Prosthetic valve dysfunction is a significant clinical event. Determining its etiological mechanism and severity can be difficult. The authors present the case of a 50-year-old man, with two mechanical valve prostheses in aortic and mitral positions, hospitalized for decompensated heart failure. He had a long history of rheumatic multivalvular disease and had undergone three heart surgeries.
On admission, investigation led to a diagnosis of severe dysfunction of both mechanical prostheses with different etiologies and mechanisms: pannus formation in the prosthetic aortic valve and intermittent dysfunction of the mitral prosthesis due to interference of a ruptured chorda tendinea in closure of the disks. The patient was reoperated, leading to significant improvement in functional class.
Em doentes portadores de próteses valvulares mecânicas, a ocorrência de disfunção protésica constitui um evento clínico relevante. Ocasionalmente, a demonstração do seu mecanismo etiológico e gravidade podem revelar-se difíceis.
Apresenta-se o caso clínico de um doente de 50 anos, portador de duas próteses valvulares mecânicas nas posições aórtica e mitral, internado por descompensação de insuficiência cardíaca. Nos antecedentes apresentava uma história longa de doença plurivalvular reumática com três cirurgias cardíacas prévias.
No internamento a investigação conduziu ao diagnóstico de disfunção grave de ambas as próteses mecânicas, sendo a etiologia e o mecanismo diferente em cada caso: recidiva de pannus no caso da prótese aórtica e bloqueio intermitente do encerramento dos discos por interposição de uma corda rota do aparelho subvalvular, no caso da prótese mitral. O doente foi reoperado, tendo havido melhoria significativa da classe funcional.
Progressive refinements in the design and hemodynamic profile of valve prostheses have led to reductions in prosthetic dysfunction.1 However, all valve prostheses continue to be associated with complications2 and dysfunction of varying severity, and this should be borne in mind when assessing the risk/benefit ratio in individual patients referred for valve replacement.3
Obstructive mechanical prosthetic valve dysfunction can be due to valve thrombosis, pannus formation or a combination of the two.4
Thrombosis is usually associated with subtherapeutic levels of anticoagulation,5 develops rapidly and is potentially fatal.6 However, cases have been reported of a more insidious course, and differential diagnosis with pannus formation is of the utmost importance since the only therapeutic option in cases of pannus causing significant hemodynamic compromise is surgery (prosthesis replacement or pannus resection in selected cases),7 while in cases of prosthesis thrombosis thrombolytic therapy can be considered.8,9
Pannus results from the intraprosthetic development of fibrovascular and/or granulation tissue, which can cause significant obstruction.2,10 Diagnosis is hindered not only by its habitually slow and insidious formation, but particularly by the fact that routine diagnostic exams such as echocardiography can document high transprosthetic gradients but do not provide adequate visualization of pannus ingrowth.11
Prosthetic valves are also liable to pathological regurgitation; in mechanical prostheses, this is usually the result of endocarditis or of technical problems during implantation.
The case presented here describes a patient with recurrent mechanical aortic prosthetic valve obstruction due to pannus formation, together with intermittent dysfunction of the mitral mechanical prosthesis due to interference of a ruptured chorda tendinea of the residual native subvalvular apparatus in closure of the disks.
Case reportA 50-year-old man, with a history of rheumatic fever at age 22 and three previous heart valve surgeries, was admitted to a cardiology ward in March 2010 for decompensated heart failure (HF).
In 1991, following a first episode of atrial fibrillation (AF), he was diagnosed with rheumatic valvular disease, with regurgitation in both the aortic and mitral valves, together with moderate left ventricular (LV) dilatation and dysfunction. At that time, he underwent his first cardiac surgery, the aortic valve being replaced by a 23-mm Duromedics mechanical prosthesis and the mitral valve being repaired with a Carpentier-Edwards ring. Echocardiography on the seventh postoperative day showed a peak gradient through the aortic prosthesis of 23 mmHg and no apparent regurgitation. The mitral valve presented only minor regurgitation and normal area by planimetry. LV dilatation (LV end-diastolic diameter [LVEDD] of 69 mm) and moderate LV systolic dysfunction persisted. After two years during which he remained asymptomatic with generally therapeutic levels of anticoagulation, he began suffering HF symptoms, which rapidly worsened to NYHA functional class III. Echocardiography revealed significantly increased transprosthetic aortic gradients (peak and mean of 90 mmHg and 57 mmHg, respectively). Function of the repaired mitral valve remained good, with minor regurgitation. LV dilatation persisted (LVEDD and LV end-systolic diameter of 67 mm and 53 mm, respectively), with moderate systolic dysfunction. Fluoroscopic assessment confirmed prosthetic dysfunction, showing incomplete closure of the prosthetic discs.
The patient was reoperated to replace the aortic valve, the diagnosis of obstruction due to pannus formation being confirmed by direct intraoperative inspection. Following removal of the prosthetic valve and resection of the pannus, a 23-mm St. Jude aortic prosthesis was implanted. Echocardiography on the fifth postoperative day showed a peak gradient through the aortic valve of 29 mmHg.
This second surgery resulted in significant functional recovery, and the patient remained asymptomatic for 10 years. HF symptoms reappeared in 2003 and progressively worsened up to 2007. At that time, echocardiography revealed worsening mitral valve disease (stenosis and valve orifice area of 1.1 cm2 by planimetry, with severe eccentric regurgitation due to marked hypomobility of the posterior leaflet) and severe tricuspid regurgitation, with estimated pulmonary artery systolic pressure (PASP) of 45 mmHg. The aortic prosthesis presented peak and mean gradients of 41 mmHg and 27 mmHg, respectively. Worsening LV dysfunction was also observed, with ejection fraction of 35%.
A third cardiac surgery was performed in May 2007, which confirmed the echocardiographic findings regarding the mitral valve. Intraoperative inspection of the aortic prosthesis revealed no significant macroscopic changes. The mitral valve anterior leaflet was removed and the posterior leaflet rolled up, together with the chordae tendineae, and sutured to the posterior region of the annulus, thus preserving the mitral subvalvular apparatus. A St. Jude mechanical prosthesis was implanted in mitral position and tricuspid annuloplasty was performed using a 34-mm Carpentier-Edwards ring.
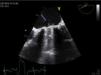
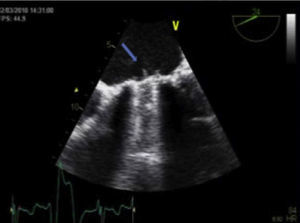
The patient was discharged in NYHA class II, but HF symptoms progressively worsened up to March 2010, and serial echocardiograms showed progressively higher gradients through the aortic prosthesis implanted in 1993. He was admitted to our department for decompensated HF, in NYHA functional class IV. On admission, he was apyretic, and cardiac auscultation revealed metallic heart sounds from the prosthetic valves and a systolic murmur of varying intensity throughout the precordium. Laboratory tests showed no elevation of inflammatory parameters; INR was 2.8. The electrocardiogram revealed AF (known since 1991), LV hypertrophy and diffuse, non-specific ventricular repolarization alterations. Transthoracic echocardiography (TTE), in which it was difficult to visualize the aortic prosthetic disks, showed Doppler parameters compatible with aortic valve obstruction. Despite variations over the cardiac cycle, gradients were higher than in previous exams (peak and mean of 70 mmHg and 40 mmHg, respectively), with a ratio of LV outflow tract velocity time integral (VTI) to aortic VTI of 0.14. A highly mobile, filamentous structure was observed seemingly adhering to the ventricular side of the mitral prosthetic ring, which did not appear to interfere with valve function, and transprosthetic gradients were not significantly elevated (peak and mean of 12 mmHg and 4 mmHg, respectively). Mitral regurgitation could not be visualized on TEE due to reverberation artifacts in the left atrium.
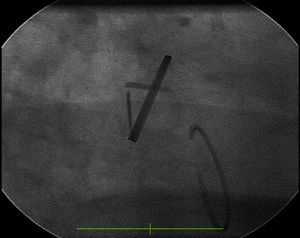
Transesophageal echocardiography performed to clarify the situation revealed mitral prosthetic dysfunction (moderate to severe intraprosthetic pathological regurgitation observed in some cardiac cycles only) due to intermittent interference of the above-described filamentous structure between the prosthetic disks, preventing normal valve function (Figure 1). Fluoroscopy confirmed intermittent incomplete closure of one of the valve disks (Figure 2).
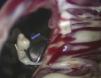
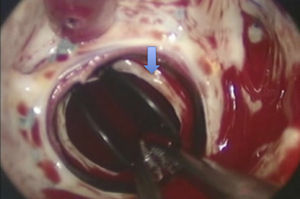
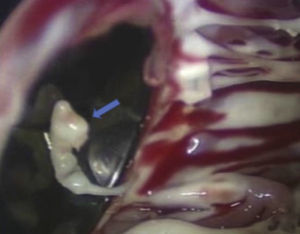
The patient was operated for the fourth time in April 2010. Pannus formation along the rim of the ventricular side of the aortic prosthetic ring (Figure 3) was confirmed intraoperatively, as well as a ruptured chorda of the native mitral subvalvular apparatus that intermittently impeded effective mitral disk closure (Figure 4). The pannus was removed, the aortic prosthesis replaced and the ruptured chorda resected.
The postoperative period was uneventful, and the patient was discharged eight days later. The discharge echocardiogram revealed normal functioning of both mechanical prostheses, with no significant changes in volumes or ventricular function compared to the preoperative period.
Two years after the last surgical intervention, the patient was in NYHA functional class II. Echocardiography in March 2012 showed the aortic and mitral prostheses to be functioning normally, with biplane LV ejection fraction of 43%. Mild tricuspid regurgitation persisted, and pulmonary artery systolic pressure was 39 mmHg.
DiscussionBoth thrombosis and pannus formation can cause obstructive prosthetic dysfunction. Differential diagnosis between these two entities is usually based on clinical and echocardiographic findings. While thrombosis can occur at any time after valve replacement surgery, it is more common for symptoms to appear soon after implantation and rapidly progress until reoperation becomes necessary, generally within a month.6 Inadequate anticoagulation is also more common in cases of thrombosis. By contrast, the late appearance of symptoms, many years after surgery, with a slower and more insidious course, favors a diagnosis of pannus. With regard to echocardiographic features, thrombi are generally visible as masses of varying size that are mobile, of low density, and adhering to the disk(s), while pannus is a fixed, dense thickening of tissue that is difficult to visualize due to its periannular location. The etiology of pannus has yet to be fully clarified,2 but is probably multifactorial. Pannus formation appears to be a biological reaction to mechanical valve prostheses, associated with various factors such as prosthesis design, biocompatibility, surgical technique, prosthesis-patient mismatch, infection, turbulent blood flow and wall shear stress.2,12,13 Interestingly, subtherapeutic anticoagulation is considered a risk factor not only for prosthesis thrombosis but also for pannus formation,2 and the two conditions can coexist.14
The hemodynamic effects of pannus depend on its location and size; in the case of mechanical aortic valves, it usually occurs on the ventricular side.4 Besides causing varying degrees of obstruction, it can occasionally lead to pathological regurgitation by preventing effective disk closure.15
Two aspects of the case presented are worth noting: the early development of pannus on the aortic prosthesis, only two years after the first surgery, and its recurrence nine years after redo valve replacement. In three series reported in the literature, of 23, 615 and 390 patients with mainly mechanical aortic prostheses (only 14 with mechanical mitral valves), mean time to reoperation due to pannus was 178±52 months, 83±52 months and 10±7.9 years, respectively,2,6,16 although a few isolated cases of rapidly progressing pannus formation have been described.10 Recurrent pannus formation has rarely been reported.
This case highlights the importance of postoperative TTE assessment to record the baseline hemodynamic parameters of prostheses for comparison with follow-up exams, particularly in cases of clinical deterioration.
The surgical techniques currently used for implantation of mechanical prostheses in mitral position are designed to spare the native subvalvular apparatus in order to preserve LV geometry and ejection fraction.11,17 Various techniques are available to achieve this, the choice of which should be based on the patient's anatomy, underlying disease and LV function, and the type of prosthesis.18 Rare cases have been reported of the mitral subvalvular apparatus or suture material interfering with prosthetic function, which can have fatal consequences,19 but in most cases, this is detected and corrected intraoperatively. This mechanism of prosthetic dysfunction is more common in rheumatic valvular disease. In our patient, mitral prosthetic dysfunction was intermittent, due to a ruptured chorda coming between the prosthetic disks during some cardiac cycles only, which could have made diagnosis more difficult. In these cycles, disk closure was incomplete, causing pathological regurgitation.
This case illustrates how diagnosing intermittent prosthetic dysfunction can be problematic, and highlights the importance of careful auscultation and thorough echocardiographic assessment. Doppler study of transprosthetic gradients at low scanning speeds over a long period, covering a large number of cardiac cycles, is essential to maximize the probability of diagnosing intermittent valve dysfunction. Fluoroscopy, a technique that is often neglected, can be useful in this context.3
It is also important to bear in mind the increased risk associated with a fourth cardiac surgery, particularly for replacement of a mechanical prosthesis, as in the case presented.20,21
The interest of this case lies in the fact that the patient simultaneously presented dysfunction of aortic and mitral mechanical prostheses, due to two different and relatively uncommon mechanisms.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to thank Dr. Raquel Gouveia and Dr. Manuel Canada of the Cardiology Department of Hospital de Santa Cruz (Centro Hospitalar Lisboa Ocidental) for their invaluable contribution in the echocardiographic investigation of this case.
Please cite this article as: Cardoso G, Trabulo M, Andrade MJ, et al. Um caso raro de dois mecanismos de disfunção protésica no mesmo doente. Rev Port Cardiol. 2013;32:1037–1041.