Over the last decade, several studies have suggested that left ventricular endomyocardial biopsy is safer and has a higher diagnostic yield than transvenous right ventricular biopsy. In addition, recent publications indicate that the transradial approach is a feasible and safe alternative to the transfemoral approach for sampling the left ventricle. We report our initial experience with transradial endomyocardial biopsy with regards to feasibility, safety and usefulness.
MethodsSingle-center registry of consecutive patients undergoing intended transradial left endomyocardial biopsy. Clinical and technical data were collected prospectively, with a particular focus on success rate and complications.
ResultsTwenty-seven patients were screened for left ventricle biopsy. Twenty (25) were selected for an intended transradial approach (mean age 51±18 years old, 22 male). Success rate was 100% with no crossover to femoral approach. There were no major complications. Two patients experienced mild radial spasm. One of them also had a run of non-sustained ventricular tachycardia.
Indication for biopsy was either myocarditis or cardiomyopathy of unknown etiology. The final diagnosis was acute lymphocytic myocarditis in five patients, chronic myocarditis in one patient, amyloid light-chain amyloidosis in four patients and transthyretin amyloidosis in six patients. Myocarditis was ruled out in eight patients and amyloidosis in one patient.
ConclusionsTransradial left ventricle endomyocardial biopsy is a very safe and feasible method of sampling the myocardium for histopathological analysis, with a good diagnostic yield and clinically meaningful results in properly selected patients.
Durante a última década, vários estudos têm sugerido que a biópsia endomiocárdica ventricular esquerda é mais segura e de superior rentabilidade diagnóstica do que a do ventrículo direito. Adicionalmente, várias publicações recentes têm introduzido a abordagem transradial como uma alternativa exequível e segura à transfemoral, para amostrar o ventrículo esquerdo. O objetivo deste estudo é reportar a experiência inicial de um centro em biópsia endomiocárdica ventricular esquerda transradial, relativamente a exequibilidade, segurança e utilidade.
MétodosRegisto unicêntrico de doentes consecutivos submetidos a biópsia endomiocárdica ventricular esquerda, com acesso de primeira intenção radial. Registaram-se os dados clínicos e técnicos, com particular foco na taxa de sucesso e complicações.
ResultadosForam submetidos a biópsia endomiocárdica ventricular esquerda 27 doentes, 25 dos quais pré-selecionados para acesso transradial (idade média 51±18, 22 homens). A taxa de sucesso foi de 100%. Não ocorreram complicações major, apenas espasmo radial em dois doentes, num dos quais se observou uma salva de taquicardia ventricular não mantida. A indicação foi miocardite ou miocardiopatia de etiologia a esclarecer. O diagnóstico final foi de miocardite aguda em cinco doentes, miocardite crónica em um doente, amiloidose AL em quatro doentes e ATTR em seis doentes. Excluiu-se miocardite em oito doentes e amiloidose em um doente.
ConclusãoA biópsia endomiocárdica ventricular esquerda transradial demonstrou ser segura, exequível e de boa rentabilidade diagnóstica, com resultados clinicamente relevantes em doentes selecionados.
The development of endomyocardial biopsy in the 1960s facilitated percutaneous sampling of the myocardium without the need for a grossly invasive procedure.1 However, in recent decades, significant advances in both invasive and non-invasive cardiac imaging have enabled the study of a wide array of cardiac diseases, without the need for a histopathological analysis of the heart. As a result, enthusiasm for myocardial biopsy has waned. Indeed, the most recent recommendations regarding endomyocardial biopsy date back to 2007, and a high level of recommendation applies in only a handful of settings.2 However, these guidelines base their recommendations on efficacy and safety data in existence at that time. Since then, two main changes have occurred: Firstly, several authors have published extensive data on an approach via the left ventricle rather than just the right ventricle, allowing for more extended myocardial sampling.3,4 Secondly, several operators started performing left ventricular (LV) biopsy using the radial approach, widely popular in the interventional community for its simplicity, comfort and increased safety.5 As a result, more recent documents call for a wider use of this technique, particularly in myocarditis,6,7 dilated cardiomyopathy8 and myocardial infarction with non-obstructive coronary artery disease.9
In this paper, we report our initial experience of the feasibility, safety and usefulness of transradial endomyocardial biopsy.
MethodsGeneral featuresThis study involved a single-center registry of consecutive patients undergoing intended transradial endomyocardial biopsy. Patients were selected for endomyocardial biopsy of the left ventricle and screened for the feasibility of radial access individually, based on the operator's clinical judgment.
Clinical data included demographics, clinical setting, imaging, complications, and final biopsy results. Technical data included access site, sheath/catheter size and shape, biopsy model and size, procedural time, fluoroscopy time, success rate and cross-over rate to femoral approach.
Statistical analysisThis is a descriptive registry; complex statistical analysis was not performed. Qualitative variables are expressed both numerically and in percentages. Continuous variables are depicted as mean ± standard deviation and range, where appropriate. SPSS Statistics 24 was used for analysis.
Procedure overviewDetailed information on technique has been published in recent papers.10–13 However, because minor variation occurs across centers and operators, an overview of the technique as it was performed is provided.
The procedure was always undertaken by the same operator, who had been previously trained in transradial intervention and in performing left myocardial biopsies. During the procedure, the patient was continuously monitored as in a routine coronary angiography (i.e. with continuous 12-lead electrocardiogram and invasive blood pressure measurement).
Routine transthoracic echocardiography was performed before, during and after the procedure. Particular attention was given to documenting and quantifying pericardial effusion and mitral regurgitation, as well as excluding the presence of LV thrombus or masses which might contraindicate the procedure.
Spasm prophylaxis with intra-arterial 0.5-1 mg of isosorbide dinitrate and/or 2.5 mg of verapamil and 5000 units of unfractionated heparin were administered.
If the patient required a coronary angiogram, a standard transradial coronary angiogram was performed using a Terumo 5/6 Fr slender sheath (external diameter 5 Fr) and standard Judkins 5 Fr coronary diagnostic catheters.
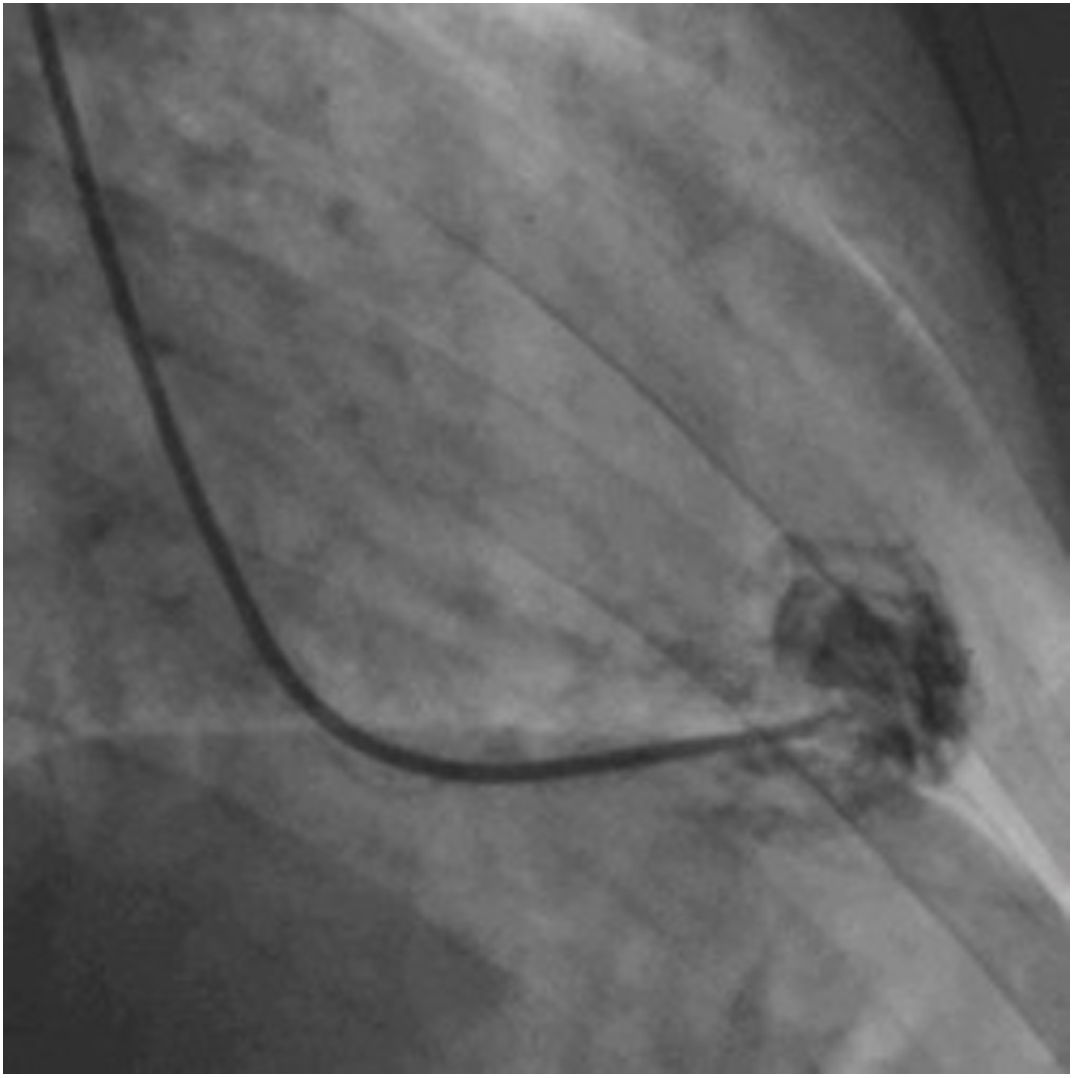
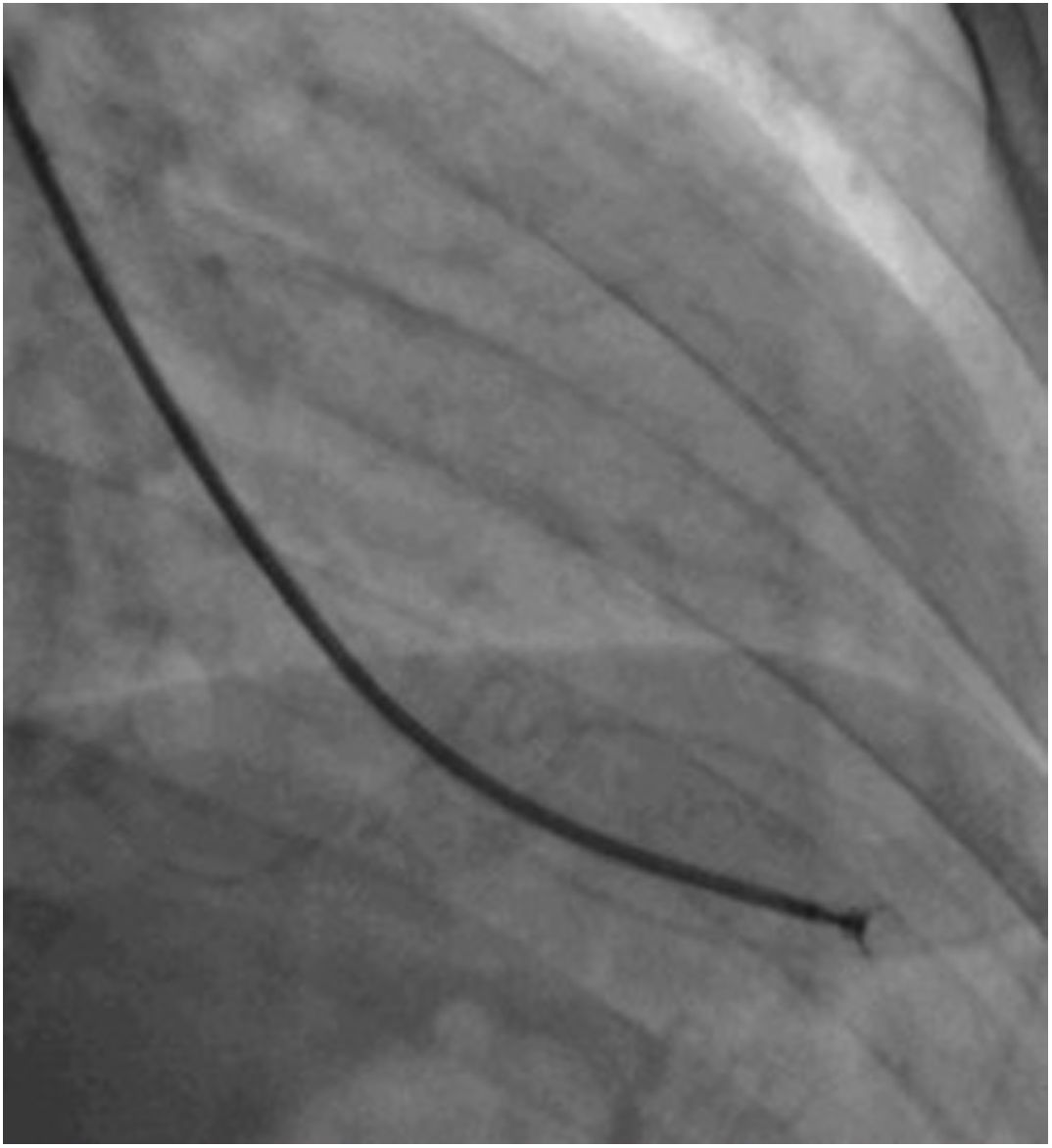
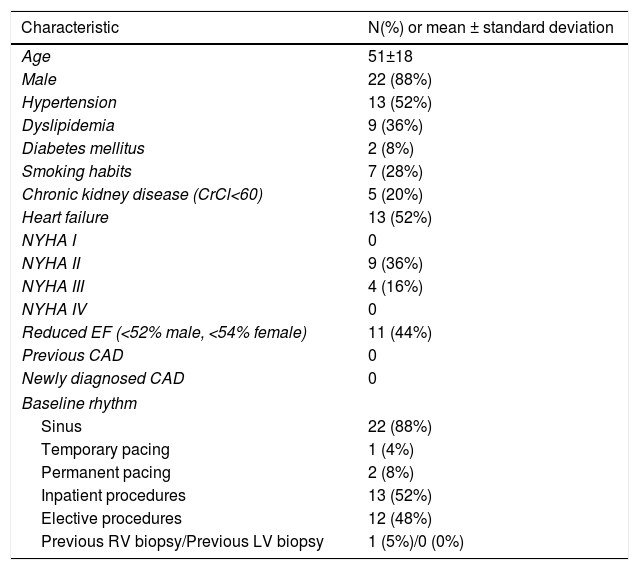
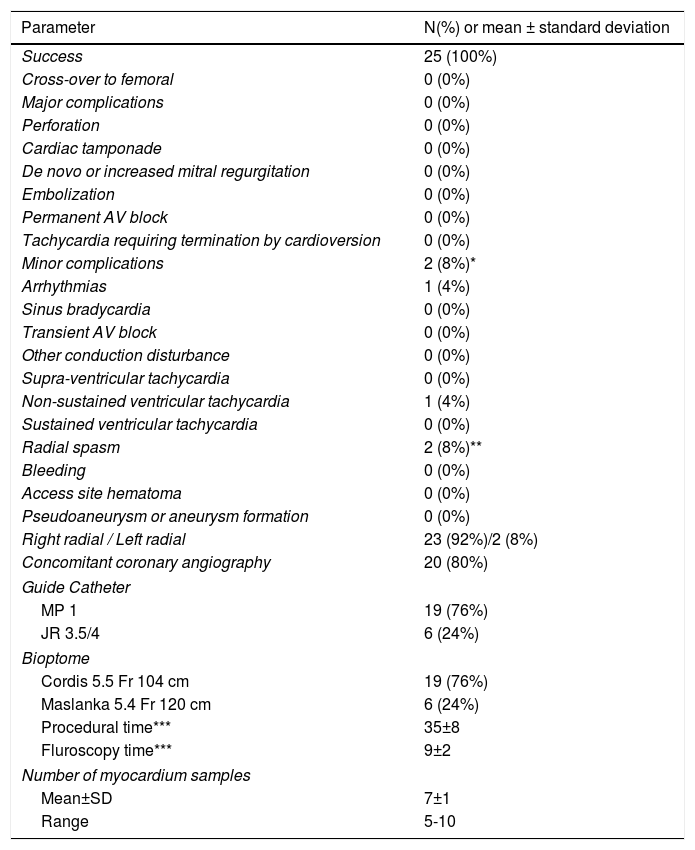
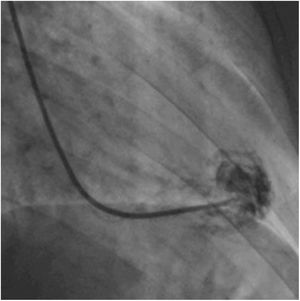
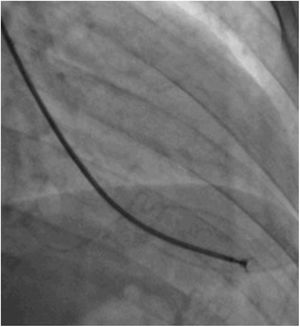
Afterwards, a 7.5 Fr (external diameter 6 Fr) Asahi Eaucath sheathless guide catheter was advanced over a standard 0.035” J wire to the ascending aorta. An MP 1 or JR 3.5/4 curve was used according to operator choice. The dilator was removed and a 5 Fr pigtail catheter was then used inside the 7.5 Fr for crossing the aortic valve and safely”landing” in the left ventricle. The guide catheter was advanced over the pigtail, which was then removed, and placed in the mid-cavity of the left ventricle. The catheter was connected to the pressure system, initial LV pressure was recorded and a minimal amount of contrast was injected (Figure 1). A 5.5 Fr Cordis 104 cm or 5.4 Fr Maslanka 120 cm bioptome was advanced and the myocardial wall was sampled (Figure 2). Back-bleed from the catheter and, if necessary, aspiration, were then performed to ensure no air bubbles were in the system. These steps were then repeated several times until the desired number of samples was obtained – a minimum of five – of which at least one was frozen for further analysis, if necessary.
All mid-apical segments of all the walls of the left ventricle were sampled by gently directing the catheter during the procedure, guided by fluoroscopy and echocardiography. First the guide is positioned using fluoroscopy and then confirmed by transthoracic echocardiography by an assisting physician. This enables detailed guide and bioptome positioning and early identification of possible complications. The process was repeated for all samples.
Hemostasis was obtained using the Terumo TR-Band.
ResultsBaseline population characteristicsTwenty-five patients were selected for intended LV endomyocardial biopsy. Two patients underwent the procedure in the setting of cardiogenic shock of unknown etiology and were deemed better suited to a transfemoral approach, as mechanical circulatory support was being considered post procedure. Transradial endomyocardial biopsy was thus attempted in 25 patients. The baseline data are illustrated in Table 1.
Baseline clinical characteristics.
| Characteristic | N(%) or mean ± standard deviation |
|---|---|
| Age | 51±18 |
| Male | 22 (88%) |
| Hypertension | 13 (52%) |
| Dyslipidemia | 9 (36%) |
| Diabetes mellitus | 2 (8%) |
| Smoking habits | 7 (28%) |
| Chronic kidney disease (CrCl<60) | 5 (20%) |
| Heart failure | 13 (52%) |
| NYHA I | 0 |
| NYHA II | 9 (36%) |
| NYHA III | 4 (16%) |
| NYHA IV | 0 |
| Reduced EF (<52% male, <54% female) | 11 (44%) |
| Previous CAD | 0 |
| Newly diagnosed CAD | 0 |
| Baseline rhythm | |
| Sinus | 22 (88%) |
| Temporary pacing | 1 (4%) |
| Permanent pacing | 2 (8%) |
| Inpatient procedures | 13 (52%) |
| Elective procedures | 12 (48%) |
| Previous RV biopsy/Previous LV biopsy | 1 (5%)/0 (0%) |
CrCl: creatinine clearance; CAD: coronary artery disease; EF: ejection fraction; LV: left ventricular; NYHA: New York Heart Association; RV: right ventricular.
All patients scheduled for transradial biopsy underwent the procedure successfully, with no cases of cross-over to a femoral approach. There were no major complications, and only two cases of minor radial spasm. One of these two patients also had a run of non-sustained ventricular tachycardia (Table 2).
Technical data, success and complications.
| Parameter | N(%) or mean ± standard deviation |
|---|---|
| Success | 25 (100%) |
| Cross-over to femoral | 0 (0%) |
| Major complications | 0 (0%) |
| Perforation | 0 (0%) |
| Cardiac tamponade | 0 (0%) |
| De novo or increased mitral regurgitation | 0 (0%) |
| Embolization | 0 (0%) |
| Permanent AV block | 0 (0%) |
| Tachycardia requiring termination by cardioversion | 0 (0%) |
| Minor complications | 2 (8%)* |
| Arrhythmias | 1 (4%) |
| Sinus bradycardia | 0 (0%) |
| Transient AV block | 0 (0%) |
| Other conduction disturbance | 0 (0%) |
| Supra-ventricular tachycardia | 0 (0%) |
| Non-sustained ventricular tachycardia | 1 (4%) |
| Sustained ventricular tachycardia | 0 (0%) |
| Radial spasm | 2 (8%)** |
| Bleeding | 0 (0%) |
| Access site hematoma | 0 (0%) |
| Pseudoaneurysm or aneurysm formation | 0 (0%) |
| Right radial / Left radial | 23 (92%)/2 (8%) |
| Concomitant coronary angiography | 20 (80%) |
| Guide Catheter | |
| MP 1 | 19 (76%) |
| JR 3.5/4 | 6 (24%) |
| Bioptome | |
| Cordis 5.5 Fr 104 cm | 19 (76%) |
| Maslanka 5.4 Fr 120 cm | 6 (24%) |
| Procedural time*** | 35±8 |
| Fluroscopy time*** | 9±2 |
| Number of myocardium samples | |
| Mean±SD | 7±1 |
| Range | 5-10 |
AV: atrioventricular; MP: Multipurpose; JR: Judkins Right.
Regarding the two above-mentioned patients who underwent programmed transfemoral LV endomyocardial biopsy, the procedure was also successful with no major or minor complications.
Biopsy indication, results and final clinical diagnosisThere were two main clinical settings for performing the procedure: myocarditis and cardiomyopathy. The details are provided in Table 3.
Indication for biopsy and final result.
| Indication – N(%) | Final Result | |
|---|---|---|
| Acute myocarditis (clinically suspected myocarditis) | 8 (32%) | 5 confirmed3 ruled out |
| New-onset HF | 4 (16%) | 2 confirmed2 ruled out |
| 3rd degree AV block | 1 (4%) | 1 Ruled out |
| No signs of HF or serious arrythmias | 3 (12%) | 3 confirmed |
| Chronic myocarditis(clinically suspected - DCM with HF) | 6 (24%) | 1 confirmed5 ruled out |
| Hypertrophic cardiomyopathy | 11 (44%) | |
| AHCM with clinical features of amyloidosis | 2 (8%) | 2 ATTR amyloidosis |
| Suspected cardiac amyloidosis with previous inconclusive studies | 9 (36%) | 4 AL amyloidosis4 ATTR amyloidosis1 ruled out |
AHCM: apical hypertrophic cardiomyopathy; ATTR: transthyretin; AL: amyloid light-chain; AV: atrioventricular; DCM: dilated cardiomyopathy; HF: heart failure.
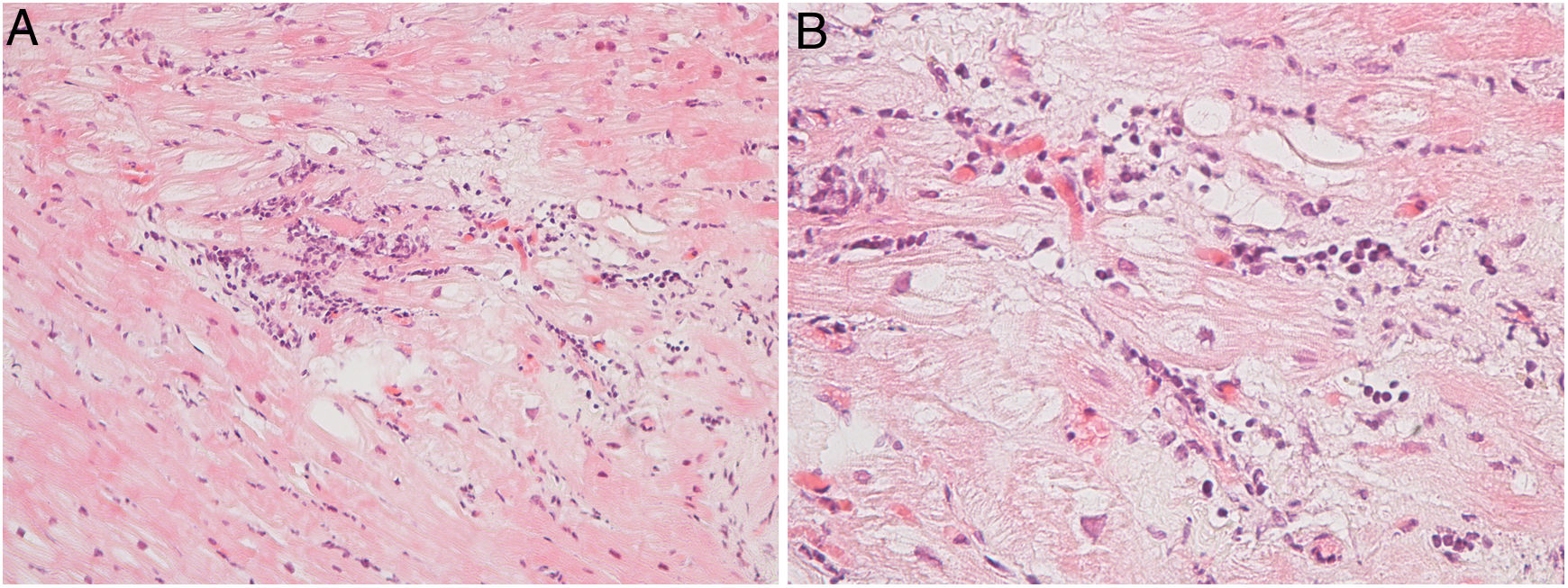
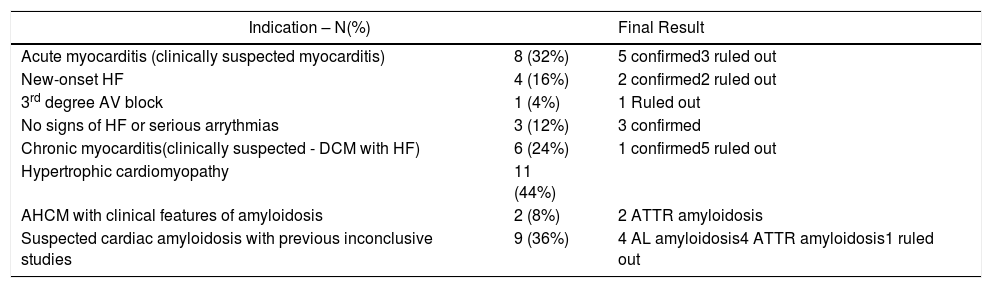
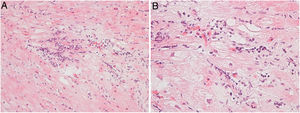
Regarding myocarditis, eight studies were performed in the setting of suspected acute myocarditis (Figure 3A and 3B).
Three patients presented with acute coronary syndrome chest pain-like symptoms. Two of them underwent the procedure because of recurrent myocarditis. The third patient underwent the procedure due to very high troponin levels and mildly impaired LV function, despite the absence of clinical signs of heart failure. All had a favorable clinical outcome with normalization of LV function after a few weeks. Their final diagnosis was acute lymphocytic myocarditis. One patient had a mildly positive parvovirus B19 result in the polymerase chain reaction (PCR) test.
Four patients underwent the procedure because of new-onset heart failure of unknown etiology. They had no other characteristics of acute myocarditis but a potentially treatable type of myocarditis, such as giant cell myocarditis, was ruled out, thereby excluding an indication for immunosuppression therapy. Two of these patients tested positive for acute lymphocytic myocarditis, one in the setting of Takayasu arteritis, and progressed favorably, with normalization of LV function after a few weeks.
One patient was a 46-year-old patient with new-onset third degree atrioventricular (AV) block. The biopsy ruled out myocarditis, but showed some degree of fibrosis, possibly related to past radiotherapy years earlier in the setting of Hodgkin's lymphoma. The result helped to explain the etiology of the AV block at such a young age and reinforced the need for a permanent pacemaker, as the cause of the bradycardia was not reversible.
Six patients underwent the procedure in the setting of dilated cardiomyopathy for ruling out chronic myocarditis. They were young patients (age range 23-56 years) refractory to medical therapy for heart failure titrated to maximum doses. Only one met the formal pathological criteria of chronic myocarditis.6 The study of viral genomes by PCR was negative.
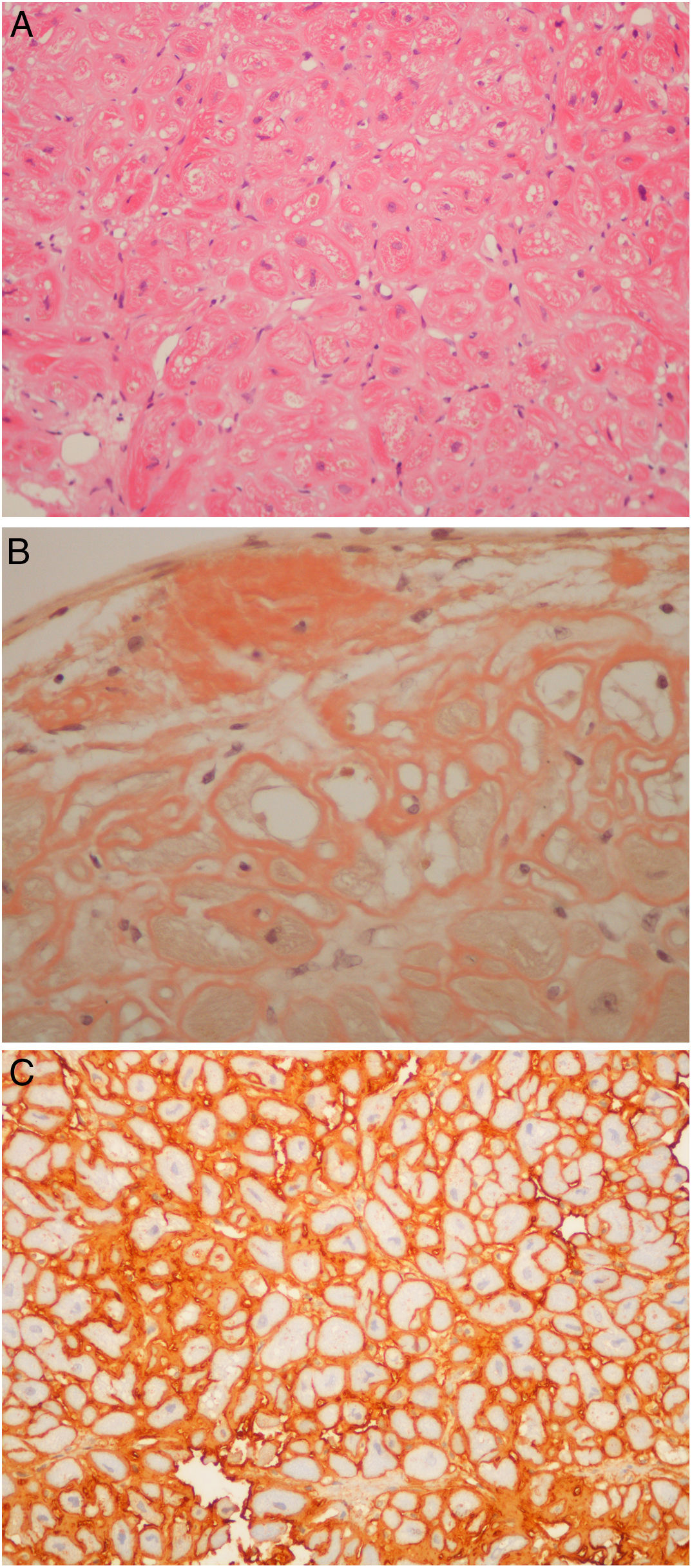
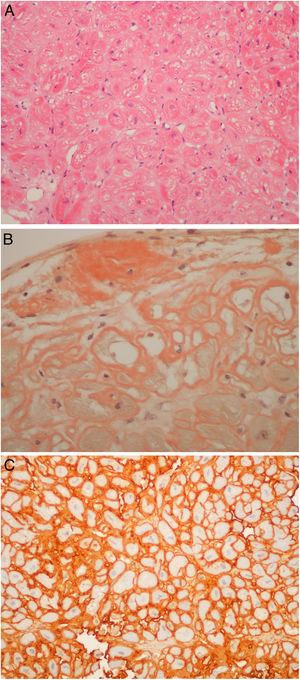
Regarding those patients who underwent the procedure in the setting of cardiomyopathy, there were two cases of apical hypertrophy with imaging and clinical features of concomitant amyloidosis. Therefore, the exact etiology of their cardiomyopathy was not fully understood. Their final diagnosis was familial transthyretin (ATTR) amyloidosis (V30M mutation) cardiomyopathy. Nine patients underwent endomyocardial biopsy due to amyloidosis (Figure 4A, and 4C) of unknown etiology after earlier studies, which included laboratory workup and an abdominal fat biopsy. The echocardiograms of eight patients were highly suggestive of amyloidosis. The final diagnosis was amyloid light-chain amyloidosis in four cases (resulting in hematology referral for the study of lymphoproliferative diseases), wild type ATTR amyloidosis in four patients. One patient was referred by another institution to the amyloidosis clinic because of an inconclusive echocardiogram together with a cardiac magnetic resonance raising the possibility of amyloidosis, which was ruled out in the cardiac biopsy. It is noteworthy that all six patients with ATTR amyloid deposits had their TTR gene sequenced.
(A) Light-chain cardiac amyloidosis. Hyalin widening of the interstitial space in the myocardial tissue (hematoxylin and eosin). (B) Extracellular amyloid deposits in the endocardium and surrounding the cardiomyocytes (Congo red staining). (C) Lambda light-chain immunostaining of the pericellular amyloid deposits (immunohistochemical method with an anti-lambda light-chain antibody).
Finally, the two previously mentioned patients, who underwent the transfemoral procedure, were also new-onset heart failure cases with cardiogenic shock after cardiac arrest; myocarditis was ruled out in both.
DiscussionIn our initial experience, transradial endomyocardial biopsy achieved excellent results, both regarding procedural success and complications. Our research is supported by an increasing amount of published data on this particular approach to endomyocardial biopsy.
Safety is the major concern when performing this technique, as many patients are often young (especially in the setting of myocarditis) and others have large ventricles with thin walls. We did not encounter safety issues, especially perforation, embolization or mitral regurgitation. This was likely the result of specific operator training both in radial intervention and LV biopsy, as well as technical safety precautions. We highlight the use of pressure monitoring, extensive back bleed and catheter aspiration, as well as peri and intra-procedural echocardiography guidance. All of these enable constant monitoring of the procedure and minimize risks.
As mentioned above, current guidelines regarding endomyocardial biopsy are now over a decade old and base their safety considerations on even older data.2 Indeed, these recommendations highlight a large series of transvenous RV biopsies with an overall 6% complication rate, a 1.2% possible or definite perforation rate and 0.4% death rate.14 Over the past decade, however, several authors from experienced European centers have published more extensive experience (ranging from 755 to 4221 procedures) totaling more than 8000 procedures.3,4,15 In addition, two of these publications provide extensive data on both left and RV biopsy, thereby providing clearer data.3,4 Overall, the published major and minor complication rate of these authors ranges from 0.12% to 0.82% and 1.35 to 5.2%, respectively, regarding RV biopsies.3,4,15 The rate of LV biopsy major and minor complications in LV biopsies was 0.33 to 0.64% and 2.2% to 2.89%, respectively.3,4 Importantly, there were no deaths in any of these series. Most major complications were perforation with subsequent tamponade. A very small number were cases of embolization. Several minor complications included bleeding or vascular-related.
These data provide valuable insights into safety. Firstly, the risk of the procedure when performed in experienced centers is low, and seemingly lower than previously published studies; secondly, LV biopsy seems at least as safe, if not safer, than RV biopsy.
Published transradial endomyocardial biopsy experience is quite recent. The earliest publications date back to 201412 and 2015.10,11 The largest series published to date was multicenter data comprising over 100 patients.13 In all of these recent case series, there were no major complications. Furthermore, the latter group published the largest radial versus femoral case series comparing both approaches, with 129 cases via radial and 134 cases via femoral access. There were no major complications and bleeding occurred exclusively in the transfemoral group.16
Our experience thus reflects the findings in contemporary data. Endomyocardial biopsy is a largely safe technique, all the more so when performed via the left ventricle, especially with the added safety benefits of the transradial approach.
In the series presented herein, the feasibility rate was 100%, with no cross-overs and only mild radial spasm observed in two patients. This was likely the result of patient selection, spasm prophylaxis administration, the use of hydrophilic catheters and the use of large size intra-luminal sheathless catheters, which allow for comfortable bioptome passage and have a reduced external caliper. Importantly, patient feedback was very positive and one patient, who had previously undergone a transjugular RV biopsy at another institution, actively voiced his preference for the transradial technique, because of improved comfort. When the procedure is performed electively, and if no complications occur, same-day discharge is possible. Our results are similar to previously published data. In fact, in the largest series published to date, cross-over to the femoral technique was required in only one patient (0.98%).13
The last issue regarding this technique is its diagnostic yield and clinical usefulness. In our experience, in which LV biopsy was always performed outside the setting of transplant, the results were clinically meaningful, as they added to the clinical question at hand. Indeed, the suspected diagnosis was either confirmed or ruled out, such as in the setting of myocarditis. Additionally, a doubtful diagnosis was clarified, such as the etiology of a cardiomyopathy, or amyloidosis infiltration and/or type in cases with predominant heart involvement and previously inconclusive studies. Finally, atypical forms of LV hypertrophy (i.e. apical) were observed in patients with ATTR, and tissue biopsy was most useful for confirming amyloidosis and ruling out hypertrophy.
Importantly, our pathologist's feedback was also very positive, as the quality and size of the samples was deemed excellent and better than when we had previously used the RV approach.
The abovementioned papers from the transfemoral era suggest that the LV approach is superior to the RV approach outside the transplant setting. Indeed, in the setting of myocarditis, the largest published series reports a 92.1% vs. 81.3% diagnostic yield,4 and in the setting of predominant LV disease, the difference is quite large and the LV approach is favored with 97.8% vs. 53%, respectively.3 Published data from the transradial approach do not allow for a comparison of left versus right approaches, however the conclusions are similar to ours: the results added to the clinical dilemma, confirming, excluding or clarifying a diagnostic hypothesis.13
Despite its advantages, the transradial LV endomyocardial biopsy technique is not without limitations. This approach requires the use of heparin, which may add to the risk of bleeding. In cases in which repeated biopsy is required, such as transplant patients, radial patency and the risk of vascular complications may become an issue with frequent procedures. In addition, the LV approach, while apparently less prone to perforation, regardless of the access site, may lead to a more dangerous scenario should perforation occur, given the much higher intracavitary pressure of the left ventricle. Also, the risk of cerebral embolization is essentially a concern of the left, rather than the right, approach, and thus it cannot be performed in the presence of LV intracavitary masses.
It is also important to point out the particular limitations of this paper. This an observational single-center study. Additionally, the sample size is relatively small given the single-center nature of the data. And finally, an ultrasound study of the radial artery was not performed after the procedure. Thus, although there was no clinical evidence of radial patency complications, these cannot be completely ruled out.
ConclusionTransradial LV endomyocardial biopsy provides a safe and feasible method of sampling the myocardium for histopathological analysis, with a good diagnostic yield and clinically meaningful results in properly selected patients. This technique should probably be the default method for endomyocardial biopsy in patients undergoing a single biopsy procedure outside the setting of cardiac transplant in a radial center.
Keypoints- -
What is known about the topic?
- ∘
The indications for endomyocardial biopsy are still debated, partly because of safety concerns and doubts about the clinical usefulness of the results;
- ∘
Recent papers suggest the safety of LV biopsy is superior to RV biopsy;
- ∘
Very recent data suggest that the transradial approach is a new and better method of sampling the left ventricle when compared to the transfemoral approach.
- -
What does this study add?
- ∘
Additional data confirming that transradial LV biopsy is feasible and safe;
- ∘
Clinical data explaining how the results were useful in a variety of clinical scenarios;
- ∘
Data to support the growing indications for endomyocardial biopsy.
The authors have no conflicts of interest to declare.