The evidence for beta-blocker use in patients after acute coronary syndrome (ACS), particularly in those with left ventricular (LV) dysfunction, dates from the late 1990s. We aimed to assess the role of beta-blockers in a contemporary population of patients with ACS.
MethodsPropensity-score matching (1:2) was performed for the use of beta-blockers in a population of consecutive patients admitted to our department with ACS. After matching, 1520 patients were analyzed. Cox regression analysis was used to assess the impact of beta-blocker use on the primary outcome (one-year all-cause mortality).
ResultsPatients who did not receive beta-blockers were less aggressively treated with other pharmacological and invasive interventions and had higher one-year mortality (20.3% vs. 7.5%). Beta-blocker use was an independent predictor of mortality, with a significant relative risk reduction of 56%. The other independent predictors were age, diabetes, LV dysfunction, heart rate, systolic blood pressure and creatinine on admission. The impact of beta-blockers was significant for all classes of LV function, including patients with normal or mildly reduced ejection fraction.
ConclusionsIn a contemporary ACS population, we confirmed the benefits of beta-blocker use after ACS, including in patients with normal or mildly to moderately impaired LV function.
A evidência para a utilização dos bloqueadores-beta em doentes após síndrome coronária aguda (SCA), particularmente em doentes com disfunção ventricular esquerda (VE) é do final dos anos 90. Foi nosso objetivo analisar o papel dos bloqueadores-beta numa população contemporânea de doentes com SCA.
MétodosFoi realizado emparelhamento de score de propensão (1:2) para a utilização de bloqueadores-beta numa população consecutiva de doentes admitidos no nosso serviço por SCA. Após emparelhamento, foram analisados 1520 doentes. Foi utilizada a análise de regressão de Cox para avaliar o impacto da utilização dos bloqueadores-beta na mortalidade de todas as causas a um ano de seguimento.
ResultadosOs doentes que não receberam bloqueadores-beta foram tratados de forma menos agressiva com outras intervenções farmacológicas e invasivas e tiveram maior mortalidade a um ano (20,3% versus 7,5%). A utilização de bloqueadores-beta foi preditor independente de mortalidade com redução significativa do risco relativo de 56%. Os restantes preditores independentes foram a idade, diabetes, disfunção VE, frequência cardíaca, pressão arterial sistólica e creatinina na admissão. O impacto dos bloqueadores-beta foi significativo em todas as classes de função VE, incluindo doentes com fração de ejeção normal ou ligeiramente reduzida.
ConclusõesNuma população contemporânea de doentes com SCA, confirmámos os benefícios da terapêutica bloqueadora-beta após SCA, incluindo em doentes com função VE normal ou com compromisso ligeiro a moderado.
angiotensin-converting enzyme inhibitor
acute coronary syndrome
angiotensin receptor blocker
atrioventricular
coronary artery bypass grafting
confidence interval
hazard ratio
left ventricular
left ventricular ejection fraction
non-ST-elevation acute coronary syndrome
percutaneous coronary intervention
ST-elevation myocardial infarction
transient ischemic attack
The role of beta-blockers is clearly established for secondary prevention in all current guidelines for the management of patients with acute coronary syndromes (ACS), particularly in the presence of left ventricular (LV) dysfunction.1,2 Beta-blockers improve outcome in coronary artery disease by reducing oxygen demand and hence ischemia, attenuating ventricular remodeling, and preventing lethal arrhythmias and sudden death. However, the majority of studies that support these effects were performed between the 1970s and the 1990s, before major advances in therapy such as the introduction of reperfusion therapy and modern pharmacotherapy.3–10 Beta-blockers have not been investigated in contemporary trials, although it is not unreasonable to extrapolate their benefits to this setting.
Each successive intervention that reduces risk reduces the absolute benefit of further interventions. Dramatic decreases in mortality were observed in the early 21st century in several ACS registries.11–17 As the baseline risk of a population decreases due to a new intervention, the incremental benefit of previous interventions needs to be re-evaluated. Currently, most ACS patients are discharged without significant residual ischemia, and the risk of lethal arrhythmias is extremely low because remodeling and even quite large reductions in LV ejection fraction (LVEF) with heart failure are not a significant problem with contemporary treatment. A more recent study in stable CAD patients challenged the use of beta-blockers, further reinforcing the need for reassessment of their benefit in a contemporary cohort of patients with ACS.18 Also, current guidelines do not provide a definite recommendation for the use of beta-blockers in patients with ACS and normal or mildly reduced LVEF (≥40%).1,2
It was our objective to assess the role of beta-blockers in a contemporary population of patients with ACS.
MethodsAll consecutive adult patients (aged ≥18 years) admitted to our intensive care unit with ACS were prospectively included in our center's ACS registry between January 2005 and November 2015 and were included in the present study. Criteria for inclusion were a history of chest pain at rest or other symptoms suggestive of ACS (the most recent episode within 48 hours of admission) with or without new or presumed new significant ST-segment or T-wave changes, new left bundle branch block and elevated biomarkers of myocardial damage with a rise and/or fall in levels. Myocardial infarction (MI) was defined according to the universal definition of type 1 MI.19 A diagnosis of ST-elevation MI (STEMI) was made in the presence of persistent (>30 min) ST-segment elevation. All other cases were considered non-ST elevation ACS (NSTE-ACS).
Data were collected in a dedicated electronic database, and included demographic, clinical and patient-management related characteristics, as well as clinical outcome. Hypertension, diabetes and hyperlipidemia were defined as either previously known or on specific therapy. If patients had smoked during the previous six months they were classified as smokers and were self-reported. Decisions on patient management strategy, including referral for coronary angiography and mode of myocardial revascularization, if any, were at the discretion of the attending physician. LVEF was obtained before discharge by echocardiography.
Follow-up was obtained for every patient who survived to discharge by reviewing medical records and/or by telephone interview with the patient or family members. The primary endpoint was all-cause mortality at one-year follow-up. In-hospital secondary endpoints were cardiac arrest, complete atrioventricular (AV) block, mechanical complications, stroke/transient ischemic attack (TIA), LV function, major bleeding (according to the Global Use of Strategies to Open Occluded Coronary Arteries [GUSTO] criteria), and all-cause mortality during the index hospitalization and at 30-day follow-up.20
All procedures contributing to this work comply with the ethical standards of the 1975 Helsinki Declaration. This research does not involve human or animal experimentation.
Statistical analysisContinuous variables are reported as means and standard deviation and were compared with the Student's t test. Normality tests were performed with the Kolmogorov-Smirnov test and homogeneity of variance was tested with Levene's test. Continuous variables without normal distribution are reported as medians and interquartile range and were compared with the Mann-Whitney test. Categorical variables are reported as percentages and differences between groups were tested with the chi-square test or Fischer's exact test, as appropriate.
Propensity-score matching was performed to adjust for the non-randomized assignment of patients to treatment and for the potential bias due to differences between the study groups. Propensity scoring helps deal with bias arising from confounding by indication, enabling a more accurate comparison of outcomes between participants with similar propensity scores based on the set of available information about that individual. A propensity score was calculated for each participant by logistic regression as the likelihood of being assigned to treatment with a beta-blocker. The model included all variables that in the logistic analysis had a p-value <0.05. A 1:2 matched analysis was then performed on the basis of each patient's estimated propensity score. Baseline and in-hospital characteristics were then compared.
Estimates of event-free survival at one-year follow-up were calculated by the Kaplan-Meier method and survival curves were compared with the log-rank test. A Cox proportional-hazards regression model was used with the p level for entry into and removal from the model set at 0.05 and 0.10, respectively (forward stepwise method with likelihood ratio statistics), to select variables that were independent predictors of all-cause mortality. Estimates of the association between predictors and endpoints are presented as hazard ratio (HR) and 95% confidence interval (CI).
For all-cause mortality, subgroup analysis was performed according to gender, age (<70 years and ≥70 years), presence or absence of diabetes, STEMI vs. NSTE-ACS, and LVEF (<35%, 35-50% and ≥50%). This categorization of LV function was used since it was the one available in our database; specific LVEF values were not available for all patients. Analysis of the interaction between beta-blocker therapy and each subgroup was performed using Cox regression models.
IBM SPSS statistical software (version 19.0.0.2) was used for all statistical analyses. All statistical tests were two-sided with a value of 0.05 for statistical significance.
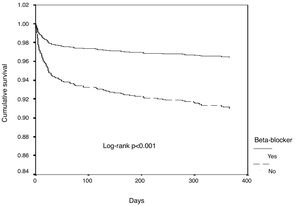
ResultsOf a total of 3536 patients included in our registry during the study period, 83.4% received beta-blocker treatment. After propensity-score matching, 1520 patients were selected for analysis. The population's characteristics were well balanced between groups (Table 1); the absolute standardized difference of less than 10% for all variables indicates adequate matching. Patients’ mean age was 66±13 years, most were male (68%), and the most frequent diagnosis was STEMI (61.6% of patients). In patients who died very early in the course of admission, before a complete echocardiogram was performed (1.3%), an admission echocardiogram or information from ventriculography was used to assess LV function. Follow-up information was obtained in 99.8% of patients. In-hospital, 30-day and one-year mortality were 7.5%, 8.7% and 11.8%, respectively. In this matched cohort, patients who did not receive beta-blockers were also less likely to receive antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs), statins and revascularization (Table 2). Also, all-cause mortality, mechanical complications and cardiac arrest were significantly more frequent in patients not treated with beta-blockers. The rate of complete AV block and stroke/TIA was similar in both groups.
Baseline clinical characteristics after propensity-score matching.
| No beta-blocker therapy (n=507) | Beta-blocker therapy (n=1013) | p | |
|---|---|---|---|
| Age, years | 66 (13) | 66 (13) | 0.914 |
| Male gender, % | 68.2 | 68.7 | 0.901 |
| Risk factors, % | |||
| Hypertension | 61.9 | 62.3 | 0.937 |
| Hyperlipidemia | 47.9 | 46.5 | 0.636 |
| Diabetes | 25.0 | 27.8 | 0.274 |
| Smoking | 33.9 | 33.5 | 0.903 |
| Previous history, % | |||
| MI | 13.2 | 15.2 | 0.337 |
| PCI | 8.9 | 9.0 | 1.000 |
| CABG | 3.4 | 4.9 | 0.199 |
| Previous revascularization | 11.8 | 12.4 | 0.798 |
| Initial presentation | |||
| Heart rate, bpm | 75 (21) | 75 (18) | 0.783 |
| SBP, mmHg | 131 (26) | 130 (27) | 0.651 |
| Killip class >I, % | 15.8 | 14.6 | 0.599 |
| Killip class IV, % | 3.4 | 2.1 | 0.132 |
| STEMI, % | 58.2 | 63.4 | 0.057 |
| Creatinine, mg/dl | 1.1 (0.8) | 1.0 (0.6) | 0.05 |
bpm: beats per minute; CABG: coronary artery bypass grafting; MI: myocardial infarction; PCI: percutaneous coronary intervention; SBP: systolic blood pressure; STEMI: ST-elevation myocardial infarction.
Treatment and outcome.
| No beta-blocker therapy (n=507) | Beta-blocker therapy (n=1013) | p | |
|---|---|---|---|
| Aspirin, % | 88.2 | 98.8 | <0.001 |
| DAPT, % | 81.1 | 94.6 | <0.001 |
| ACEI/ARB, % | 66.7 | 91.0 | <0.001 |
| Statin, % | 78.3 | 95.2 | <0.001 |
| PCI, % | 68.2 | 80.6 | <0.001 |
| CABG, % | 1.2 | 3.1 | 0.038 |
| Revascularizationa, % | 69.4 | 83.6 | <0.001 |
| Cardiac arrest, % | 11.6 | 7.5 | 0.010 |
| Complete AV block, % | 2.8 | 2.7 | 1.000 |
| LVEF, % | 0.970 | ||
| >50 | 65.7 | 66.1 | |
| 35-50 | 23.7 | 23.1 | |
| <35 | 10.7 | 10.8 | |
| Mechanical complications, % | 13.2 | 5.6 | <0.001 |
| Stroke/TIA, % | 1.6 | 0.7 | 0.099 |
| Major bleeding, % | 4.7 | 3.0 | 0.107 |
| In-hospital mortality, % | 15.0 | 3.8 | <0.001 |
| 30-day mortality, % | 16.2 | 4.9 | <0.001 |
| One-year mortality, % | 20.3 | 7.5 | <0.001 |
Revascularization includes PCI and/or CABG.
ACEI/ARB: angiotensin converting enzyme inhibitor/angiotensin receptor blocker; AV: atrioventricular; CABG: coronary artery bypass grafting; DAPT: double antiplatelet therapy; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; TIA: transient ischemic attack.
In univariate Cox regression analysis, the use of beta-blockers was associated with better outcome in the overall population (HR 0.34, 95% CI 0.25-0.45, p<0.001). In the multiple Cox proportional-hazards regression model, the use of beta-blockers remained an independent predictor of better outcome, together with the use of ACEIs/ARBs (Table 3 and Figure 1). Age, heart rate, systolic blood pressure, diabetes, LVEF, ACEI/ARB use, renal function and mechanical complications were the other independent predictors of all-cause mortality in patients with ACS.
Univariate and multivariate Cox regression analysis.
| HR (95% CI) | p | HR (95% CI) | p | |
|---|---|---|---|---|
| Age (per 10-year increase) | 1.82 (1.59-2.08) | <0.001 | 2.03 (1.72-2.39) | <0.001 |
| Male gender | 0.59 (0.44-0.79) | <0.001 | ||
| Diabetes | 2.07 (1.54-2.79) | <0.001 | 1.99 (1.44-2.76) | <0.001 |
| STEMI | 1.31 (0.96-1.79) | 0.088 | - | - |
| Heart rate (per 10-bpm increase) | 1.33 (1.26-1.42) | <0.001 | 1.17 (1.09-1.25) | <0.001 |
| SBP (per 10-mmHg increase) | 0.87 (0.82-0.92) | <0.001 | 0.92 (0.86-0.98 | 0.017 |
| Killip class >1 | 3.82 (2.82-5.18) | <0.001 | - | - |
| LVEF (decrease) | 2.76 (2.31-3.29) | <0.001 | 1.84 (1.49-2.28) | <0.001 |
| Creatinine | 1.34 (1.24-1.45) | <0.001 | 1.27 (1.13-1.42) | <0.001 |
| DAPT | 0.51 (0.34-0.75) | 0.001 | - | - |
| ACEI/ARB | 0.32 (0.24-0.44) | <0.001 | 0.52 (0.35-0.76) | 0.001 |
| Beta-blocker | 0.34 (0.25-0.45) | <0.001 | 0.44 (0.31-0.62) | <0.001 |
| Statin | 0.38 (0.27-0.55) | <0.001 | - | - |
| Revascularization | 0.49 (0.37-0.67) | <0.001 | - | - |
| Mechanical complication | 12.32 (9.12-16.64) | <0.001 | 4.83 (3.30-7.08) | <0.001 |
ACEI/ARB: angiotensin converting enzyme inhibitor/angiotensin receptor blocker; bpm: beats per minute; CI: confidence interval; DAPT: double antiplatelet therapy; HR: hazard ratio; LVEF: left ventricular ejection fraction; SBP: systolic blood pressure; STEMI: ST-elevation myocardial infarction.
In the subgroup analysis (Figure 2), all subgroups had a better outcome with the use of beta-blockers, including patients with normal or mildly to moderately reduced LVEF, in both univariate and multivariate analysis.
DiscussionSeveral trials and meta-analyses have demonstrated that beta-blockers reduce mortality and reinfarction by 20-25% in those who have recovered from MI.3–10 Over 52000 patients were randomized in clinical trials studying beta-blockers in acute MI, covering a range of beta-blockers, and largely conducted in the pre-reperfusion era. The available data at that time suggested trends toward reductions in mortality, reinfarction and cardiac arrest, if used in patients without contraindications. A review of secondary prevention trials of beta-blocker therapy both in the acute phase and as secondary prevention showed an overall benefit, with a relative risk reduction of 19% in mortality, particularly for secondary prevention (23% relative risk reduction).10 The Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial definitively demonstrated the benefit of beta-blockers in patients with LV dysfunction (LVEF <40%) after MI with or without clinical signs of heart failure.9 However, since the 1980s, aspirin, P2Y12 inhibitors, thrombolysis followed by primary angioplasty, high-dose statins, enoxaparin, mineralocorticoid receptor antagonists, ACEIs, implantable cardioverter-defibrillators, and early revascularization for NSTE-ACS have all been introduced. These changes in management were followed by dramatic decreases in mortality in the early 21st century in several registries.11–17
COMMIT, a large trial performed in the reperfusion era, showed no difference in the rate of the composite endpoint of death, reinfarction, or cardiac arrest in the metoprolol group compared with the placebo group, but significant reductions occurred in reinfarction and episodes of ventricular fibrillation.21 A meta-analysis that included earlier studies and low-risk patients from the COMMIT trial showed reductions of 13% in all-cause mortality, 22% in reinfarction, and 15% in ventricular fibrillation or cardiac arrest.21 However, in order to achieve these benefits safely, it is important to avoid early administration of beta-blockers to patients with relative contraindications.
Another paper challenged the beneficial effect of beta-blockers after ACS.18 In an observational study with propensity-score matching, with a median follow-up of 44 months in stable outpatients with and without coronary artery disease, the use of beta-blockers was not associated with a lower risk of cardiovascular events (primary outcome: a composite of cardiovascular death, nonfatal MI or nonfatal stroke; secondary outcome: the primary outcome plus hospitalization for atherothrombotic events or revascularization; tertiary outcomes: all-cause mortality, cardiovascular mortality, nonfatal MI, nonfatal stroke, and hospitalization separately), including in the cohort with prior MI. However, in those with recent MI (≤1 year), beta-blocker use was associated with a lower incidence of the secondary outcome (odds ratio 0.77).
In view of these uncertainties and contradictions and the limited evidence, the purpose of our study was, based on a real-world contemporary population of patients from an ACS registry, to assess whether beta-blocker therapy is still beneficial, on top of all guideline-recommended therapies. We observed not only that the magnitude of benefit is highly significant, with a relative risk reduction in one-year all-cause mortality of 56%, but also that this benefit is the same for STEMI and NSTE-ACS patients, and most importantly is independent of LV function.
A recent study based on a UK registry showed that in survivors of hospitalization with MI who did not have heart failure or LV systolic dysfunction, the use of beta-blockers was not associated with a lower risk of death up to one year.22 This result is clearly different from ours, but their sample has different characteristics. Ours had a predominance of patients with STEMI, and Dondo et al.’s study only included patients who survived to discharge. Their main strength is that it has a very large sample of patients and also used propensity-score matching. However, the cutoff used for systolic dysfunction was LVEF <30%, and they therefore included patients with normal and mildly and moderately impaired systolic function in the same analysis. For this reason, we also performed a substudy of patients who survived to discharge. In those patients, a tendency for some benefit was found for beta-blocker use in patients with LVEF 35-50% (HR 0.46, 95% CI 0.20-1.06, p=0.069) and with LVEF >50% (HR 0.42, 95% CI 0.18-0.96, p=0.036). Surprisingly, no benefit was found, in terms of all-cause mortality at one-year follow-up, in patients with LVEF <35% (HR 0.25, 95% CI 0.05-1.26, p=0.09) (p=0.391 for the interaction). However, our study is clearly underpowered for this analysis, particularly in the group with severe LV dysfunction. Thus, although our results are only partially in agreement with the findings of Dondo et al., both studies can only be viewed as hypothesis-generating and the question should be addressed in larger studies, preferably randomized clinical trials. The different results in patients who survived to discharge highlight the importance of early implementation of beta-blockers, which appear to have a major impact early in the course of disease, independently of LV function.
In our population, more patients in the beta-blocker group underwent percutaneous coronary intervention (PCI) than in the group without beta-blockers (68.0% vs. 80.6%). This difference could be explained by the fact that STEMI was slightly more frequent in the beta-blocker group, and coronary anatomy in NSTE-ACS is more often unsuitable for PCI. Also, aspirin and double antiplatelet therapy were used much less in patients not taking beta-blockers, as were other drugs with significant impact on outcome, such as ACEIs and statins. This may be explained by the fact that in-hospital death was significantly more frequent and in some cases occurred very soon after admission (in some cases before PCI); the rate of major complications (mechanical complications, stroke/TIA and major bleeding) is another possible explanation for our findings, because these complications might have precluded the use of some drugs.
LimitationsThis was an observational and non-randomized study. However, propensity-score matching enables the resulting limitations to be mitigated to some extent. It was also a single-center study, and so its findings may not be applicable to other populations with different baseline characteristics, especially since our population had a predominance of STEMI cases, which is not the case in many other centers. Ours is a tertiary center with cases referred from many other hospitals in the region for urgent invasive treatment of ACS. Thus, some caution is advised when translating our findings to other cohorts.
From our registry, it was not possible to perform detailed analysis of the type and dosage of beta-blockers, compliance with treatment over time, or the reasons for not prescribing a beta-blocker. Concerning the latter, a study in the 1990s reported the presence of contraindications in 18% of MI survivors, the most common of which were bronchial asthma or chronic obstructive lung disease (7%), heart failure controlled only by >80 mg of furosemide daily or digoxin (7%), sinus bradycardia (4%), AV block (5%) and hypotension (5%).5 In that study, only 51% of MI survivors were discharged on a beta-blocker and although 82% had no contraindication, only 58% of this group received a beta-blocker. Moreover, most patients received a significantly lower dosage than those shown to be effective in reducing mortality. It is also important to remember that in the 1990s, most available beta-blockers were not cardioselective. A more recent study that analyzed the reasons recorded for not prescribing a beta-blocker (with a rate of beta-blocker prescription after MI in the overall population of 80%) showed that half of the small number of patients who did not receive beta-blockers had contraindications.11 The rate of beta-blocker prescription in this study was similar to ours and thus only about 10% of the overall population would be expected have contraindications, which probably would not have affected our results. Regarding compliance, two recent papers showed that overall long-term compliance with beta-blockers is high – after one year, the proportion of patients still on a beta-blocker had fallen by only 4%, with around 80% of MI patients still taking the drug.23,24 The same authors also report that if the medication is not prescribed at discharge it is highly unlikely to be prescribed later. For this reason, the lack of information on compliance probably does not significantly affect our results.
ConclusionsOur study, in a contemporary ACS population, confirms that the benefits of beta-blocker use after ACS on top of all other guideline-recommended treatments are still significant, particularly when prescribed early. This is true not only in patients with LV dysfunction but also in patients with normal or mild to moderate LV dysfunction.
FundingThis research did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.









 LVEF: left ventricular ejection fraction;
LVEF: left ventricular ejection fraction; 

