Acetylsalicylic acid (ASA) has both antithrombotic and anti-inflammatory effects, the latter being achieved when administered at higher doses (500 mg, 1000 mg per os). However, it is not known whether the anti-inflammatory effect decreases the antithrombotic potency of ASA. This experimental study intends to assess whether ASA maintains its antithrombotic effect when administered in an anti-inflammatory dose.
MethodsTwenty healthy volunteers were recruited and randomized into four groups. Each group ingested ASA 100 mg, 300 mg, 500 mg, and 1000 mg respectively. Their basal platelet function was measured using PFA-200 technology and reassessed one hour after ASA ingestion.
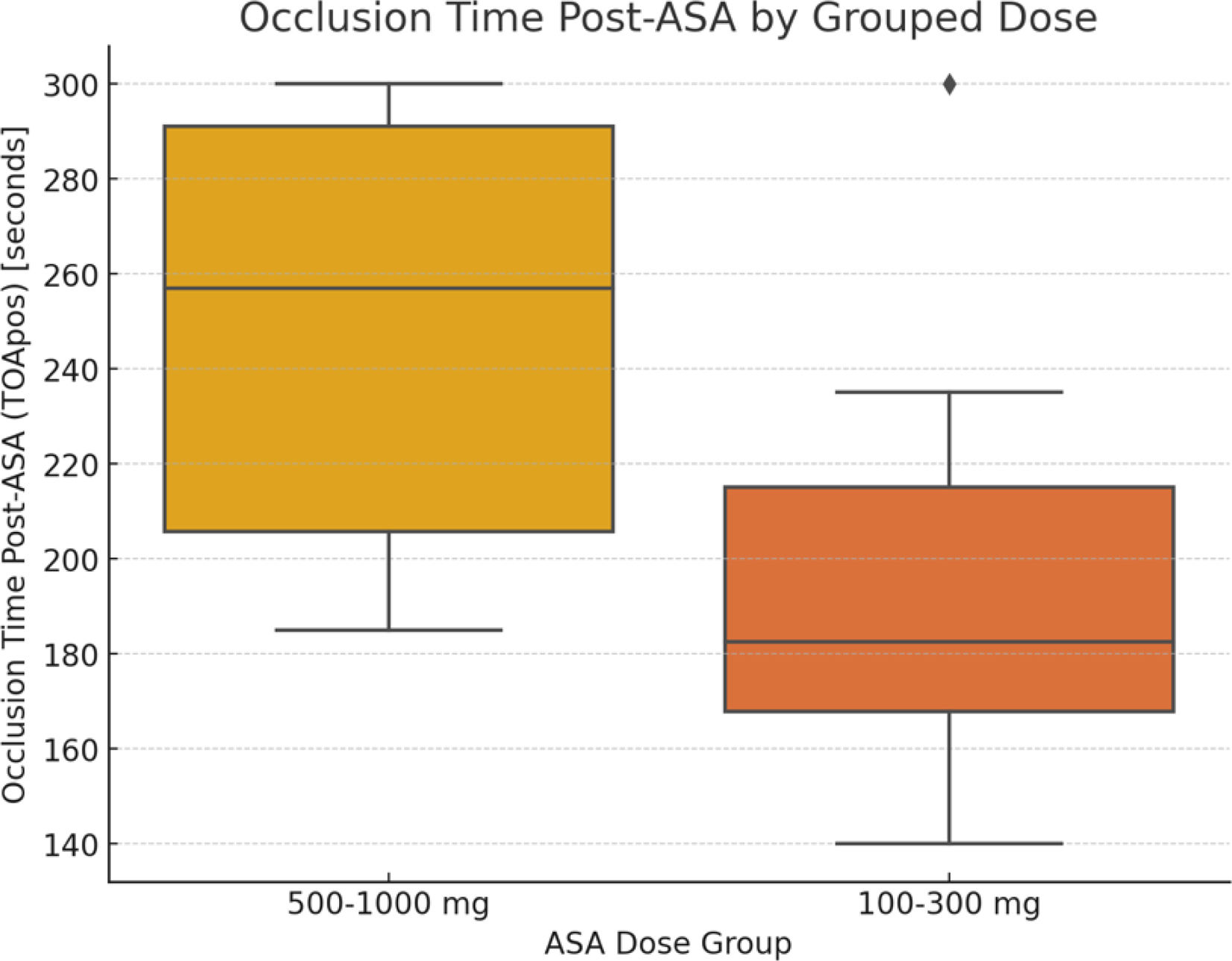
ResultsThe volunteers were all antiaggregated after ingestion of ASA, regardless of the dose taken. No statistical significance was found regarding age or gender. A subanalysis was performed, comparing the two groups that ingested lower dosages (100 mg and 300 mg) against the group that ingested higher dosages (500 mg and 1000 mg). The results were statistically significant, suggesting higher dosages may correspond to a higher antithrombotic effect.
ConclusionsAcetylsalicylic acid maintains its antithrombotic effect when administered in an anti-inflammatory dose. There is no clear association between the potency of antithrombotic effect and the ASA dose administered; however, our subanalysis suggests that higher dosages may correspond to a higher potency of antiaggregation.
O ácido acetilsalicílico (AAS) possui um duplo efeito enquanto agente antiagregante e anti-inflamatório, este último adquirido quando administrado em doses elevadas (500, 1000mg per os). Contudo, não é conhecido se, com o ganho do efeito anti-inflamatório (em doses altas), ocorre perda do efeito antiagregante. O presente estudo experimental pretende esclarecer se o AAS mantém o seu efeito antiagregante, quando administrados em doses anti-inflamatórias.
MétodosVinte voluntários saudáveis foram recrutados e randomizados em quatro grupos. Cada grupo ingeriu AAS 100mg, 300mg, 500mg e 1000mg, respetivamente. A função plaquetária basal dos voluntários foi mensurada, utilizando a tecnologia PFA-200, e reavaliada uma hora após a ingestão do AAS.
ResultadosOs voluntários ficaram todos antiagregados após a toma do AAS, independentemente da dose ingerida. Não se verificaram diferenças estatisticamente significativas entre géneros e idades. Uma subanálise foi realizada, comparando, por um lado, os dois grupos que ingeriram doses baixas (100 e 300mg) com os dois grupos que ingeriram doses altas (500mg e 1000mg). Os resultados foram estatisticamente significativos, sugerindo que uma dose mais elevada corresponde a uma maior potência de efeito antiagregante.
ConclusãoO AAS mantém o seu efeito antiagregante mesmo quando administrado em doses anti-inflamatórias. Não há uma clara associação entre a potência do efeito antiagregante e a dose de AAS administrada, todavia, a subanálise realizada parece sugerir que doses mais altas se traduzem numa maior potência de antiagregação.
We rely on the antithrombotic effect of acetylsalicylic acid (ASA) in a number of diseases, including in the setting of cardiovascular protection through the irreversible inhibition of platelet cyclooxygenase (COX)-1 and blockade of Thromboxane A2 (TXA2) production.1 Aside from this antithrombotic effect, other beneficial properties are known, such as its anti-inflammatory effect, when administered in higher doses (500 mg, 1000 mg) through the COX-2 pathway. However, it is not known whether the anti-inflammatory effect decreases the antithrombotic potency of ASA.
Many studies regarding the mechanisms of action of ASA have been conducted since the late 19th century. Some of these tried to answer the question of whether these two main properties were able to coexist or if they were mutually exclusive in vivo. Over the years, scientific findings have supported the anti-inflammatory properties of ASA even when administered in lower doses (50–100 mg), although through inhibition of immune mediated responses2 and not through the COX-2 pathway. However, the reverse scenario has not been confirmed to date: whether the antithrombotic effect remains even when ASA is administered at higher (anti-inflammatory) doses.
In modern clinical practice, the aforementioned gap in evidence may lead to an unnecessary use of two drugs (one antithrombotic and one anti-inflammatory) that could be replaced by ASA alone if this question were answered. This scenario presents frequently in cardiology departments: patients with post-infarct pericarditis or cases where the cardiologist is in doubt between non-ST segment elevation myocardial infarction vs. myopericarditis. In such cases, the use of ASA could ensure both antithrombotic and anti-inflammatory effects. Since the antithrombotic effect of ASA has not been scientifically proven for higher doses, currently ASA 100 mg is used as an antithrombotic agent and ibuprofen as an anti-inflammatory. This solution is, however, flawed, given that recent evidence has shown that the administration of nonsteroidal anti-inflammatory drugs (NSAIDs) could interact with ASA by binding reversibly to COX-1 and thus impair its antithrombotic effect.3 This experimental study sought to assess whether ASA maintains its antithrombotic effect when administered in anti-inflammatory dose (500 mg, 1000 mg), and thus the simultaneous need for NSAIDs can be avoided in the clinical settings exemplified above.
MethodsTwenty healthy volunteers were recruited among our hospital staff (nurses, interns, residents, senior doctors, cardio-pneumology technicians and medical assistant professionals). All volunteers signed an informed consent form and approval from our clinical investigation and ethics committee was obtained. The volunteers were then checked for exclusion criteria: having a medical indication for ASA; having taken ASA in the eight days prior to the experiment; having a known allergy to ASA; intake of chocolate, garlic, ginger, blueberries, ginkgo biloba in the 24 hours prior to the experiment; intake of NSAIDs, abacavir, abatacept, abciximab, abiraterone, acamprosate, acarbose, acebutolol, aceclofenac, acemetacin, acenocumarol in the 48 hours prior to the experiment; body mass index supra or infra-normal and thrombocytopenia <150000 platelets/μL. The volunteers had their platelet function assessed in a qualitative manner, using PFA-200 Innovance technology, at the beginning of the experiment.
The volunteers were then randomized into four groups, each with five participants. Participants from groups 1, 2, 3 and 4 ingested ASA 100 mg, 300 mg, 500 mg and 1000 mg respectively, in a blind experiment. One hour after ingestion, the volunteers’ platelet function was reassessed. PFA-200 evaluates platelet function through platelet occlusion time (OT), which is measured in milliseconds (ms). Normal platelet function translates into OT of 82–150 ms. If the OT is >150 ms, the patient is antiaggregated.
Statistical analysisDescriptive statistics were used to summarize the demographic data, including age and sex distribution among the volunteers. Continuous variables, such as OT, were expressed as mean±standard deviation and median with interquartile range (IQR), as appropriate. To assess the effect of ASA on OT, a paired t-test was used to compare the baseline OT with the post-ASA OT across the entire sample. A one-way analysis of variance (ANOVA) was conducted to evaluate differences in post-ASA OT among the four different ASA dose groups (100 mg, 300 mg, 500 mg, and 1000 mg). Post-hoc analysis using the Tukey HSD test was performed to identify pairwise differences between dose groups. Additionally, the lower dose groups (100 mg and 300 mg) were combined and compared to the higher dose groups (500 mg and 1000 mg) using an independent t-test to further explore dose-dependent effects on OT. These groups consolidations were deemed necessary to improve sample size and thus enable the improvement of the statistical power for the analysis of broader dose-dependent trends. Also, from the clinical perspective, the distinction between “low-dose” (typically 75–300 mg) and “high-dose” (500–1000 mg) ASA reflects practical dosing thresholds in clinical practice, which is in keeping with the main objective of this study. Effect size was calculated using Cohen's d to quantify the magnitude of the difference between baseline and post-ASA OTs. To evaluate the potential influence of sex and age (<40 years old or >40 years old) on post-ASA OT, a two-way ANOVA was conducted. Independent t-tests were used to directly compare the mean post-ASA OT between males and females. All statistical tests were two-tailed, and a p<0.05 was considered statistically significant. Data analyses were performed using SPSS (IBM SPSS statistics, Version 29.0).
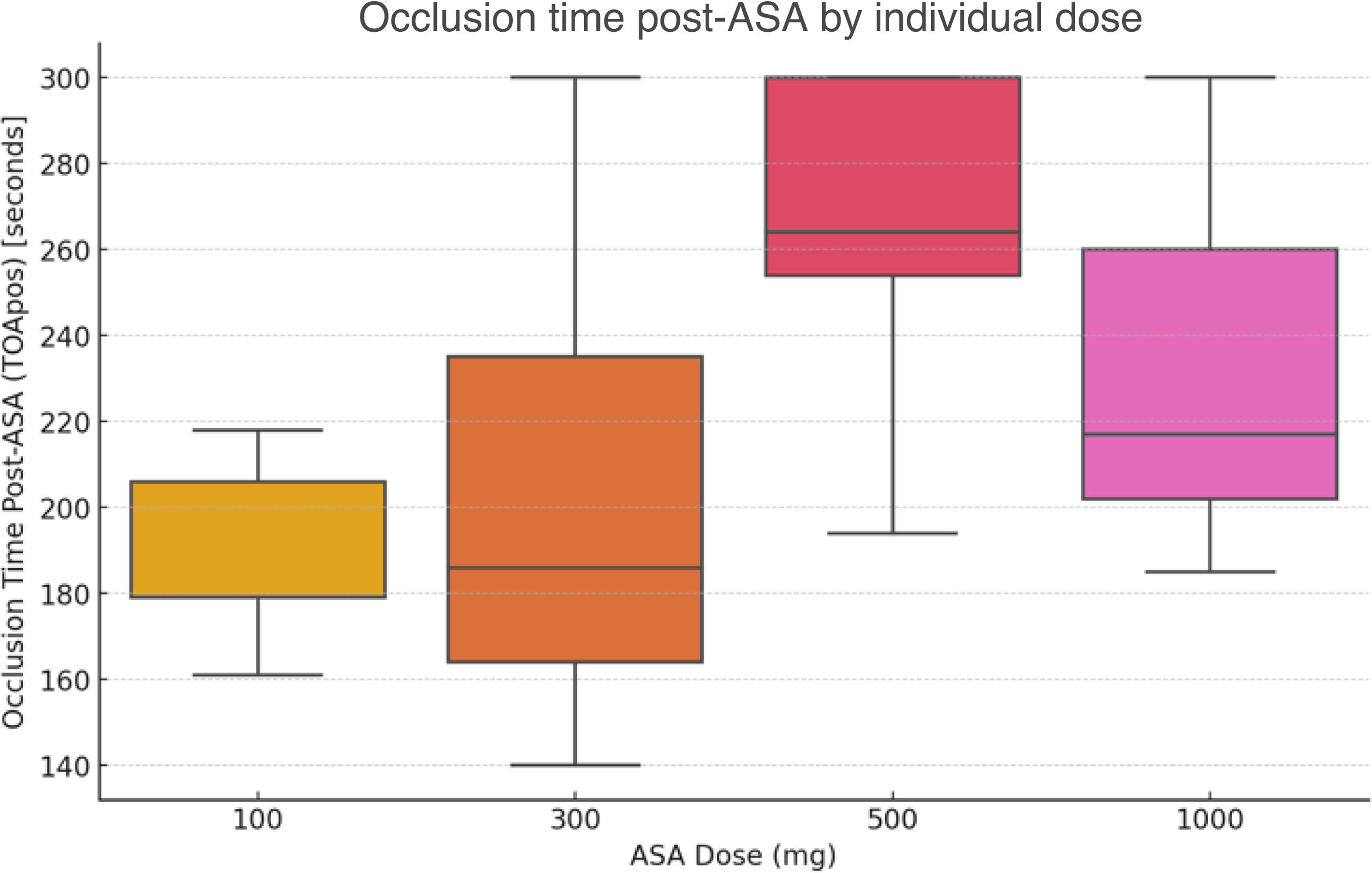
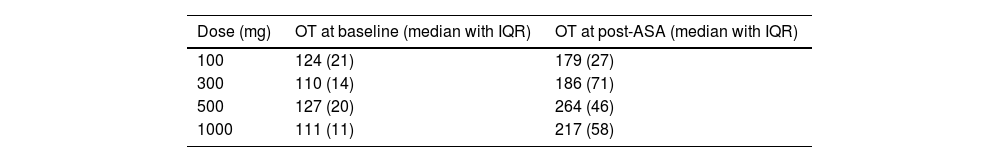
ResultsVolunteers were aged between 26 and 53 years old, with a mean age of 39.3 years; 80% were female (n=16). Prior to ASA administration, 19 out of 20 volunteers had OT within the reference range (Figure 1). In all four groups, volunteers reached an OT >150 ms (Figure 2) with a global mean OT of 222.2, median 211.5 (IQR 77.5 ms). OT according to ASA dose at baseline and after ASA intake are summarized in Table 1.
The effect of ASA on OTs was analyzed across different dose levels. When comparing the baseline OT with the post-ASA OT for the entire sample, there was a significant increase from a mean of 120.7±20.5 ms at baseline to 222.2±51.5 ms post-ASA (p<0.0001). When comparing the mean OT across individual dose groups (100 mg, 300 mg, 500 mg, and 1000 mg), the results of a one-way ANOVA did not show statistically significant differences (p=0.103).
To further explore the potential effect of aspirin dosage, the lower dose groups (100 mg and 300 mg) were combined and compared against the higher dose groups (500 mg and 1000 mg). The mean OT for the combined lower dose group was 196.8±44.2 ms, while the mean OT for the combined higher dose group was 247.6±45.4 ms (p=0.023). The effect size, measured by Cohen's d, for the difference between baseline OT and post-ASA OT is 2.59, suggesting a large effect size from ASA on OT.
While males have a slightly higher mean OT post-ASA compared to females, this difference is not statistically significant in this sample (females 218.5±52.3 ms vs. males 237.0±52.7 ms, p=0.535). The ANOVA analysis shows that sex (p=0.535) and age (p=0.779) do not have a statistically significant effect on OTs post-ASA.
DiscussionAcetylsalicylic acid is a potent antithrombotic agent which has been studied for the past 50 years and has fulfilled a major role in cardiovascular diseases, particularly in the setting of coronary artery disease. Its mechanisms of action have been extensively studied since the late 1960s and it is recognized that ASA acts through irreversible inhibition of both COX enzymes.4 ASA achieves maximum platelet inhibition mainly through the COX-1 pathway, about one to two hours after a loading dose. However, in regard to its anti-inflammatory effect, it is known that it is achieved at higher dosages, mainly through COX-2 inhibition pathway. It is not known whether the ASA antithrombotic effect remains patent when it gains its anti-inflammatory properties due to lack of evidence.
Our experimental study enrolled twenty healthy volunteers at four different dosages of ASA to check whether the antithrombotic effect remained present throughout. Our results showed that all volunteers were under ASA antithrombotic effect regardless of the dose administered.
In a more detailed analysis of the data, Figure 1 reveals an apparent stepwise increase in OT between 100 mg and 500 mg, which could raise the question of the existence of a potential threshold for antiplatelet effect as dose increases. However, with our limited sample size, we lacked the statistical power to confirm these trends or to establish a clear dose threshold for maximal antiplatelet effect. Further studies are required to address this particular question. While this finding may indeed hold clinical relevance, our primary aim was to answer the broader question of whether the antithrombotic effect of ASA is preserved at high (anti-inflammatory) doses. We, therefore, conducted pairwise comparisons between “low-dose” and “high-dose” categories, which allowed us to enhance statistical power and align the analysis with clinically relevant dosing strategies. This approach enabled us to address the study's main objective, which was to confirm the maintenance of ASA's antithrombotic effect at high (anti-inflammatory) doses, even if detailed dose–response relationships require further investigation.
However, it is important to point out the limitations of this experiment: (1) it was a pilot study with a very limited number of volunteers which prevents the results from being extrapolated to larger cohorts; unfortunately no formal sample size estimation was performed for this study, given the number of participants was determined by financial and logistical constraints; (2) even though our findings suggest a dose-dependent antiaggregation effect of ASA, the study's sample size may not be sufficient to determine conclusively a threshold for antiplatelet effect equivalence between higher doses; (3) the method used to assess the presence of the antithrombotic effect was a qualitative assessment of platelet function and not through direct quantification of TXA2 and other prostanoids in the blood stream; additionally, PFA-200 technology is operator-dependent, requiring a well-trained team of technicians to process the samples and avoid interpretation error. In other words, it is not possible to rule out human error. As a final limitation, it is important to highlight that despite having conducted an in vivo experiment, we used healthy and young volunteers (low mean age) and not patients with a recent acute coronary syndrome (usually older patients) or myopericarditis. The levels of prostanoids, the turnover of COX enzymes in different cell types and even ASA pharmacokinetics may be different in sick patients in comparison with the healthy and young volunteers in our experiment.
ConclusionIn our in vivo experimental study, using a qualitative platelet function assessment technology, we concluded that ASA maintains its antithrombotic effect when administered in an anti-inflammatory dose. There is no clear association between the potency of the antithrombotic effect and the ASA dose administered; however, our subanalysis suggests that higher dosages correspond to higher potency of antiaggregation.
This was a pilot study, intended as exploratory research, that supports the maintenance of the antithrombotic effect of ASA in higher dosages. We believe it provides a foundation for future research with larger and better-powered cohorts to confirm these results.
Ethical approvalHospital Ethics Committee Prof. Doutor Fernando Fonseca, EPE.
Declaration of generative AI and AI-assisted technologies in the writing processNo AI or AI-assisted devices were used in the writing process of this manuscript.
FundingThis research was supported by combined contributions from:
- -
Cardiology Department of Hospital Prof. Doutor Fernando Fonseca, EPE, by providing financial funding allowing us to purchase of the laboratory kits required for the analysis of blood samples with the PFA-200 Innovance technology equipment.
- -
Blood Service Department of Hospital Prof. Doutor Fernando Fonseca, EPE, by providing financial funding enabling us to purchase of the laboratory kits required for the analysis of blood samples by the PFA-200 Innovance technology equipment.
- -
SIEMMENS, for offering twenty laboratory kits required for the analysis of blood samples with the PFA-200 Innovance technology equipment.
The authors declare no conflicts of interest.
We thank the Department of Cardiology and the Blood Service Department of Hospital Prof. Doutor Fernando Fonseca, Amadora, Lisbon, for their support and availability for the conduct of this experimental study.
We also thank all the volunteers that agreed to participate, leading to outstanding cooperation with the technical team.