Some studies suggest that patients with low flow low gradient (LF-LG) aortic stenosis (AS) may achieve worse results after undergoing transcatheter aortic valve implantation (TAVI).
PurposeTo compare outcomes between LF-LG AS and high gradient (HG) AS patients submitted to TAVI.
MethodsInclusion of 480 consecutive patients who underwent TAVI between 2008 and 2020 at a single tertiary center. Patients were divided into high gradient AS and LF-LG AS; and baseline characteristics and outcomes after the procedure were collected and compared between groups.
ResultsPatients with LF-LG AS had worse baseline characteristics, with higher Society of Thoracic Surgeons score (p=0.008), New Euroscore II (p<0.0001), and NT pro-natriuretic peptide B (p=0.001), more frequent left ventricular ejection fraction (LVEF) <40% (p<0.0001), coronary artery disease (p<0.0001), including previous myocardial infarction (p=0.002) and coronary artery bypass graft (p<0.0001), poor vascular accesses (p=0.026) and periprocedural angioplasty (p=0.038). In a multivariate analysis, adjusted to differences in baseline characteristics, LF-LG AS was associated with worse functional class at one year (p=0.023) and in the long-term (p=0.004) and with heart failure hospitalizations at one year and in the long-term (p=0.001 and p<0.0001). In a sub-analysis including only patients with LF-LG AS, those with LVEF <40% had the worst outcomes, with more global (p=0.035) and cardiovascular (p=0.038) mortality.
ConclusionPatients with LF-LG AS have worse short and long-term outcomes, even when adjusted for baseline characteristic differences. The sub-group of patients with LVEF <40% have the most ominous global outcomes.
Estudos sugerem que doentes com estenose aórtica (EA) com baixo fluxo-baixo gradiente (BF-BG) têm piores resultados após implantação de válvula aórtica percutânea (VAP).
ObjetivoComparar resultados entre doentes com EA com BF-BG e gradiente elevado (GE) submetidos a VAP.
MétodosForam incluídos 480 doentes submetidos a VAP entre 2008 e 2020 num centro terciário. Os doentes foram divididos em EA BF-BG e GE e as características basais e os resultados após o procedimento foram comparados entre grupos.
ResultadosDoentes com EA BF-BG têm piores características basais, com valores mais elevados de STS score (p=0,008), New Euroscore II (p<0,0001), e NT pro-BNP (p=0,001), mais frequentemente fração de ejeção do ventrículo esquerdo (FEVE) <40% (p<0,0001), doença coronária (p<0,0001), incluindo enfarte do miocárdio (p=0,002) e cirurgia de revascularização (p<0,0001), maus acessos vasculares (p=0,026) e angioplastia coronária periprocedimento (p=0,038). Em análise multivariável, ajustando as diferenças nas características basais, a EA BF-BG associou-se a pior capacidade funcional a um ano (p=0,023) e longo prazo (p=0m004) e com hospitalização por insuficiência cardíaca (IC) a um ano e longo prazo (p=0,001 e p<0,0001). Numa subanálise incluindo apenas os doentes com EA BF-BG, aqueles com FEVE<40% tiveram os piores resultados, com mais mortalidade global (p=0,035) e cardiovascular (p=0,038).
ConclusãoOs doentes com EA BF-BG têm piores resultados em curto e longo prazo, mesmo quando ajustado para as diferenças nas características basais. O subgrupo de doentes com FEVE < 40% tem os piores resultados globais.
Transcatheter aortic valve implantation (TAVI) has transformed the treatment of patients with severe aortic stenosis (AS) and is the preferred treatment strategy in high risk patients.1 Since the first percutaneous aortic valve was implanted in 2002, there has been significant advances in TAVI technology, expanding its indications, leading to more patients being treated, including those at the extremes of the risk spectrum (from low to very high risk).2–6 The low flow low gradient (LF-LG) severe aortic stenosis (AS) subgroup of patients is usually categorized in the high risk group and these cases are often characterized by significant difficulties, including frequent delay in diagnosis and treatment.7 The literature suggests that despite having low gradients, these patients may actually have an advanced stage of the disease. The associated prognosis is dismal and mortality rates can reach 76% if not treated.7–11 Despite having worse outcomes than high gradient (HG) AS patients, several studies have shown that prognosis is improved with surgical aortic valve replacement (AVR).12–15 As surgery is high risk in these patients, TAVI has emerged as a very attractive treatment option. However, there is still a paucity and often contradictory data on long-term TAVI outcomes in this sub-group of patients.16–19 The aim of this study was to assess short and long-term TAVI outcomes in patients with LF-LG and compare them with the HG AS TAVI population.
Materials and methodsStudy populationBetween 2008 and 2020 all consecutive patients aged ≥18 years with symptomatic severe AS treated with TAVI at a single university center were prospectively included in a dedicated TAVI database. Patients with incomplete echocardiographic data, normal flow low gradient AS or aortic regurgitation predominance were then excluded from analysis.
The decision to perform TAVI was discussed by a heart team, considering both surgical AVR and TAVI risk. Patient evaluation included medical history, physical examination, 12-lead electrocardiography, blood analysis, transthoracic echocardiography (TTE), coronary angiography and cardiac, thoracic, abdominal and pelvic computed tomography (CT) with contrast.
EchocardiographyEchocardiographic measurements were obtained pre-TAVI and at hospital discharge. All images and measurements were acquired from the standard views and digitally stored for offline analysis. Left ventricle ejection fraction was calculated using the Simpson's Rule. Aortic valve (AV) velocity and gradients were assessed with Doppler TTE using the Bernoulli's principle. Stroke volume (SV) was measured using the velocity time integral in the LV outflow tract with pulsed-wave Doppler; then aortic valve area (AVA) was calculated by means of the continuity equation.
The diagnosis of severe AS was made according to the guidelines if:6,20
- -
AVA <1 cm2 or <0.6 cm2/m2 and a mean AV gradient ≥40 mmHg or peak velocity jet ≥4m/s (HG AS)
- -
AVA <1 cm2 or <0.6 cm2/m2 and a mean AV gradient <40 mmHg (LF-LG AS) if the following criteria were met:
- -
Classic LF-LG (cLF-LG) when LVEF <50% and:
- -
Dobutamine stress echocardiography with contractile reserve (increase ≥20% in SV) and a mean AV gradient increase for ≥40 mmHg maintaining an AVA <1 cm2 or
- -
AV score calcium >2000 Agatston Units (AU) in men or >1300 AU in women on CT.21
- -
- -
Paradoxical LF-LG (pLF-LG) when LVEF ≥50% and SV <35 mL/m2, if high AV score calcium as defined for cLF-LG.
- -
The anesthetic technique was individualized, and the procedure was performed under general anesthesia, continuous sedation or local anesthesia with no sedation. Transesophageal echocardiography guidance was not routinely used. The access route (transfemoral, trans-subclavian, transaortic or transcava) was selected according to the results of the CT angiography.
Post-procedural careAfter the procedure all patients were admitted to an intensive care unit for at least 24 hours. Data on procedural success and periprocedural complications were collected for each patient according to the Valve Academic Research Consortium 2 (VARC-2) criteria.22
Patients were treated with antithrombotic therapy, consisting of: dual antiplatelet therapy (acetylsalicylic acid plus clopidogrel) for one to three months, followed by simple antiplatelet therapy; or simple antiplatelet therapy if high bleeding risk; or oral anticoagulation if there was another clinical indication.
Follow-upClinical follow-up and post discharge events were analyzed at clinical visits, through phone contact and assessing medical records. Patients were followed up at one, six and 12 months at a TAVI outpatient clinic at our center, and at least yearly thereafter by an attending cardiologist. All clinical events were defined according to the VARC-2 criteria.22
Statistical analysisContinuous variables were reported as the mean (standard deviation) and were compared using the two-sample t-test. Categorical variables were expressed as frequencies and percentages and were compared using the chi-squared or Fisher's exact test, as appropriate.
Univariate Cox proportional hazard regression model with corresponding 95% confidence intervals was performed to assess the effect of LF-LG AS on multiple outcomes and a multivariate Cox regression model was used to assess the same effect with adjustment for differences in baseline characteristics.
A propensity score matching analysis was performed to control for the possible bias induced by the heterogenicity among the groups’ baseline characteristics, which may influence the occurrence of LF-LG AS. The propensity scores were estimated for each patient using a multivariable logistic regression and patients were then matched 2:1 using the nearest neighbor method and similarity between baseline characteristics in this matched population confirmed. Then the effect of LF-LG AS on the outcomes was assessed using the Cox regression model.
A sub-analysis of the LF-LG AS group to compare outcomes in reduced ejection fraction (rEF), LVEF<40% vs. preserved or mildly reduced ejection fraction (pEF) LVEF≥40%, considering the cut-off for LVEF used in heart failure (HF) guidelines,23 was employed with the same statistical methods.
A two-sided p value <0.05 was considered significant. Statistical analysis was performed with SPSS for Windows version 26 (IBM Corp, Armonk, New York).
ResultsDuring the enrollment period, a total of 517 patients underwent TAVI at our center. Of these, 37 patients were excluded from the analysis: 21 had incomplete echocardiographic data, 10 had normal flow low gradient AS, five had an aortic bioprothesis disfunction, with a predominance of regurgitation, and one patient had moderate AS with severe aortic regurgitation). A total of 480 patients were included in the statistical analysis.
Baseline and procedural characteristicsBaseline demographic, echocardiographic, tomographic, laboratorial and procedural characteristics are shown in Table 1. Of 81 patients (16.9%) with LF-LG AS, 39 had pLF-LG and 42 had cLF-LG. A dobutamine stress echocardiography was performed in five of them, all with contractile reserve and mean gradient rising to ≥40 mmHg). Patients with LF-LG AS had worse baseline characteristics, a higher Society of Thoracic Surgeons score and New Euroscore II risk, higher NT pro natriuretic peptide B, more frequent LVEF <40% and tricuspid annular plane systolic excursion <17 mm, coronary artery disease (including previous myocardial infarction and coronary artery bypass graft), poor vascular accesses and periprocedural angioplasty. Periprocedural complications were similar between groups (14.8% in LF-LG and 16.3% in HG, p=0.741). After the procedure, 56.4% of patients with initial rEF saw an improvement to ≥40%, but a significant difference was still found in mean LVEF pre-hospital discharge (47% in LF-LG vs. 55% in HG patients, p<0.0001) between groups.
Baseline demographic, echocardiographic, tomographic, laboratorial and procedural characteristics of all patients.
| All patients (n=480) | LF-LG patients (n=81) | HG patients (n=399) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), mean (SD) | 82 (7) | 81 (6) | 82 (7) | 0,314 |
| Male (n, %) | 207 (43,1) | 40 (49,4) | 167 (41,9) | 0,212 |
| Body mass index (kg/m2), mean (SD) | 27,06 (4,62) | 26,46 (3,90) | 27,19 (4,74) | 0,141 |
| Hypertension (n, %) | 402 (83,8) | 68 (84,0) | 334 (83,7) | 0,957 |
| Diabetes mellitus (n, %) | 174 (36,3) | 28 (34,6) | 146 (36,6) | 0,730 |
| Dyslipidemia (n, %) | 327 (68,1) | 59 (72,8) | 268 (67,2) | 0,318 |
| Smoker (n, %) | 60 (12,5) | 13 (16,0) | 47 (11,8) | 0,289 |
| Coronary artery disease (n, %) | 194 (40,5) | 47 (58,0) | 149 (36,9) | <0,0001 |
| Previous myocardial infarction (n, %) | 79 (16,5) | 23 (28,4) | 56 (14,1) | 0,002 |
| Previous percutaneous angioplasty (n, %) | 104 (21,7) | 23 (28,4) | 81 (20,3) | 0,107 |
| Previous CABG (n, %) | 73 (15,2) | 24 (29,6) | 49 (12,3) | <0,0001 |
| Previous valvular surgery (n, %) | 29 (6,0) | 7 (8,6) | 22 (5,5) | 0,281 |
| Peripheral artery disease (n, %) | 82 (17,1) | 18 (22,2) | 64 (16,0) | 0,178 |
| Chronic kidney disease (n, %) | 241 (50,2) | 44 (54,3) | 197 (49,4) | 0,417 |
| COPD (n, %) | 114 (23,8) | 21 (25,9) | 93 (23,3) | 0,614 |
| Pacemaker (n, %) | 39 (8,1) | 10 (12,3) | 29 (7,3) | 0,127 |
| Atrial fibrillation (n, %) | 165 (34,4) | 35 (43,2) | 130 (32,6) | 0,066 |
| NYHA III-IV (n, %) | 349 (72,7) | 61 (75,3) | 288 (75,2) | 0,564 |
| New Euroscore II (%), mean (SD) | 6,95 (6,83) | 10,43 (9,02) | 6,25 (6,08) | <0,0001 |
| STS score (%), mean (SD) | 5,94 (4,65) | 7,69 (4,49) | 5,60 (4,12) | 0,008 |
| Beta-Blocker (n, %) | 231 (48,2) | 54 (67,5) | 177 (44,4) | <0,0001 |
| Diuretic (n, %) | 353 (73,7) | 64 (80,0) | 289 (72,4) | 0,161 |
| ACEI/ARB (n, %) | 340 (71,0) | 55 (68,8) | 285 (71,4) | 0,630 |
| Echocardiography | ||||
| Maximum aortic valve gradient (mmHg), mean (SD) | 83 (25) | 56 (28) | 89 (20) | <0,0001 |
| Mean aortic valve gradient (mmHg), mean (SD) | 52 (15) | 31 (6) | 56 (13) | <0,0001 |
| Aortic valve area (cm2), mean (SD) | 0,68 (0,21) | 0,75 (0,18) | 0,66 (0,21) | 0,001 |
| LVEF <40% (n, %) | 62 (12,9) | 27 (33,3) | 35 (8,8) | <0,0001 |
| LVEF (%), mean (SD) | 53 (11) | 46 (14) | 54 (9) | <0,0001 |
| TAPSE <17mm (n, %) | 42 (9,2) | 16 (21,1) | 26 (6,8) | <0,0001 |
| PASP (mmHg), mean (SD) | 44 (13) | 44 (13) | 44 (13) | 0,889 |
| Aortic regurgitation (moderate to severe) (n, %) | 76 (16,1) | 15 (19,0) | 61 (15,5) | 0,439 |
| Mitral regurgitation (moderate to severe) (n, %) | 83 (17,6) | 15 (19,2) | 68 (17,3) | 0,676 |
| Tricuspid regurgitation (moderate to severe (n, %) | 61 (13,1) | 14 (18,2) | 47 (12,1) | 0,145 |
| Bicuspid aortic valve (n, %) | 16 (3,6) | 4 (5,3) | 12 (3,3) | 0,402 |
| Cardiac angio-CT | ||||
| Aortic valve calcium score (AU), mean (SD) | 2524 (1510) | 1846 (1338) | 2658 (1508) | <0,0001 |
| Poor vascular accesses (n, %) | 44 (10,5) | 13 (17,8) | 31 (9,0) | 0,026 |
| Blood analysis | ||||
| Hemoglobin (g/dL), mean (SD) | 12,0 (1,8) | 12,2 (2,0) | 11,9 (1,8) | 0,323 |
| Creatinine (mg/dL), mean (SD) | 1,2 (0,7) | 1,30 (0,66) | 1,19 (0,69) | 0,205 |
| NTproNPB (pg/mL), mean (SD)* | 4480 (6856) | 11252 (13122) | 3095 (3640) | 0,001 |
| NPB (pg/mL), mean (SD)** | 754 (1209) | 1139 (1801) | 676 (1036) | 0,004 |
| Procedural Characteristics | ||||
| Transfemoral route (n, %) | 447 (93,3) | 74 (92,5) | 373 (93,5) | 0,748 |
| Pre dilation (n, %) | 173 (56,4) | 25 (42,4) | 148 (59,7) | 0,016 |
| Pos dilation (n, %) | 150 (33,1) | 23 (29,9) | 127 (33,8) | 0,507 |
| Valve in Valve (n, %) | 16 (3,3) | 3 (3,7) | 13 (3,3) | 0,839 |
| General anesthesia (n, %) | 215 (45,6) | 38 (47,5) | 177 (45,3) | 0,715 |
| Periprocedural coronary angioplasty (n, %) | 25 (11,7) | 8 (21,6) | 17 (9,6) | 0,038 |
ACEI: angiotensin converser enzyme inhibitor; ARB: aldosterone receptor blocker; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; LVEF: left ventricle ejection fraction; NTproPNB: N-terminal pro-B type natriuretic peptide; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; SD: standard deviation; TAPSE: tricuspid annular plane systolic excursion.
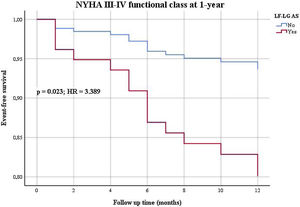
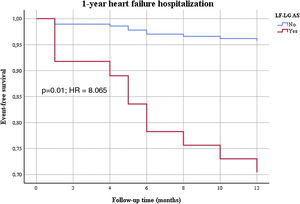
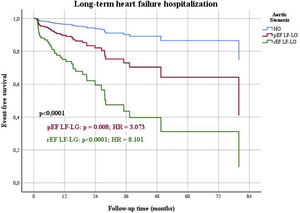
Patients were followed for a mean period of 21±21 months (minimum 0 and maximum 112 months). In univariable analysis (Table 2), LF-LG AS was associated with worse one year mortality, one year and long-term functional class, and one year and long-term heart HF hospitalizations. When adjusted to the differences in baseline characteristics, in a Cox regression multivariable analysis (Table 3), LF-LG AS was still associated with worse functional class at one year (Figure 1) and in the long-term, and with one year (Figure 2) and long-term HF hospitalizations.
Differences in outcomes after transcatheter aortic valve implantation according to high gradient aortic stenosis versus low flow low grade aortic stenosis.
| All patients (n=480) | LF-LG patients (n=81) | HG patients (n=399) | p value | |
|---|---|---|---|---|
| Global mortality+HF hospitalization (n, %) | 220(45.8) | 47(58.0) | 173(43.4) | 0.016 |
| 1-year mortality+HF hospitalization (n, %) | 65(14.7) | 22(29.3) | 43(11.7) | <0.0001 |
| Global mortality (n, %) | 125(26.1) | 23(28.4) | 102(25.6) | 0.605 |
| Intra-hospital mortality (n, %) | 29(6.0) | 5(6.2) | 24(6.0) | 0.957 |
| 30-day mortality (n, %) | 30(6.3) | 6(7.4) | 24(6.0) | 0.637 |
| 1-year mortality (n, %) | 56(12.5) | 16(21.3) | 40(10.8) | 0.012 |
| Long-term mortality (n, %) | 94(21.2) | 20(26.7) | 74(20.1) | 0.205 |
| 1-year HF hospitalization (n, %) | 25(5.7) | 13(17.6) | 12(3.3) | <0.0001 |
| Long-term HF hospitalization (n, %) | 41(9.3) | 18(24.3) | 23(6.3) | <0.0001 |
| 1-year NYHA III-IV (n, %) | 27(6.2) | 13(17.8) | 14(3.8) | <0.0001 |
| Long-term NYHA III-IV (n, %) | 37(8.4) | 15(20.5) | 22(6.0) | <0.0001 |
HF: heart failure; NYHA: New York Heart Association.
Univariate and multivariate Cox regression analysis of outcomes after transcatheter aortic valve implantation according to high gradient versus low flow low gradient aortic stenosis.
| Univariable analysis | Multivariable analysis* | |||||
|---|---|---|---|---|---|---|
| p-value | HR | CI 95% | p-value | Adjusted HR | CI 95% | |
| Global mortality+HF hospitalization (n, %) | <0.0001 | 1.862 | 1.345-2.578 | 0.059 | - | - |
| 1-year mortality+HF hospitalization (n, %) | <0.0001 | 2.862 | 1.691-4.844 | 0.017 | 3.354 | 1.245-9.033 |
| 1-year mortality (n, %) | 0.041 | 1.888 | 1.025-3.477 | 0.246 | - | - |
| 1-year HF hospitalization (n, %) | <0.001 | 6.456 | 2.890-14.423 | 0.001 | 8.065 | 2.457-26.472 |
| Long-term HF hospitalization (n, %) | <0.0001 | 5.068 | 2.717-9.453 | <0.0001 | 7.980 | 2.485-25.628 |
| 1-year NYHA III-IV (n, %) | <0.0001 | 5.098 | 2.395-10.852 | 0.023 | 3.389 | 1.186-9.680 |
| Long-term NYHA III-IV (n, %) | <0.0001 | 4.142 | 2.141-8.014 | 0.004 | 5.063 | 1.701-15.069 |
CI: confidence interval; HF: heart failure; HR: hazard ratio; NYHA: New York Heart Association.
After propensity score matching (121 HG and 79 LF-LG AS patients), the outcomes remained very similar, with worse functional class at one year (p=0.033) and long-term (p=0.018), and with one year (p=0.032) and long-term (p=0.005) HF hospitalizations.
Sub-analysis of low flow low gradient aortic stenosis patientsIn a first sub-analysis considering only patients with LF-LG, despite significant differences in baseline characteristics in cLF-LG and pLF-LG, there were no differences when outcomes (mortality, HF hospitalizations and functional class) were compared between these two sub-groups.
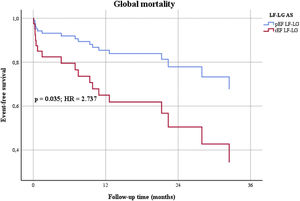
However, when patients were sub-divided considering LVEF ≥ or <40% (pEF or rEF) significant differences were found. Patients with rEF were younger and had worse baseline characteristics, with higher risk scores and natriuretic peptides. They were more commonly smokers and had peripheral artery disease more frequently (Table 4). There were no differences in periprocedural complication rates (22.2% in rEF versus 11.1% in pEF, p=0.185). In the sub-group of patients with rEF, there was an improvement in mean LVEF from 28% to 34%, but it remained inferior to pEF patients (53%, p<0.0001). About one third of rEF patients recovered LVEF≥40%. Despite similar procedural success and younger age, after multivariable Cox regression analysis (Tables 5 and 6), adjusting for the different baseline characteristics, there were significant differences in global (Figure 3) and cardiovascular mortality.
Baseline demographic, echocardiographic, tomographic, laboratorial and procedural characteristics of LF-LG AS patients.
| All LF-LG (n=81) | rEF LF-LG (n=27) | pEF LF-LG (n=54) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), mean(SD) | 81(6) | 78(7) | 82(6) | 0.005 |
| Male (n, %) | 40(49.4) | 17(63.0) | 23(42.6) | 0.084 |
| Body mass index (kg/m2), mean(SD) | 26.46(3.90) | 25.69(3.72) | 26.84(3.97) | 0.212 |
| Hypertension (n, %) | 68(84.0) | 20(74.1) | 48(88.9) | 0.087 |
| Diabetes mellitus (n, %) | 28(34.6) | 11(40.7) | 17(31.5) | 0.409 |
| Dyslipidemia (n, %) | 59(72.8) | 19(70.4) | 40(74.1) | 0.724 |
| Smoker (n, %) | 13(16.0) | 9(33.3) | 4(7.4) | 0.003 |
| Coronary artery disease (n, %) | 47(58.0) | 19(70.4) | 28(51.9) | 0.111 |
| Previous myocardial infarction (n, %) | 23(28.4) | 11(40.7) | 12(22.2) | 0.081 |
| Previous percutaneous angioplasty (n, %) | 23(28.4) | 10(37.0) | 13(24.1) | 0.223 |
| Previous CABG (n, %) | 24(29.6) | 9(33.3) | 15(27.8) | 0.606 |
| Previous valvular surgery (n, %) | 7(8.6) | 2(7.4) | 5(9.3) | 1.000 |
| Peripheral artery disease (n, %) | 18(22.2) | 12(44.4) | 6(11.1) | 0.001 |
| Chronic kidney disease (n, %) | 44(54.3) | 18(66.7) | 26(48.1) | 0.115 |
| COPD (n, %) | 21(25.9) | 8(29.6) | 13(24.1) | 0.591 |
| Pacemaker (n, %) | 10(12.3) | 5(18.5) | 5(9.3) | 0.232 |
| Atrial fibrillation (n, %) | 35(43.2) | 10(37.0) | 25(46.3) | 0.428 |
| NYHA III-IV (n, %) | 61(75.3) | 24(88.9) | 37(68.5) | 0.057 |
| New Euroscore II (%), mean(SD) | 10.43(9.02) | 15.90(10.95) | 7.64(6.33) | 0.001 |
| STS score (%), mean(SD) | 7.69(4.49) | 10.03(7.11) | 6.48(5.85) | 0.022 |
| Beta-Blocker (n, %) | 54(67.5) | 20(76.9) | 34(63.0) | 0.212 |
| Diuretic (n, %) | 64(80.0) | 22(84.6) | 42(77.8) | 0.562 |
| ACEI/ARB (n, %) | 55(68.8) | 19(73.1) | 36(66.7) | 0.562 |
| Ecochardiography | ||||
| Maximum aortic valve gradient (mmHg), mean(SD) | 56(28) | 47(10) | 60(32) | 0.054 |
| Mean aortic valve gradient (mmHg), mean(SD) | 31(6) | 29(6) | 33(6) | 0.004 |
| Aortic valve area (cm2), mean(SD) | 0.75(0.18) | 0.70(0.18) | 0.77(0.19) | 0.136 |
| LVEF (%), mean(SD) | 46(14) | 28(8) | 54(8) | <0.0001 |
| TAPSE <17mm (n, %) | 16(21.1) | 11(45.8) | 5(9.6) | <0.0001 |
| PASP (mmHg), mean(SD) | 44(13) | 44(12) | 44(14) | 0.916 |
| Aortic regurgitation (moderate to severe) (n, %) | 15(19.0) | 5(19.2) | 10(18.9) | 0.969 |
| Mitral regurgitation (moderate to severe) (n, %) | 15(19.2) | 8(30.8) | 7(13.5) | 0.067 |
| Tricuspid regurgitation (moderate to severe) (n,%) | 14(18.2) | 3(11.5) | 11(21.6) | 0.360 |
| Bicuspid aortic valve (n, %) | 4(5.3) | 2(8.0) | 2(3.9) | 0.594 |
| Cardiac angio-CT | ||||
| Aortic valve calcium score (AU), mean(SD) | 1846(1338) | 2076(1726) | 1746(1146) | 0.416 |
| Poor vascular accesses (n, %) | 13(17.8) | 6(28.6) | 7(13.5) | 0.127 |
| Blood analysis | ||||
| Hemoglobin (g/dL), mean(SD) | 12.2(2.0) | 11.9(2.0) | 12.3(2.0) | 0.389 |
| Creatinine (mg/dL), mean(SD) | 1.30(0.66) | 1.44(0.74) | 1.23(0.62) | 0.191 |
| NTproNPB (pg/mL), mean(SD)* | 11 252(13 122) | 21 943(11 405) | 5907(10 981) | 0.080 |
| NPB (pg/mL), mean(SD)** | 1139(1801) | 2163(2607) | 592(763) | 0.008 |
| Procedural Characteristics | ||||
| Transfemoral route (n, %) | 74(92.5) | 23(88.5) | 51(94.4) | 0.384 |
| Pre dilation (n, %) | 25(42.4) | 7(38.9) | 18(43.9) | 0.720 |
| Pos dilation (n, %) | 23(29.9) | 5(19.2) | 18(35.3) | 0.145 |
| Valve in Valve (n, %) | 3(3.7) | 1(3.7) | 2(3.7) | 1.000 |
| General anesthesia (n, %) | 38(47.5) | 13(50.0) | 25(46.3) | 0.756 |
| Periprocedural coronary angioplasty (n, %) | 8(21.6) | 2(15.4) | 6(25.0) | 0.685 |
ACEI: angiotensin converser enzyme inhibitor; ARB: aldosterone receptor blocker; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; LVEF: left ventricle ejection fraction; NTproNPB: N terminalis pro natriuretic peptide B; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; SD: standard deviation; TAPSE: tricuspid annular plane systolic excursion. *used since 2020; NPB: Natriuretic peptide B, **used until 2019.
Differences in outcomes after transcatheter aortic valve replacement according to reduced ejection fraction low flow low gradient versus preserved ejection fraction low flow low gradient aortic stenosis.
| All LF-LG (n=81) | rEF LF-LG (n=27) | pEF LF-LG (n=54) | p-value | |
|---|---|---|---|---|
| Global mortality+HF hospitalization (n, %) | 47(58.0) | 19(70.4) | 28(51.9) | 0.111 |
| 1-year mortality+HF hospitalization (n, %) | 22(29.3) | 9(40.9) | 13(24.5) | 0.156 |
| Global mortality (n, %) | 23(28.4) | 13(48.1) | 10(18.5) | 0.005 |
| Cardiovascular mortality (n, %) | 11(13.6) | 7(25.9) | 4(7.4) | 0.036 |
| Intra-hospital mortality (n, %) | 5(6.2) | 4(14.8) | 1(1.9) | 0.040 |
| 30-day mortality (n, %) | 6(7.4) | 5(18.5) | 1(1.9) | 0.014 |
| 1-year mortality (n, %) | 16(21.3) | 5(22.7) | 11(20.8) | 0.849 |
| Long-term mortality (n, %) | 20(26.7) | 6(27.3) | 14(26.4) | 0.939 |
| 1-year HF hospitalization (n, %) | 13(17.6) | 6(27.3) | 7(13.5) | 0.154 |
| Long-term HF hospitalization (n, %) | 18(24.3) | 9(40.9) | 9(17.3) | 0.031 |
| 1-year NYHA III-IV (n, %) | 13(17.8) | 7(31.8) | 6(11.8) | 0.040 |
| Long-term NYHA III-IV (n, %) | 15(20.5) | 7(31.8) | 8(15.7) | 0.118 |
HF: heart failure; NYHA: New York Heart Association.
Univariate and multivariate Cox regression analysis of outcomes after transcatheter aortic valve implantation in reduced ejection fraction low flow low gradient versus preserved ejection fraction low flow low gradient aortic stenosis.
| Univariable analysis | Multivariable analysis* | |||||
|---|---|---|---|---|---|---|
| p-value | HR | CI 95% | p-value | Adjusted HR | CI 95% | |
| Global mortality (n, %) | 0.007 | 3.138 | 1.373-7.172 | 0.035 | 2.737 | 1.071-7.124 |
| Cardiovascular mortality (n, %) | 0.023 | 4.191 | 1.224-14.351 | 0.038 | 4.340 | 1.081-17.420 |
| Intra-hospital mortality (n, %) | 0.159 | - | - | - | - | - |
| 30-day mortality (n, %) | 0.030 | 10.829 | 1.265-92.721 | 0.681 | - | - |
| 30-day cardiovascular mortality (n, %) | 0.221 | - | - | - | - | - |
| Long-term HF hospitalization (n, %) | 0.032 | 2.881 | 1.098-7.559 | 0.078 | - | - |
| 1-year NYHA III-IV (n, %) | 0.040 | 3.136 | 1.052-9.351 | 0.189 | - | - |
CI; confidence interval; HF: heart failure; HR: hazard ratio; NYHA: New York Heart Association.
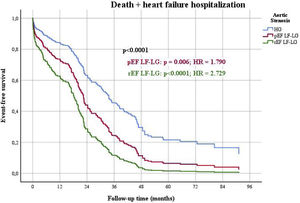
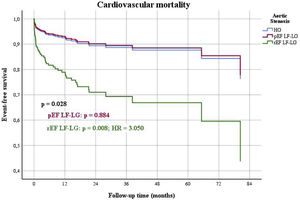
When outcomes were compared among the three groups of patients (HG as control, pEF LF-LG and rEF LF-LG), in a multivariable Cox regression analysis (Table 7), there were significant differences in the composite end-point of death and HF hospitalizations (Figure 4), mostly due to HF hospitalizations which were significantly different among the three groups (Figure 5), while global and cardiovascular mortality were only significantly different in the rEF LF-LG sub-group (Figure 6).
Multivariate Cox regression analysis of outcomes after transcatheter aortic valve replacement according to high gradient aortic stenosis versus preserved ejection fraction low flow low gradient and reduced ejection fraction low flow low gradient aortic stenosis.
| p for the model | p for pEF LF-LG | HR | CI 95% | p for rEF LF-LG | HR | CI 95% | |
|---|---|---|---|---|---|---|---|
| Global mortality+HF hospitalization (n, %) | <0.0001 | 0.006 | 1.790 | 1.184-2.704 | <0.0001 | 2.729 | 1.647-4.522 |
| 1-year mortality+HF hospitalization (n, %) | <0.0001 | 0.009 | 2.359 | 1.235-4.507 | 0.001 | 4.160 | 1.860-9.307 |
| Global mortality (n, %) | 0.103 | 0.999 | - | - | 0.034 | 2.142 | 1.058-4.338 |
| Global cardiovascular mortality (n, %) | 0.028 | 0.884 | - | - | 0.008 | 3.050 | 1.333-6.976 |
| 1-year HF hospitalization (n, %) | <0.0001 | 0.001 | 4.810 | 1.864-12.415 | <0.0001 | 12.151 | 4.486-32.909 |
| Long-term HF hospitalization (n, %) | <0.0001 | 0.008 | 3.073 | 1.346-7.016 | <0.0001 | 8.101 | 3.579-18.339 |
| 1-year NYHA III-IV (n, %) | <0.0001 | 0.031 | 2.881 | 1.098-7.554 | <0.0001 | 8.900 | 3.490-22.695 |
| Long-term NYHA III-IV (n, %) | <0.0001 | 0.037 | 2.505 | 1.057-5.935 | <0.0001 | 6.224 | 2.569-15.079 |
HF: heart failure; NYHA: New York Heart Association.
Included in the analysis: coronary artery disease, previous myocardial infarction, coronary artery bypass graft, peripheral artery disease, smoking, beta-blockers, TAPSE<17 mm.
Multivariate Cox regression analysis for death+heart failure hospitalizations in high gradient versus preserved ejection fraction low flow low gradient and reduced ejection fraction low flow low gradient aortic stenosis. pEF: preserved ejection fraction; rEF: reduced ejection fraction; LF-LG: low flow low gradient; HG: high gradient; HR: hazard ratio.
In this study, we showed that patients with LF-LG AS had a significantly worse prognosis after TAVI when compared to HG AS. No differences were found in outcomes when comparing pLF-LG with cLF-LG (considering the definition in valvular heart disease guidelines,20 with a LVEF cut-off of 50%). The most ominous outcomes were found in the rEF (LVEF<40%) sub-group of patients.
Previous studies have revealed a mortality and clinical functional status benefit from aortic valve replacement (AVR) in patients with LF-LG AS, compared to medical treatment alone, even in patients with low ejection fraction.11–13,15,23,24 Mortality is still high in this sub-group of patients, with a described peri-operative mortality of 16-22%, one year mortality of approximately 20% and long-term mortality of 22-50%.12–14,24
Considering this scenario, it has been suggested that TAVI could be an acceptable option for this high surgical risk group. However, data comparing TAVI versus medical treatment and TAVI versus surgery are scarce in this specific population, and there are still some concerns about TAVI results in patients with LF-LG AS compared with HG AS. There are some discrepancies in the published studies, with most of them showing worse outcomes in LF-LG, but with little data suggesting similar results, especially when comparing pLF-LG with HG.11,17–19,25–30 In our study, we performed a comprehensive assessment of TAVI outcomes in patients with LF-LG AS versus HG AS, including mortality rates, HF hospitalizations and NYHA functional class, and a sub-analysis of the same outcomes in rEF LF-LG AS versus pEF LF-LG AS. In line with previously published data, our study had a relatively high rate of patients with LF-LG AS (16.9%), and is consistent with most reports, showing worse prognosis in patients with LF-LG AS compared to HG AS, particularly concerning HF hospitalizations and functional status.11,17,18,25–28 This is particularly evident in the sub-group of patients with low ejection fraction, which has significantly higher mortality rates.
It can be hypothesized that LF-LG AS patients have worse outcomes because they represent a more advanced stage of the valvular disease. Other explanation could be the concomitant presence of AS and intrinsic myocardial disease, especially in patients with rEF LF-LG, who may already have low LVEF independent of AS or in whom the intrinsic myocardiopathy would mean the left ventricle is unable to deal with the increased overload.28 In fact, Ben-Dor et al. showed that the rEF LF-LG group of patients had more frequently associated conditions that adversely affect LV function, such as previous myocardial infarction, diabetes mellitus and renal disfunction.28 In our study, we also found a statistically significant difference concerning previous myocardial infarction between patients with rEF LF-LG and pEF LF-LG AS. There is also the possibility that some of these patients did not actually have severe AS, in whom the prognosis was determined by intrinsic cardiomyopathy resulting in a slight or no benefit from valve intervention. Identifying this restricted sub-group of patients could be challenging but would mean targeting the suitable treatment at the intrinsic cardiomyopathy, not exposing patients to the risks of complications associated with a futile TAVI procedure.
Despite the worse outcomes when compared to HG AS, also in line with other studies, our data suggest that TAVI in these high risk sub-group of patients may have a global beneficial effect.11,16,25,27,28 In fact, although caution should be taken when comparing results from different studies with different populations, in the TAVI procedure long-term mortality is lower (26.7% in LF-LG AS and 27.3% in patients with reduced LVEF) than that described for medical treatment.8–11 When compared to the results described in literature with the alternative surgical therapy, TAVI is associated with lower peri-procedural mortality (6.2%) and similar one year and long-term mortality.12–15,24 Moreover, in the sub-group of patients with rEF LF-LG, there was an improvement in LVEF right after the procedure, with approximately one third of the patients recovering the function >40%. These results are also described in other studies.11,25,28,31
These good global results suggest that TAVI may be a viable option to consider when treating LF-LG patients. However, an effort is required to identify the limited number of patients who would not benefit from this therapeutic approach.
LimitationsThis study is a single-center retrospective data analysis with its inherent limitations. Although we made a simple comparison with published results, we did not use medical treatment and surgical AVR groups. As we had a limited number of patients with rEF LF-LG AS that had LVEF>40% after the procedure, we were unable to determine predictors of LVEF recovery and to compare outcomes in these cases, which could be helpful when selecting patient for intervention.
ConclusionPatients with LF-LG AS who underwent TAVI have worse prognosis when compared to HG AS, particularly when they have reduced EF. Despite this, mortality is lower than described in the literature for medical treatment alone, and peri-procedural mortality is inferior to that described for surgical AVR. Furthermore, a significant clinical improvement occurs, including a recovery of LVEF in one third of rEF LF-LG patients. More studies are needed to improve the selection of patients with LF-LG who will undergo TAVI, surgical AVR or medical therapy.
Conflicts of interestThe authors have no conflicts of interest to declare.