Decoding the genetic basis of coronary artery disease (CAD) through an intermediate phenotype – coronary calcification – can help us to better understand this deadly disease and enable the creation of better therapeutic strategies. This work aims to assess the relationship between a set of single nucleotide polymorphisms (SNPs) previously associated with CAD and coronary artery calcium (CAC) score in a Portuguese asymptomatic population.
MethodsA prospective study was conducted in a cohort of 1284 subjects (aged 59.3±8.9 years, 73.6% males) without CAD. CAC score was performed using cardiac computed tomography. Thirty-three SNPs were genotyped using TaqMan real-time PCR. Anthropometric, conventional, and biochemical risk factors were evaluated. Bivariate and multivariate regression analysis estimated variables associated with the CAC score.
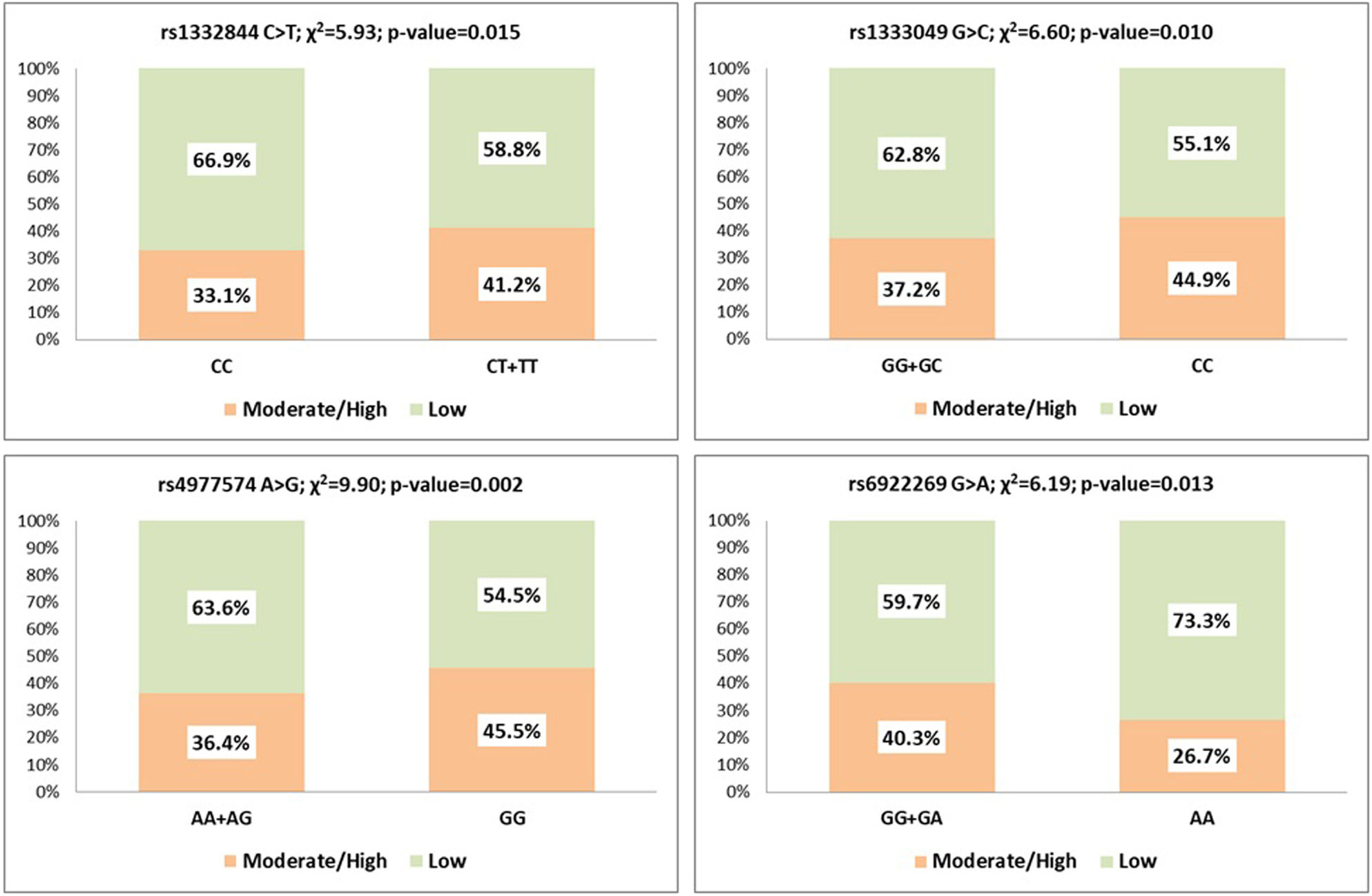
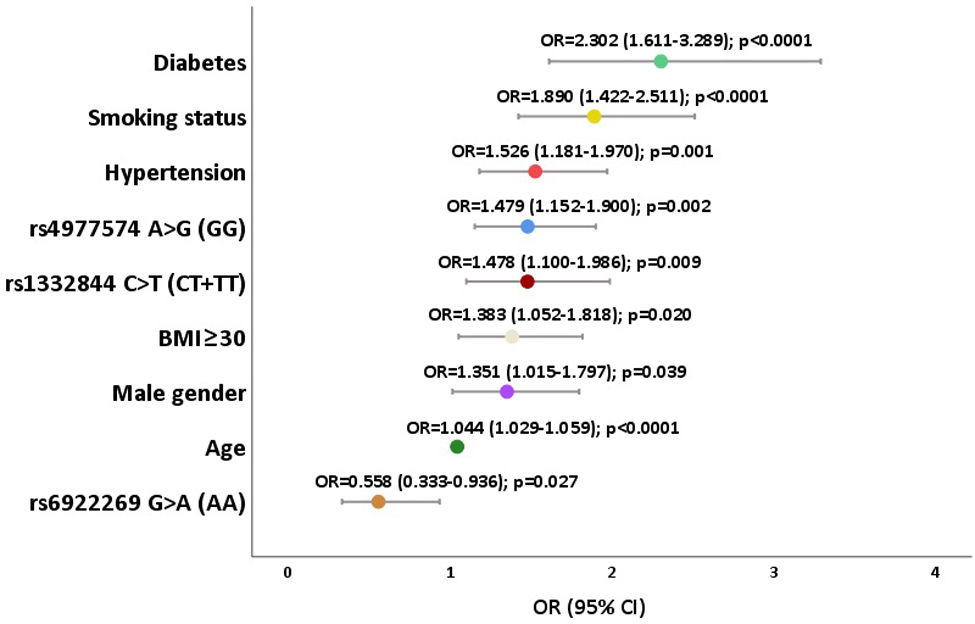
ResultsPHACTR1 rs1332844 C>T, a downstream regulator of the endothelin-1 gene, showed a significant association with CAC score (p=0.015), together with CDKN2B-AS1 variants rs4977574 A>G (p=0.002) and rs1333049 G>C (p=0.010) in the 9p21.3 locus. MTHFD1L rs6922269 G>A variant encoding a mitochondrial enzyme responsible for homocysteine remethylating showed protection against artery calcification (p=0.013). After multivariate logistic regression, PHACTR1 rs1332844 (CT+TT) (OR=1.478; p=0.009) and CDKN2B-AS1 rs4977574 (GG) (OR=1.479; p=0.002) remained in the equation as independently associated with arterial calcification. MTHFD1L rs6922269 (AA) also remained associated with a lower CAC score (OR=0.558; p=0.027).
ConclusionThis study showed that three genetic variants previously linked with CAD are associated with CAC in asymptomatic populations. Understanding these genetic factors, combined with conventional risk factors, could guide lifestyle changes or pharmacologic interventions to mitigate CAD risk before the disease becomes clinical.
O estudo da base genética da doença arterial coronária (DAC) através de um fenótipo intermediário, a calcificação coronária, pode ajudar a melhorar a compreensão desta patologia e permitir o desenvolvimento de estratégias terapêuticas mais eficazes. Este trabalho visa avaliar a relação entre um conjunto de SNPs, previamente associados à DAC, e o score de cálcio coronário (CACs) numa população assintomática portuguesa.
MétodosEstudo prospetivo realizado numa coorte de 1284 indivíduos (59,3±8,9 anos; 73,6% homens) sem DAC aparente. O CACs foi realizado por tomografia computadorizada cardíaca. Trinta e três SNPs foram genotipados. Foram avaliados fatores de risco antropométricos, convencionais e bioquímicos. A análise bivariada e multivariada estimaram as variáveis associadas ao CACs.
ResultadosO PHACTR1 rs1332844 C>T, um regulador do gene endotelina-1, mostrou associação significativa com o CACs (p=0,015), junto com as variantes CDKN2B-AS1 rs4977574 A>G (p=0,002) e rs1333049 G>C (p=0,010) do locus 9p21.3. O MTHFD1L rs6922269 G>A que codifica uma enzima mitocondrial responsável pela remetilação da homocisteína mostrou proteção contra a calcificação arterial (p=0,013). Após regressão logística multivariada, rs1332844 (CT+TT) (OR=1,478; p=0,009) e rs4977574 (GG) (OR=1,479; p=0,002) permaneceram independentemente associados à calcificação arterial. O rs6922269 (AA) permaneceu significativamente associado a valores mais baixos de CACs (OR=0,558; p=0,027).
ConclusãoEste estudo mostrou que três variantes genéticas previamente associadas à DAC estão associadas à calcificação arterial coronária numa população assintomática. Compreender esses fatores genéticos, juntamente com os fatores de risco convencionais, pode orientar mudanças no estilo de vida ou intervenções farmacológicas de forma a mitigar o risco de DAC clínica.
Coronary artery disease (CAD) is a complex cardiovascular disease involving an interplay of genetic and environmental stimuli over a lifetime. Genetic factors contribute significantly to the risk of CAD, and in the past decade, there has been major progress in this area. Based on family and twin studies, the heritability of CAD has been estimated between 40% and 60%, a method that yields high precision despite potential bias.1
The advances in understanding the genetics of CAD and other complex diseases have been driven by high-throughput DNA microarray technology, which uses chips containing up to a million single-nucleotide polymorphisms (SNPs).2 These SNPs evaluated in genome-wide association studies (GWAS) tag common genetic variations found in at least 5% of the population and have shown that CAD is primarily driven by the combined effect of many common risk alleles, each having a negligible impact rather than by rare variants with large effects.3 A key priority is to use high-throughput methods to explore the molecular and cellular functions of the new loci identified. In addition to offering functional insights into disease mechanisms, this kind of study and association has clinical applications, such as determining biomarker causality and helping find new therapies.4
These genetic discoveries have highlighted many candidate genes and pathways that are dysregulated in CAD, including those involved in lipid metabolism, diabetes and obesity, hypertension, oxidation and inflammation, mitosis and cellular proliferation, vascular remodeling, extracellular matrix, cell growth and differentiation and plaque calcification.5
Vascular calcification (VC) is an independent risk factor for CVD. Arterial calcification of the aorta, coronary, carotid and peripheral arteries become more prevalent with age. GWAS have identified genome regions linked to VC and myocardial infarction risk, such as the 9p21 region linked to vascular disease and inflammation associated with VC.6
Arthur Agatston and Warren Janowitz published the first technique for scoring CAC scans in 1990. They proposed a method for measuring the amount of arterial calcification based on a specific score that measured the densities and extent of calcium in coronary arteries with computed tomography (CT). The CAC score is a non-invasive coronary artery examination to quantify the amount of calcium in the coronary arteries using an ECG-gated non-contrast-enhanced computed tomography scan (cardiac CT). The amount of calcium is generally related to the quantity of atheroma in the coronary vessels.7 This method supports cardiovascular risk calculation and, consequently, better clinical decision-making. Standard guidelines recommend using both absolute and percentile-based scores for risk stratification. For example, the American Heart Association/American College of Cardiology (AHA/ACC) cholesterol guideline suggests initiating statins for intermediate-risk adults with a CAC score >100 or in the 75th percentile. According to the European Guidelines on Heart Disease Prevention in clinical practice, the CAC score can be used to predict heart disease risk in asymptomatic adults at intermediate risk. The presence of a high CAC score is indicative of a significant risk of developing atherosclerotic cardiovascular disease and CAD.8
It is known that the absence of coronary calcification does not exclude obstructive CAD or the need for clinically indicated coronary revascularization among patients with clinical suspicion of CAD. However, in asymptomatic individuals, the absence of CAC is associated with a very low incidence of cardiovascular events.9
Despite the increase in direct-to-consumer testing for genetic variants of cardiovascular disease and the increased knowledge in this field, there are still limited data evaluating the association between genetic variants and CAC score, considering it a measure of subclinical atherosclerosis. Coronary artery calcification predicts future symptomatic CAD, and detecting genetic risk factors associated with CAC may lead to new therapeutic routes focused on prevention.10
ObjectivesThe objective of this study was to assess the independent relationship between a set of SNPs previously associated with CAD and the CAC score in an asymptomatic population without apparent coronary heart disease.
MethodsStudy design and populationA prospective single-center study was performed at the Research Center of Funchal Hospital Center with asymptomatic individuals without known CAD belonging to the control group of the GENEMACOR study (Genes in Madeira and Coronary Disease).11 The study population consisted of 1284 subjects, all Caucasian/White, with a mean age of 59.3±8.9 years, 73.6% male, recruited based on electoral rolls, followed-up between 2001 and 2023 (average 7.0±5.0 years).
This study followed the Declaration of Helsinki and was approved by the Ethics Committee and Institutional Board of Funchal Hospital Center under protocol 50/2012. Written informed consent was attained from all subjects at the time of enrollment.
Assessment of the cardiovascular risk factorsDemographic, traditional, and clinical risk factors: age, gender, smoking status, alcohol abuse, body mass index (BMI), physical inactivity, dyslipidemia, hypertension and type 2 diabetes, were inquired in a presential clinical appointment and have been described previously.12
Biochemical risk factorsBlood samples were performed after 12 hours of fasting at the Hospital's Central Laboratory according to the standard methods. Blood samples were placed in dry tubes and centrifuged for half an hour at 3500 g to determine total cholesterol, HDL, LDL, triglycerides, and glucose analysis, and then quantified by an enzymatic technique using an autoanalyzer ‘AU 5400’ (Beckman Coulter). Biochemical markers such as lipoprotein-a, apolipoprotein B, and high-sensitivity C-reactive protein were measured by immunoturbidimetry using an automatic ‘AU 5400’ (Beckman Coulter) system.
Determination of the coronary artery calcium scoreComputed tomography was made in all subjects through an Aquilion 64 scanner (Toshiba Medical Systems, Ōtawara, Tochigi Prefecture, Japan). The scanning with prospective ECG gating was performed during a breath-hold using 64 slices with a collimated slice thickness of 3 mm. A breath-hold typically lasted seven to eight seconds. The effective radiation dose associated with a CAC scan should average about 1.0–1.5 mSv and not exceed 3.0 mSv. The final data reconstructions were performed on the Vitrea workstation (Vital Images, Minnetonka, MN, USA; software version 5.1). Calcification was calculated using the Agatston scale by an expert trained in multi-slice CT of the heart. In our study and according to the Hoff Nomogram, the CAC score was categorized into two groups, low: 0≤CAC<100 and P<50 and moderate/high: CAC≥100 or P>50.13
Genetic analysisIn the present study, we used 33 SNPs previously associated with CAD by gene candidate studies and/or GWAS which had already been investigated in the GENEMACOR Study.12 The characteristics and putative function of each genetic variant are described in the Supplementary Table S1.
Genotyping was performed in peripheral leukocyte DNA using the TaqMan genotyping assay (Applied Biosystems, USA) with allelic discrimination. Labeled probes and primers pre-established by the supplier (7300 Real-Time PCR System, Applied Biosystems, USA) were used without prior knowledge of the individual's clinical data. The quality of the genotyping technique was checked by including one non-template control in each plate of 96 wells and a blind duplicate, accounting for 20% of all samples.
Statistical analysisDescriptive and comparative analysisContinuous variables were defined as means (±SD) or medians (min–max), as appropriate. Categorical variables were displayed as frequencies and proportions. The Student t-test and Mann–Whitney test were applicable to compare continuous data, and the χ2 test was used to compare categorical variables. Bivariate analyses evaluated associations between genotypes and demographic, biochemical and clinical risk factors (χ2 test).
Multivariate logistic regression defines the association between independent variables and the dependent variable. It included the following established predictors: age, gender, obesity (BMI ≥30 kg/m2), physical inactivity, smoking status, alcohol >300 g/week, hypertension, dyslipidemia and type 2 diabetes. All analyses were performed using SPSS version 25 (SPSS Inc. Chicago, IL). Statistical significance was set at 5% (p<0.05).
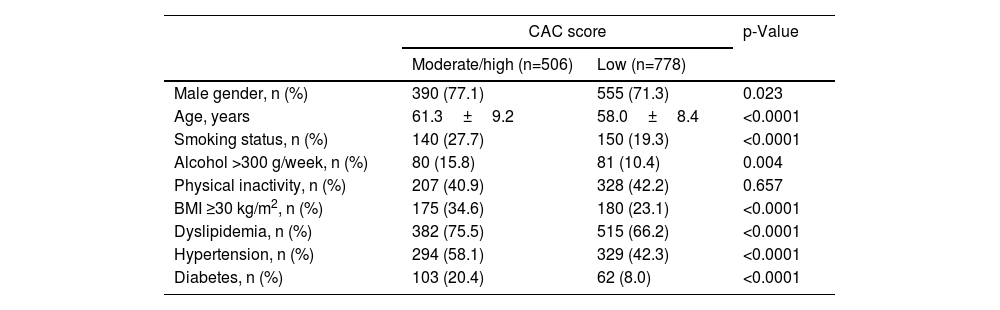
ResultsTraditional risk factorsA comparison of the individuals with moderate/high vs low CAC scores concerning traditional risk factors showed that age (p<0.0001), male gender (p=0.023), smoking status (p<0.0001), alcohol >300 g/week (p=0.004), BMI ≥30 kg/m2 (p<0.0001), dyslipidemia (p<0.0001), hypertension (p<0.0001), and type 2 diabetes (p<0.0001) are associated with moderate/high CAC score. The physical inactivity was unrelated to elevated coronary calcification (p=0.657) (Table 1).
Traditional risk factors for coronary artery calcium score.
| CAC score | p-Value | ||
|---|---|---|---|
| Moderate/high (n=506) | Low (n=778) | ||
| Male gender, n (%) | 390 (77.1) | 555 (71.3) | 0.023 |
| Age, years | 61.3±9.2 | 58.0±8.4 | <0.0001 |
| Smoking status, n (%) | 140 (27.7) | 150 (19.3) | <0.0001 |
| Alcohol >300 g/week, n (%) | 80 (15.8) | 81 (10.4) | 0.004 |
| Physical inactivity, n (%) | 207 (40.9) | 328 (42.2) | 0.657 |
| BMI ≥30 kg/m2, n (%) | 175 (34.6) | 180 (23.1) | <0.0001 |
| Dyslipidemia, n (%) | 382 (75.5) | 515 (66.2) | <0.0001 |
| Hypertension, n (%) | 294 (58.1) | 329 (42.3) | <0.0001 |
| Diabetes, n (%) | 103 (20.4) | 62 (8.0) | <0.0001 |
BMI: body mass index; CAC: coronary artery calcium. Age presented as mean±standard deviation; statistically significant for p<0.05.
After bivariate analysis, PHACTR1 rs1332844 C>T (dominant model) showed a significant association with CAC score (p=0.015), as well as CDKN2B-AS1 rs4977574 A>G (p=0.002) and rs1333049 G>C (p=0.010). MTHFD1L rs6922269 G>A showed protection against coronary artery calcification (p=0.013) (Figure 1).
Variables independently associated with coronary artery calcium scoreWe constructed a statistical model with all variables with significant CAC score association in the bivariate analysis, including the genetic variants rs1332844, rs4977574 and rs6922269, adjusted to the confounders: age, male gender, smoking status, alcohol abuse, obesity (BMI ≥30), dyslipidemia, hypertension, and type 2 diabetes.
After multivariate logistic regression (Figure 2), PHACTR1 rs1332844 CT+TT and CDKN2B-AS1 rs4977574 GG remained in the equation as CAC score risk variants (OR=1.478; 95%CI: 1.100–1.986; p=0.009 and OR=1.479; 95%CI: 1.152–1.900; p=0.002, respectively). This means that an individual with CT or TT genotype of PHACTR1 gene has a 47.8% higher probability of having a high CAC score when compared with those who have CC genotype. The same interpretation can be read for CDKN2B-AS1, as the GG genotype increases the risk of higher CAC score by 47.9%. Individuals with MTHFD1L rs6922269 AA are more protected from arterial calcification (OR=0.558; 95%CI: 0.333–0.936; p=0.027), indicating that individuals with the AA genotype exhibit a 44.2% reduced risk of having a high CAC score. The other variables significantly and independently associated with CAC score were: age (OR=1.044; 95%CI: 1.029–1.059; p<0.0001), male gender (OR=1.351; 95%CI: 1.015–1.797; p=0.039), smoking status (OR=1.890; 95%CI: 1.422–2.511; p<0.0001), BMI ≥30 (OR=1.383; 95%CI: 1.052–1.818; p=0.020), hypertension (OR=1.526; 95%CI: 1.181–1.970; p=0.001), and type 2 diabetes (OR=2.302; 95%CI: 1.611–3.289; p<0.0001) (Figure 2).
DiscussionGenome-wide association studies have identified several loci linked to coronary artery calcification, some shared with those for coronary atherosclerosis. The mechanisms of VC are complex and varied. Inflammation, endoplasmic reticulum stress, cell senescence, autophagy, apoptosis, and genetic factors promote vascular remodeling and calcification.14 Recent findings regarding the genetics of CAC mention this process should give a better understanding of the origin of CAC and could ultimately establish a basis for improved prevention and treatment of asymptomatic coronary atherosclerosis.15
The primary clinical implication of our findings is the potential to enhance cardiovascular risk prediction in asymptomatic individuals. As genetic testing becomes more accessible, genetic risk scores will likely emerge as a valuable tool for cardiovascular risk stratification, complementing traditional risk factors.
A previous study by Lange et al.,16 provided evidence that chromosomal regions 6p21.3 and 10q21.3 harbored genes associated with CAC and subclinical coronary atherosclerosis. VC is dynamic and regulates extraosseous ossification progress, currently recognized as an independent predictor of CVD mortality and morbidity. Endothelial cells (ECs) that directly respond to changes in flow shear stress and blood composition are the innermost layer of blood vessels. With vascular smooth muscle cells, ECs preserve vascular homeostasis. Increased evidence shows that ECs have unique roles in VC due to their high plasticity. The CAC score is recognized as a particular feature of coronary atherosclerosis and is associated with the conventional risk factors involving atherosclerosis and CAD.
Yazdi et al. recently demonstrated that CAC score is a marker for risk factors associated with atherosclerosis; smokers and diabetic patients have significantly high CAC score. The older patients and males, as well as hypertensive patients, moved to the moderate category. According to this author, smoking seems to be the strongest predictor for high CAC, and type 2 diabetes is the second strongest predictor for severe CAC score.17
Our study showed, as expected, that risk factors like age, male gender, smoking status, alcohol, obesity, dyslipidemia, hypertension, and type 2 diabetes are associated with the group of moderate/high CAC scores. However, type 2 diabetes is the strongest risk factor, and smoking is the second most potent risk factor. On the other hand, physical inactivity is not a significant risk factor for arterial calcification.
After multivariate logistic regression adjustment, three genetic variants of our cohort showed independent and significant association with CAC: PHACTR1 rs1332844 C>T and CDKN2B-AS1 rs4977574 A>G presented a risk for moderate/high CAC score, and MTHFD1L rs6922269 G>A displayed a significant and independent protection factor for arterial calcification.
All three SNPs identified as associated with the CAC score have been previously reported in the literature as linked to CAD. Notably, the CDKN2B-AS1 rs4977574 A>G variant is also associated with myocardial infarction and early-onset myocardial infarction. The effect sizes and corresponding studies are detailed in Supplementary Table S2.
As we outlined in the present study, the PHACTR1 rs1332844 variant showed to be a CAC score-related gene associated with higher levels of coronary artery calcification, representing a risk factor for the start of atherosclerotic plaque development in an asymptomatic population. It is shaped by a phosphatase and actin regulator belonging to a highly conserved PHACTR family and was first discovered as a phosphatase 1-binding protein in the brain in 2004.18 Several large-scale GWAS from different cohorts and research groups in the last decade showed that SNPs at the PHACTR1 locus are significantly associated with CAD and myocardial infarction. Cell biological studies unravel that PHACTR1 mediates endothelial inflammation and monocyte adhesion by activating NF-kB dependent intercellular adhesion molecule1 (ICAM1) and vascular cell adhesion molecule1 (VCAM1) expression. This point highlights the potential to prevent endothelial dysfunction and atherosclerosis by targeting PHACTR1 expression.19 Recent research demonstrates that PHACTR1 functions as a transcriptional corepressor of endothelial cell-specific peroxisome proliferator-activated receptor γ (PPARγ), repressing its transcriptional activity. PPARγ is a nuclear receptor and transcription factor expressed in various tissues, including vascular cells, where it mediates diverse processes, including adipogenesis, cell proliferation and differentiation, as well as anti-inflammatory and antifibrotic actions.20
Jiang et al.21 investigated this gene with a transgenic mice model with deletion of the Phactr1 exon (Phactr1−/−). They compared the “knockout” model with wild-type mice, showing that PHACTR1 is highly expressed in murine ECs, especially in regions with disturbed flow, such as arterial branch points more susceptible to endothelial dysfunction and plaque formation, and Phactr1 appears to be a key player in this process. The authors also found that the protective effects of PHACTR1 depletion on endothelial activation and atherosclerosis were reversed when a PPARγ antagonist was administered. This point suggests that PHACTR1s protective effects may be mediated through the PPARγ signaling pathway. By blocking PPARγ activity, the protective impact of PHACTR1 depletion was lost, leading to increased endothelial activation, atherosclerotic development, and posterior plaque calcification.21
The other SNP significantly associated with CAC score, in our work, was rs4977574 A>G located at 9p21 locus in an intronic region of the CDKN2B-AS1 gene that has been linked to an augmented risk of CAD, coronary artery calcification and plaque calcification, essential features of atherosclerosis. Plaque formation represents the pathognomonic hallmark of atherosclerosis caused by the malfunction of ECs regulated by long non-coding RNAs (lncRNAs). LncRNA cyclin-dependent kinase inhibitor 2B antisense RNA 1 (CDKN2B-AS1) is predominately expressed in tissues associated with CAD, including cardiac, vascular ECs and human monocyte-derived macrophages.22 It is a transcription factor that affects the regulation of genes implicated in vascular smooth muscle cell proliferation, inflammation, and the overall process of atherosclerosis, including plaque calcification. Evidence has suggested that genetic variants of CDKN2B-AS1 play critical roles in various diseases, including atherosclerosis, diabetes, and some types of cancer.23
Kavousi et al., conducting a multi-ancestry GWAS study with 26909 individuals of European ancestry and 8867 individuals of African descent, investigated coronary artery calcification, identifying initially eleven genetic risk loci associated with CAC. They confirmed these findings using a single-nucleus ATAC-seq dataset in healthy and diseased coronary arteries, identifying credible CAC variants at novel loci overlapping cell type-specific peak-to-gene links. These results demonstrated eight relevant loci genes in the vascular wall, providing candidate regulatory mechanisms for CAC associations. Among them, similarly to our group, recognized PHACR1 and CDKN2B-AS1, two loci associated with CAC. The authors conclude that it is possible to identify druggable targets for CAC besides improving CAC's genetic architecture and extending the understanding of the biological pathways underlying CAC formation, which may point to new therapeutic avenues for preventing clinical disease. Several CAC-specific genes represent targets of the druggable genome, which should be considered for preclinical studies of CAC. By querying the drug-gene interaction database (DGIdb) and DrugBank database, it can be revealed that approved compounds are under investigation targeting other novel loci.24
Finally, MTHFD1L (methylenetetrahydrofolate dehydrogenase-like 1 (NADP+ dependent) encodes a protein that is involved in the synthesis of tetrahydrofolate in mitochondria. THF is crucial in the de novo synthesis of purines and thymidylate and the turnover of methionine from homocysteine (HC). The MTHFD1L rs6922269 G>A variant showed, in our study, protection against coronary calcification.
The protective effect of the mutated MTHFD1L gene is a paradox: This mutation is associated with decreased efficacy of the gene and higher HC levels, which is linked to higher vascular risk. But, in the present paper, we study vascular wall calcification as an intermediate endpoint for the vascular risk, not the risk itself.
We must remember that vascular disease depends on two primary components. One is plasmatic, which is responsible for acute events, platelet activation, LDL oxidation, and endothelial dysfunction, all linked to high HC levels. The other component, arterial wall degradation, is evaluated with a calcium score and is associated in clinical terms with stable lesions, and chronic angina/vascular disease seems to be less dependent on HC levels.
On the other hand, the MTHF effect depends on the levels of folic acid, the major cofactor of methionine/HC metabolism. In a population with a good level of folic acid, a component included in several enriched foods (e.g., cereals), the mutation can have a minor or no influence on HC metabolism and vascular risk. This can be the case in the present study.
Likewise, the literature does not consistently correlate this variant with CAD phenotypes, such as increased carotid intima-media thickness or angiographically proven CAD.25 So, more epidemiological studies could present more detailed arguments explaining why this inconsistency may arise due to population characteristics, environmental factors, and interactions with other genetic and epigenetic markers, adding weight to the statement.
Strengths and limitationsAs far as we know, this is the first study in a Portuguese population that establishes a correlation between a set of genetic polymorphisms and the CAC score. This work analyzed a cohort of more than 1200 non-symptomatic subjects without apparent CAD who were randomly selected from electoral rolls.
It is well established that coronary calcification, as assessed by CAC score, precedes the clinical manifestation of CAD. These findings may be significant, highlighting the potential role of these SNPs in both the development and prevention of CAC and CAD.
However, our study has its limitations. It included only 33 SNPs previously associated with CAD from our database. Recent studies identified many novel CAD loci of GWAS significance that are not yet included in our database. It is important to test other SNPs to understand which genetic variants are associated with subclinical coronary atherosclerosis.
Our cohort was selected from Madeira Island, a genetically unique population, so we cannot guarantee that it is representative of the entire Portuguese or European population. Multicenter studies with larger sample sizes from different regions must confirm the presented results.
ConclusionsThe present work showed that two genetic variants of PHACTR1 and CDKN2B-AS1 are associated with CAC, along with clinical and environmental risk factors. On the other hand, MTHFD1L revealed protection against coronary calcification. Understanding the genetic basis of vessel calcification can enable precocious lifestyle or pharmacologic changes to attenuate risk before the disease becomes clinical. More research in this field is critical to understanding the genetic basis of CAD through an intermediate phenotype, coronary arterial wall calcification.
Conflicts of interestThe authors have no conflicts of interest to declare.