to include a prospective validation of the model in future research.
Study limitationsThe study has the limitations inherent to its retrospective design, which means that the associations encountered may not be causal in nature. Furthermore, the odds ratios calculated are only an approximation of the real relative risk, which can only be calculated using a prospective methodology. It is thus essential to carry out a prospective study of potentially reversible risk factors for AKI after cardiac surgery, in order to obtain a more accurate assessment of the independent variables used in our model in terms of risk and causality. Another limitation is the fact that this was a single-center study, with a small number of patients, particularly newborns, who are the most susceptible to renal damage following cardiac surgery. This highlights the need for multicenter studies in this setting.
ConclusionAKI is common after cardiac surgery and is associated with poor short-term outcomes, with significantly longer ICU stay and mechanical ventilation, and increased mortality. Younger age and higher postoperative serum creatinine, blood urea nitrogen and lactates were powerful predictors of renal injury in this population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Dr. Tiago Costa and the Research Center of Centro Hospitalar de Lisboa Central for their assistance in the statistical analysis.
Please cite this article as: Cardoso B, Laranjo S, Gomes I, Freitas I, Trigo C, Fragata I, et al. Insuficiência renal aguda no contexto de cirurgia cardíaca pediátrica: fatores de risco e prognóstico. Proposta de um modelo preditivo. Rev Port Cardiol. 2016;35:99–104.