Pacemaker lead endocarditis is an uncommon complication after pacemaker implantation, but is associated with high rates of morbidity and mortality. The authors describe the case of a 68-year-old woman with a double-chamber pacemaker since 2007, admitted to an internal medicine department for spondylodiscitis and Staphylococcus aureus bacteremia. During hospitalization, she had an episode of syncope; the 12-lead electrocardiogram showed pacemaker malfunction with ventricular undersensing and loss of capture. A transesophageal echocardiogram showed images compatible with vegetations on the pacemaker leads. After antimicrobial therapy, the patient developed acute renal failure with subsequent multiple organ failure and death. A high index of clinical suspicion is required for early diagnosis and appropriate treatment of cardiac device-related infective endocarditis.
A endocardite de sondas de pacemaker ocorre raramente após implantação de pacemaker, mas é uma complicação com altas taxas de morbilidade e mortalidade. Os autores descrevem o caso de uma doente de 68 anos, portadora de pacemaker de dupla câmara desde 2007, internada num serviço de medicina interna por espondilodiscite e bacteriemia por Staphylococcus aureus. Durante o internamento, apresentou um episódio de síncope; o eletrocardiograma de 12 derivações apresentava sinais de disfunção de pacemaker, com undersensing e falhas de captura ventricular. Foi submetida a ecocardiogramas transtorácico e transesofágico, que evidenciaram imagens compatíveis com vegetações nas sondas do pacemaker. Após antibioterapia dirigida, desenvolveu quadro de insuficiência renal aguda e posterior falência multiorgânica, acabando por falecer. Este caso alerta para a necessidade um alto índice de suspeição para se efetuar o diagnóstico precoce e o tratamento mais célere desta entidade.
The development of cardiac implantable electronic devices (CIEDs) has considerably improved patient survival and quality of life.1 The use of these devices has increased in recent years, as their functions and indications have widened.1–4 This increase, together with the growing number of older patients with more comorbidities,1 has led to a rise in the rates of device infection.5–8 Pacemaker lead endocarditis is an uncommon but serious infectious complication9 with an incidence reported in the literature between 0.13 and 19.9%.9,10
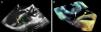
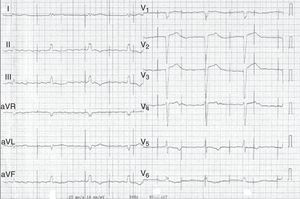
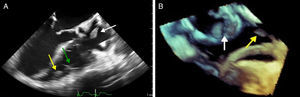
Case reportWe present the case of a 68-year-old woman with hypertension, type 2 diabetes and dyslipidemia, admitted to an internal medicine department for spondylodiscitis in L2/L3. Relevant history included radical right mastectomy for breast cancer 32 years previously, followed by radiotherapy (with chronic pleurisy and two reconstructive surgeries, the second in 2013, for graft necrosis and radiogenic ulcer of the anterior wall of the right hemithorax); myocardial infarction in 2001 with single-vessel disease treated by angioplasty of the right coronary artery and moderate to severe left ventricular systolic dysfunction (New York Heart Association class I); and implantation of a double-chamber permanent pacemaker (Identity DC, St. Jude Medical) via the right cephalic vein, programmed in DDD mode and with >99% ventricular pacing, following which sinus rhythm was maintained. In the two months before admission she had been admitted multiple times to the emergency department for left low back pain, which had worsened in the previous week. She presented inability to walk, fever (38.2°C) and elevated systemic inflammatory markers (C-reactive protein 299.4 mg/l and white cell count 21400/μl, with 88.9% neutrophils). Computed tomography of the lumbar spine confirmed the diagnosis of spondylodiscitis. Blood cultures were positive for methicillin-resistant Staphylococcus aureus and antibiotic therapy was begun with vancomycin and ceftazidime. On the 11th day of hospitalization she had an episode of syncope, attributed to pacemaker malfunction (Figure 1). Ventricular lead impedance remained within normal values (397 Ω), but ventricular undersensing and loss of ventricular capture were observed, the ventricular lead threshold having been exceeded; the sensing was corrected and ventricular capture assured by increasing the energy of the ventricular impulse. Transthoracic echocardiography (TTE) showed akinesia of the apex and the inferior and septal walls, with severe left ventricular systolic dysfunction and right ventricular systolic dysfunction, as well as isoechoic masses on the pacemaker leads. Transesophageal echocardiography (TEE) revealed mobile masses on the atrial lead, the largest 20 mm×7 mm in size, compatible with vegetations, and masses attached to the ventricular lead, one by the entrance to the inferior vena cava measuring 13 mm×8 mm and the other on the tip of the lead by the apex of the right ventricle, measuring 14 mm×6 mm (Figure 2A and B; Videos 1-4). There were no images suggestive of valve vegetations.
(A) Two-dimensional transesophageal echocardiogram in 3-chamber view (120°) showing vegetations on the atrial lead (white arrow) and on the tip (yellow arrow) of the ventricular lead (green arrow); (B) transesophageal echocardiogram in three-dimensional zoom mode and bicaval view (120°) showing multiple vegetations on the atrial lead (white arrow) and the ventricular lead (yellow arrow).
In view of the number of vegetations, following discussion with the reference center it was decided that percutaneous device extraction was not feasible and the case was referred for cardiac surgery. Cardiac catheterization performed for preoperative assessment showed a long chronic occlusion of the mid-proximal third of the right coronary artery. While awaiting surgery, the patient developed acute renal failure that did not improve after therapeutic adjustment, and she died from multiple organ failure.
DiscussionThis case describes pacemaker lead endocarditis probably originating from the patient's radiogenic ulcer of the anterior wall of the right hemithorax.
Endocarditis accounts for around 10% of all pacemaker-related infections.1,11 Although uncommon, it is associated with high morbidity and mortality.9,12
Various studies have identified risk factors for CIED-associated infections.1,2,4,13–16 Among patient-related factors, renal failure (defined as glomerular filtration rate <60 ml/min) has the strongest relationship; others include diabetes, heart failure, use of oral anticoagulants and prolonged use of corticoids.
Procedure-related factors are also important, particularly fever in the 24 hours before the procedure, use of temporary pacing, and early reintervention.17 Operator experience can affect the risk of infection.
Repeated surgical intervention on pacemaker systems is the main isolated risk factor for pacemaker lead endocarditis,12,13 although a large study (the REPLACE registry)18 showed that rates of severe infection are low (0.8%) in these procedures.
Prompt diagnosis is essential. Clinically, pacemaker lead endocarditis is characterized by persistent bacteremia originating from a focus of infection located on the lead or tricuspid valve or in an area of the endocardium in contact with the leads. Fever, signs of pocket inflammation and systemic manifestations such as respiratory or rheumatic disturbances are the most frequent symptoms, but endocarditis should be considered in all patients with pacemakers and infection that is unexplained or resistant to initial treatment.9,13 Pacemaker malfunction, as seen in the case presented, is a rare manifestation of this entity. Laboratory tests show elevated parameters of systemic inflammation, although these are non-specific. Presentation can be acute (at up to six weeks after pacemaker implantation, which may facilitate diagnosis) or chronic (more than six weeks after implantation).
It used to be thought that such infections were mostly caused by S. aureus in the acute phase and S. epidermis in the chronic phase,9,13 but according to the most recent guidelines, the two pathogens do not differ in terms of time after implantation.19
TEE is the imaging modality of choice when there is a high clinical suspicion of pacemaker lead endocarditis, since it has higher sensitivity (around 90%) for detecting lead vegetations than TTE, with which it is harder to visualize the right atrium, superior vena cava and vegetations.1,2,9,12,13 Three-dimensional (3D) echocardiography can show the relations between the various anatomical structures and is better at revealing intracavitary leads, since it is less prone to the artefacts associated with TEE and TTE. Precise localization of vegetations in patients with CIEDs helps with therapeutic decision-making, although the absence of vegetations on 3D echocardiography does not completely exclude endocarditis.20 Several prognostic factors are better assessed by TTE, such as the presence of pericardial effusion, ventricular dysfunction, and pulmonary artery systolic pressure.1
In the case described, the clinical manifestations, together with S. aureus bacteremia and pacemaker malfunction, raised suspicion of the diagnosis, confirmed by TEE, which showed various vegetations on the atrial and ventricular pacemaker leads. The rise in ventricular pacing threshold may have been due to changes in the lead/myocardium interface caused by the presence of vegetations, particularly that located on the tip of the ventricular lead.
With regard to treatment, removal is not necessary so long as the infection is superficial and the device is not involved; a 7-10 day cycle of antibiotic therapy is acceptable.1 Complete removal is recommended when there is established infection of the device itself, irrespective of the site of infection, since recurrence is common in cases treated conservatively,21–24 although some cases have been reported in which conservative treatment was sufficient.24
There is some debate concerning the approach to adopt for device removal. Technological advances in recent years have made percutaneous removal the method of choice for lead extraction. However, this approach should only be used in centers with extensive experience in the procedure and with cardiothoracic surgery facilities available, since there is a risk of complications such as cardiac tamponade, hemothorax and pulmonary embolism, although these are estimated to occur in less than 2% of cases.2 Thoracotomy to remove a CIED is limited to cases in which percutaneous removal is not possible or when there are vegetations larger than 2 cm on the leads,1 due to the risk of pulmonary embolism using a percutaneous approach. In the case presented, after presentation and discussion with the reference center, percutaneous extraction of the pacemaker and leads was not considered feasible and it was decided to proceed with surgical extraction, which was not performed due to the onset of multiple organ failure.
Antibiotics are considered an adjuvant therapy in infections of this type, as complete removal of the device should not be postponed. Since Staphylococcus spp. are responsible in most cases (it is estimated that around half of patients with S. aureus bacteremia and a CIED will develop device infection1), empirical antibiotic coverage with vancomycin is recommended until the results of microbiological tests are available due to the possibility of methicillin resistance.1,2,4 There is insufficient evidence to determine the ideal duration of antibiotic therapy. Factors influencing the decision include the extent of infection, the micro-organism involved, the presence and duration of bacteremia (if present, it is advisable to prolong intravenous antibiotic therapy for two weeks after device removal), and associated complications, such as valvular involvement, venous thrombosis or osteomyelitis; in such cases, therapy should be prolonged for 4-6 weeks.1
When necessary, a new CIED should be implanted on the contralateral side. In the case presented, the patient was in sinus rhythm at the time of initial implantation and throughout follow-up. Following the episode of syncope, the ECG showed atrial flutter and pacemaker malfunction. Since the patient died so soon after diagnosis of endocarditis, we cannot know how the patient's rhythm would have evolved, but considering the change in electrical conduction, another pacemaker would probably have been implanted if the planned treatment had been performed.
The ideal timing to implant a new device has not been established, but it is generally accepted that it should not take place before blood cultures are negative (in patients with previously positive cultures) and the pocket infection is under control. Some authors4 suggest that there are three approaches to device removal: urgent (immediate), early (after a short period of antibiotic therapy), and postponed (after 4-8 weeks of antibiotic therapy to treat the infection and reduce the size of any vegetations).
Mortality from this type of infection varies considerably, and there are no precise estimates due to the heterogeneity of studies, but it tends to be higher in patients treated conservatively.11,21–27
ConclusionAlthough pacemaker lead endocarditis is an uncommon complication, fever and/or bacteremia in a patient with a pacemaker or other CIED should raise suspicion and thus permit an early diagnosis. Echocardiography, preferably transesophageal, is essential for a diagnosis of endocarditis, while 3D echocardiography has additional value for accurate localization of vegetations on CIEDs, which can be decisive in therapeutic decision-making. The clinical presentation in this case alerted us to the possibility of this rare diagnosis. Treatment should consist of percutaneous or surgical removal of the pacemaker and leads and appropriate antibiotic therapy.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Guedes H, Pereira A, Pontes dos Santos R, Marques L, Moreno N, Castro A, et al. Um caso complexo de endocardite de sondas de pacemaker. Rev Port Cardiol. 2017;36:775.e1–775.e5.