Transapical off-pump NeoChord DS1000™ implantation is a minimally invasive surgical mitral valve repair (MVr) procedure to treat degenerative mitral regurgitation (MR), which is performed using the NeoChord DS1000™ system with two and three-dimensional transesophageal echocardiographic guidance on a beating heart. It has been demonstrated to be safe and effective in carefully selected patients.
ObjectiveThe authors aim to analyze short-term clinical and echocardiographic results after mitral valve repair using the NeoChord™ system.
MethodsAll patients that underwent transapical off-pump mitral valve repair with NeoChord™ implantation at our center, between December 2017 and December 2019, were included. The procedure was performed by left minithoracotomy, under general anesthesia. All patients presented severe primary MR due to flail/prolapse of one leaflet (anterior or posterior).
ResultsEighteen patients were included in the analysis, the mean age was 65±15 years, 72% were male. The mean EuroSCORE II was 1.9±1.6. All patients had New York Heart Association (NYHA) class ≥ II. Mean effective regurgitant orifice area was 1.0±0.4 cm2, with a mean regurgitant volume 146±42 mL, and a mean leaflet-to-annulus index of 1.29±0.14. MR was due to leaflet prolapse in 50% (N=9), and flail leaflet in 50% (N=9). Anatomic type A (isolated P2 defect) was the predominant form in 66.5% (N=12). Successful repair, defined by none, trace or mild mitral regurgitation, by implantation of two to four neochordae, was achieved in all 18 patients. No major complications arose intra-procedurally. The median follow-up was 194 days. NYHA class was ≤II in 94.5% patients at six-month follow-up, which represented a significant improvement in symptomatic status (p=0.002). At follow-up, 72% of patients (N=13) had grade ≤2 MR. There was a significant reduction in mean indexed left atrium volume (63±7 mL/m2 vs. 45±6 mL/m2, p=0.038), mean indexed left ventricular end-diastolic volume (87±7 mL/m2 vs. 79±9 ml/m2, p=0.001), and pulmonary arterial systolic pressure (44±4 vs. 31±8 mmHg, p=0.002). The re-intervention rate was 11.1% (N=2, both patients underwent reintervention, either a re-do NeoChord™ or conventional MV repair on-pump surgery). No major adverse cardiac or cerebrovascular events were registered.
ConclusionsIn selected patients, minimally invasive MVr using the NeoChord™ system is safe, effective and reproducible. Early clinical and echocardiographic results suggest a significant symptomatic improvement, sustained MR grade decrease, and favorable left cardiac chamber remodeling, with low re-intervention rates. These results warrant further confirmation in larger cohorts, on longer period of follow-up.
A implantação de neocordas por via transapical sem circulação extracorporal é uma técnica minimamente invasiva de reparação valvular mitral (RVM). É utilizada no tratamento de insuficiência mitral (IM) degenerativa, com recurso ao dispositivo NeoChord DS1000™. O procedimento é realizado sob orientação de ecocardiografia transesofáfica (ETE) 2D e 3D e demonstrou ser seguro e eficaz em doentes cuidadosamente selecionados.
ObjetivoOs autores pretendem analisar os resultados clínicos e ecocardiográficos a curto prazo da RVM minimamente invasiva com o sistema NeoChord™.
MetodosForam incluídos todos os doentes submetidos a RVM com implantação de neocordas por via transapical no nosso centro, entre dezembro de 2017 e dezembro de 2019. O procedimento foi realizado por minitoracotomia esquerda, sob anestesia geral. Todos os doentes apresentaram IM primária grave por flail ou prolapso de um dos folhetos da válvula mitral.
ResultadosDezoito doentes foram incluídos na análise, com uma idade média de 65±15 anos, 72% do sexo masculino. O score EuroSCORE II médio foi 1,9±1,6. Todos os doentes tinham classe funcional NYHA ≥ II. O EROA médio foi de 1,0±0,4 cm2, com um volume regurgitante médio de 146±42mL e um índice folheto-anel de 1,29±0,14. A causa da IM foi prolapso de um dos folhetos em 50% dos doentes (N=9) e flail de um dos folhetos em 50% (N=9). O tipo anatómico A (defeito isolado de P2) foi a forma predominante, em 66,5% dos casos (N=12). O sucesso do procedimento, definido por IM mínima ou ligeira com implantação de 2 a 4 neocordas, foi atingido em todos os doentes. Não se registaram complicações major intraprocedimento. O follow-up (F-UP) médio foi de 194 dias. A classe de NYHA foi ≤ II em 94,5% dos doentes aos seis meses de F-UP, representando uma melhoria significativa comparativamente à classe funcional basal (p=0,002). Ao longo do F-UP, 72% dos doentes (N=13) mantiveram IM grau ≤ II. Verificou-se uma redução significativa no volume auricular esquerdo (63±7 mL/m2versus 45±6 mL/m2, p=0,038), volume telediastólico do ventrículo esquerdo (87±7 mL/m2versus 79±9ml/m2, p=0,001) e da PSAP (44±4 versus 31±8 mmHg, p=0,002). A taxa de reintervenção foi de 11,1% (N=2, um dos doentes tendo sido submetido a re-do NeoChord™ e outro a cirurgia convencional de RVM). Não foram registados eventos adversos major cardio ou cerebrovasculares.
ConclusãoEm doentes selecionados, a RVM minimamente invasiva com recurso ao sistema NeoChord™ revelou-se segura, efetiva e reprodutível. Os resultados clínicos e ecocardiográficos a curto prazo sugerem uma melhoria sintomática significativa, redução sustentada do grau de IM e remodelagem favorável das câmaras cardíacas esquerdas, com uma taxa reduzida de reintervenção. Estes resultados merecem confirmação ulterior em coortes maiores de doentes e ao longo de um follow-up mais prolongado.
Mitral valve regurgitation (MR) is the second most common valve disease requiring intervention in developed countries.1 It can be either a disease of the mitral valve and/or subvalvular apparatus (primary MR) or arise secondary to left ventricular (LV) and/or left atrium (LA) geometry disruption, most frequently with chamber dilation (secondary MR). Primary MR is most often a consequence of degenerative MV disease, with prolapse and/or flail of one or more leaflet segments.1
According to the prevailing guidelines,1,2 surgical indication for primary severe MR is dictated by symptom status, in addition to the consequences of MR on LV and/or LA dimensions, LV systolic dysfunction, or pulmonary artery systolic pressure (PASP).
Mitral valve repair (MVr) is the preferred approach to treat severe MV disease secondary to leaflet flail or prolapse, supported by a long-term survival benefit over MV replacement.3 The surgical community has shifted from resection of abnormal leaflet tissue with precise valve anatomy reconstruction toward a more conservative approach, involving implantation of polytetrafluoroethylene neochordae to support the prolapsing segments’ free edge, with no or limited resection. Also, with patients being referred for surgery earlier, the need for routine adjunct annuloplasty has become debatable. Some authors state that in degenerative MR, early surgical intervention without annuloplasty, may preserve better the three-dimensional dynamic interplay of the ventriculo-annular continuity.4
Acute post-operative LV dysfunction observed in some patients after MV surgery may be directly related to cardiopulmonary bypass (CBP) and cardioplegia, which have known profound consequences in myocardial oxidative stress, free radical injury and inflammation. These observations, together with treated patients being evermore complex and frail, has raised interest in minimally invasive techniques for MVr.
A new minimally invasive MVr technique that allows transapical off-pump neochord implantation is now available and has been demonstrated to be reproducible and safe in short-term follow-up. The procedure is performed using the NeoChord DS1000TM system (NeoChord, Inc., Eden Praire, MN) using transesophageal echocardiography (TEE) guidance. The procedure has proven to be safe, effective and reproducible in selected patients, both in the short5 and in the medium term.6
In this study, we aim to characterize early Portuguese experience with this technique, providing early clinical and echocardiographic results.
MethodsPatient selectionWe prospectively collected data from all patients who underwent NeoChord DS 1000™ system implantation in our center, between December 2017 and December 2019, after obtaining written informed consent.
Exclusion criteria included: Secondary MR, absence of adequate tissue overlap (leaflet-to-annulus ratio of less than 1.15), multiple jets in different locations, type I MR according to the Carpentier classification (leaflet perforation, associated or not to endocarditis), significant leaflet and annulus calcifications, bileaflet prolapse, prolapse/flail predominantly at the commissural regions.
Clinical data and endpoints definitionBaseline patient surgical risk profile was assessed by EuroSCORE II calculation. Symptomatic status was assessed using New York Heart Association (NYHA) functional class grading system.
Procedural success was defined as implantation of at least two neochordae with residual MR ≤ 2+. All clinical safety and efficacy endpoints definitions were in accordance with Mitral Valve Academic Research Consortium criteria.7,8 Primary safety endpoints included in-hospital mortality, perioperative complications and major adverse events. Primary efficacy endpoints included residual MR no more than moderate (grade ≤2+), freedom from reoperation for recurrent severe MR, and clinical improvement in NYHA functional class at discharge and at six months’ follow-up (median follow-up length; minimum one month, maximum 20 months).
Echocardiographic dataAll patients underwent baseline transthoracic and transesophageal echocardiographic assessment, using a commercially available ultrasound device (iE33®, Philips Medical Systems, Andover, MA, USA). Three cardiac cycles were stored in cine-loop format for subsequent off-line analysis.
Mitral regurgitation severity, mechanism, and consequences over left cardiac chamber dimensions, LV function, and pulmonary artery systolic pressure, were extensively characterized. MR severity was graded using a comprehensive multiparametric approach, according to the European Society of Cardiology's 2017 guidelines (1), from grade 1 (mild MR) to 4 (severe MR). This evaluation included qualitative (jet color flow analysis), semi-quantitative (vena contracta width, systolic flow reversal in pulmonary veins), and quantitative (effective regurgitant orifice area and regurgitant volume, calculated with the PISA method) variables which were then integrated with the abovementioned indices of expected consequences in cardiac chamber pressure, morphology, and function.
Three anatomic subtypes of degenerative MR were further itemized according to a previously described classification,9 relevant to procedural outcome prediction: Type A (flail/prolapse limited to P2 segment), type B (multi-segment disease of posterior leaflet, involving at least 2 scallops), type C (anterior leaflet disease), and type D.
All linear and volume measurements were collected using a commercially available offline workstation (syngo® Dynamics, Siemens Medical Solutions, Erlangen, Germany), and were performed by the same operator, in order to minimize inter-observer variability. A second operator randomly endorsed the first measurements.
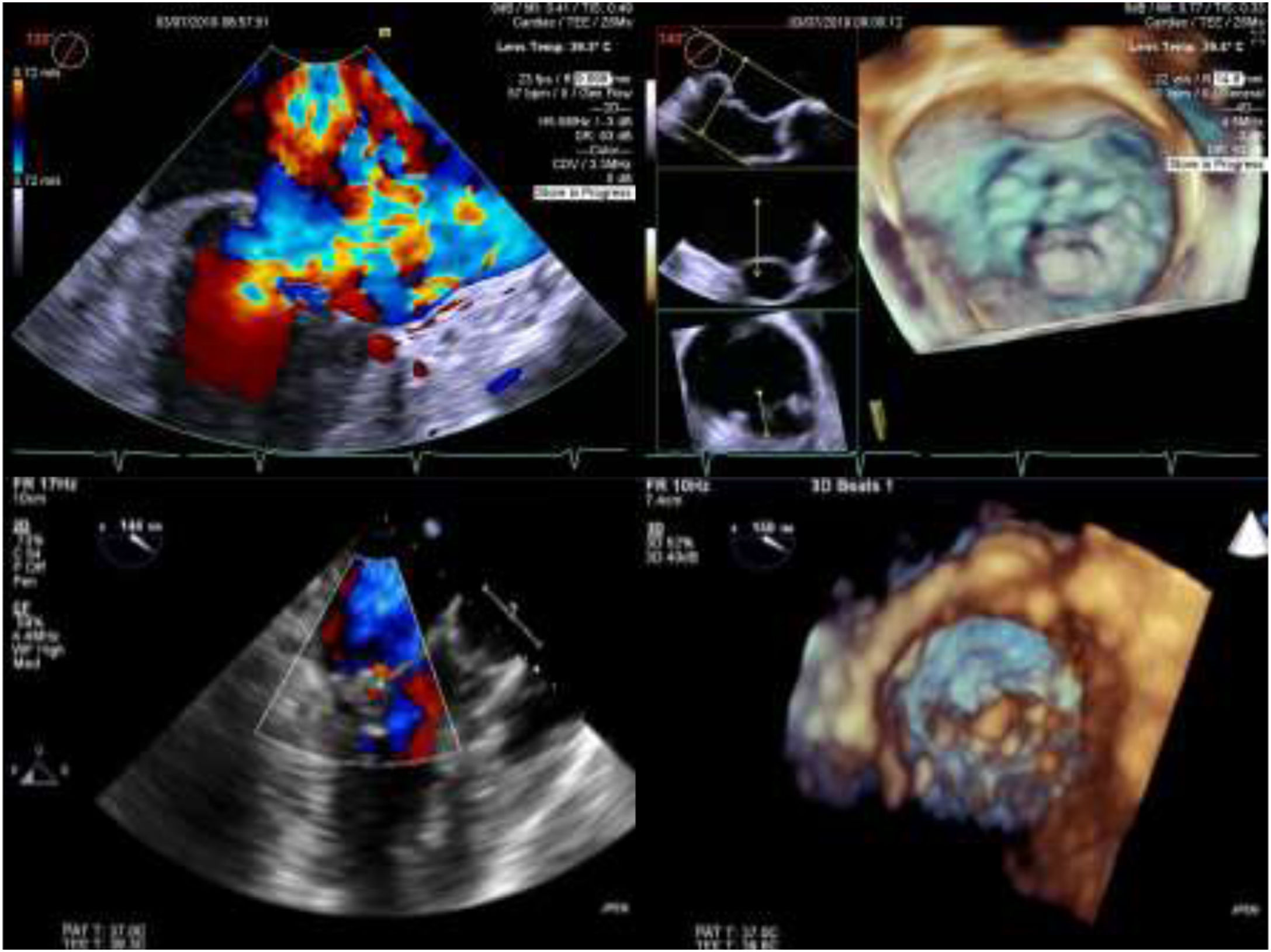
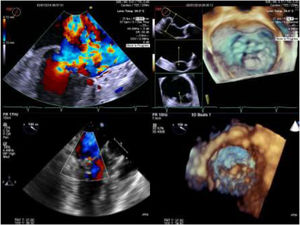
Intra-operative two dimensional and three-dimensional TEE were routinely used to guide neochordae navigation, attachment, and adequate tensioning.10Figure 1 shows an example of intraprocedural TEE result. All patients had a comprehensive transthoracic echocardiographic evaluation by the time of discharge, and between three to six months follow-up.
Two and three-dimensional transesophageal echocardiogram is essential for off-pump transapical neochord implantation planning and real-time guiding. Left upper panel: baseline severe mitral regurgitation jet, eccentrically oriented toward interatrial septum, due to posterior leaflet prolapse. Right upper panel: Surgical view of mitral valve from an atrial perspective, showing P2 prolapse (arrow). Left lower panel: Mild grade mitral regurgitation after two neochordae implantation. Right lower panel: Three-dimensional view of mitral valve following neochordae implantation confirmed correction of P2 prolapse.
Neochordae implantation is performed under general anesthesia, without the use of CBP, and with advanced hemodynamic monitoring. TEE is used for real-time guidance. Intraoperative blood salvage is available for use, when the precepted blood volume loss is of hemodynamic relevance but was not routinely employed.11 Access to the LV apex is achieved through a left lateral thoracotomy in the fifth intercostal space (3-4 cm incision). Two purse-string sutures are placed slightly posterolateral from the apex, and the ventriculotomy is performed. At this stage, the NeoChord DS1000™ device is inserted in the LV. TEE imaging is used to allow safe device navigation, in order to minimize papillary muscle and native MV chord entanglement, when trying to reach the flailing segment free border, usually at the level of posterior mitral valve leaflet. The NeoChord™ is then deployed, following fiber optic confirmation of adequate leaflet capture. Usually, the procedure involves the implantation of at least two neochordae, distributed along the free margin of the prolapsing scallop(s). As a critical final step, the neochordae are set in tension under direct real-time echocardiographic guidance. All procedures were performed by the same team (two cardiac surgeons and one echocardiography imaging specialist).
Statistical analysisData are expressed as mean and standard deviation for quantitative variables or number (n) and percentage (%) for categorical variables. All p values are two-tailed and a significance level of 5% was used. Statistical analysis was performed using SPSS statistics 24 (IBM Corp, Armonk, NY).
ResultsEighteen patients were treated with neochord implantation at our center over the above-mentioned period. The majority (72%) were male, and the mean age was 65±15 years (minimum 45 years, maximum 84 years).
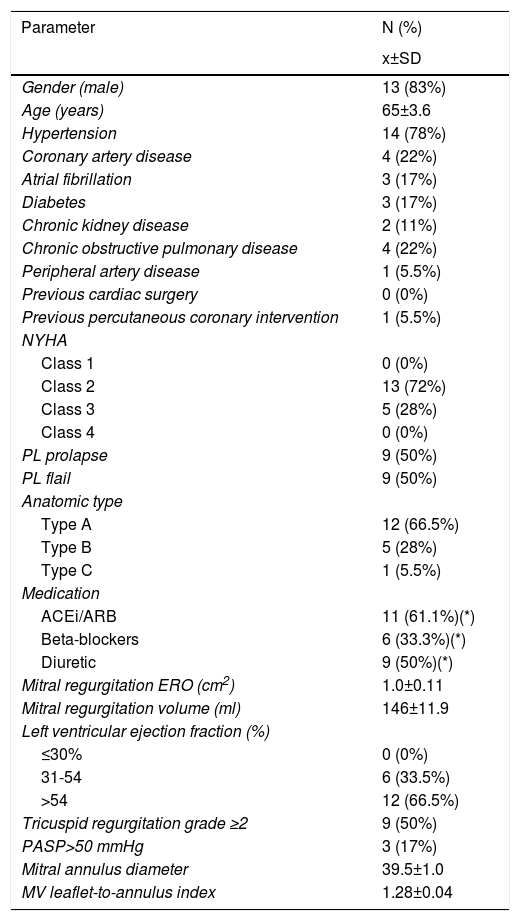
The mean EuroSCORE II was 1.9±1.6. The prevalence of AF was 16.7%. All patients were in NYHA class≥II (N=18). Other relevant demographic and clinical variables are presented in Table 1.
Baseline clinical and echocardiographic characteristics.
| Parameter | N (%) |
|---|---|
| x±SD | |
| Gender (male) | 13 (83%) |
| Age (years) | 65±3.6 |
| Hypertension | 14 (78%) |
| Coronary artery disease | 4 (22%) |
| Atrial fibrillation | 3 (17%) |
| Diabetes | 3 (17%) |
| Chronic kidney disease | 2 (11%) |
| Chronic obstructive pulmonary disease | 4 (22%) |
| Peripheral artery disease | 1 (5.5%) |
| Previous cardiac surgery | 0 (0%) |
| Previous percutaneous coronary intervention | 1 (5.5%) |
| NYHA | |
| Class 1 | 0 (0%) |
| Class 2 | 13 (72%) |
| Class 3 | 5 (28%) |
| Class 4 | 0 (0%) |
| PL prolapse | 9 (50%) |
| PL flail | 9 (50%) |
| Anatomic type | |
| Type A | 12 (66.5%) |
| Type B | 5 (28%) |
| Type C | 1 (5.5%) |
| Medication | |
| ACEi/ARB | 11 (61.1%)(*) |
| Beta-blockers | 6 (33.3%)(*) |
| Diuretic | 9 (50%)(*) |
| Mitral regurgitation ERO (cm2) | 1.0±0.11 |
| Mitral regurgitation volume (ml) | 146±11.9 |
| Left ventricular ejection fraction (%) | |
| ≤30% | 0 (0%) |
| 31-54 | 6 (33.5%) |
| >54 | 12 (66.5%) |
| Tricuspid regurgitation grade ≥2 | 9 (50%) |
| PASP>50 mmHg | 3 (17%) |
| Mitral annulus diameter | 39.5±1.0 |
| MV leaflet-to-annulus index | 1.28±0.04 |
ACEi: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers; ERO: effective regurgitant orifice; MV: mitral valve; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; PL: posterior leaflet.
(*) At 6 month-follow-up, 55.5% of patients were under ACEi or ARB, 50% under beta-blocker, and 22.2% were taking diuretics.
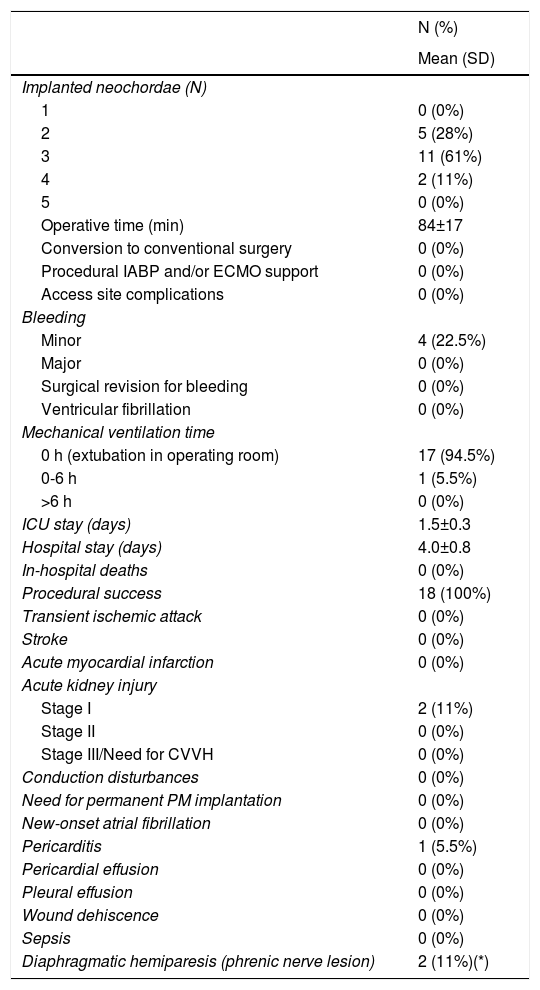
In pre-procedural echocardiographic evaluation, mean effective regurgitant orifice area was 1.0±0.4 cm2, with a mean regurgitant volume 146±42 mL, and a mean leaflet-to-annulus index (LAI) of 1.29±0.14. MR was due to leaflet prolapse in 50% (N=9), and flail leaflet in 50% (N=9). Anatomic type A (isolated P2 defect) was the predominant form in 66.7% (N=12). Successful repair, defined by none, trace or mild mitral regurgitation, by implantation of two to four neochordae, was achieved in all 18 patients. No major complications arose intra-procedurally. Mean procedural time was 84±17 minutes. Mean intensive care unit stay was 1.5±0.3 days and mean hospitalization length was 4.0±0.8 days. Relevant technical and clinical peri-procedural outcomes are depicted in Table 2.
Periprocedural (in-hospital or up to 30-day) technical issues and outcomes.
| N (%) | |
|---|---|
| Mean (SD) | |
| Implanted neochordae (N) | |
| 1 | 0 (0%) |
| 2 | 5 (28%) |
| 3 | 11 (61%) |
| 4 | 2 (11%) |
| 5 | 0 (0%) |
| Operative time (min) | 84±17 |
| Conversion to conventional surgery | 0 (0%) |
| Procedural IABP and/or ECMO support | 0 (0%) |
| Access site complications | 0 (0%) |
| Bleeding | |
| Minor | 4 (22.5%) |
| Major | 0 (0%) |
| Surgical revision for bleeding | 0 (0%) |
| Ventricular fibrillation | 0 (0%) |
| Mechanical ventilation time | |
| 0 h (extubation in operating room) | 17 (94.5%) |
| 0-6 h | 1 (5.5%) |
| >6 h | 0 (0%) |
| ICU stay (days) | 1.5±0.3 |
| Hospital stay (days) | 4.0±0.8 |
| In-hospital deaths | 0 (0%) |
| Procedural success | 18 (100%) |
| Transient ischemic attack | 0 (0%) |
| Stroke | 0 (0%) |
| Acute myocardial infarction | 0 (0%) |
| Acute kidney injury | |
| Stage I | 2 (11%) |
| Stage II | 0 (0%) |
| Stage III/Need for CVVH | 0 (0%) |
| Conduction disturbances | 0 (0%) |
| Need for permanent PM implantation | 0 (0%) |
| New-onset atrial fibrillation | 0 (0%) |
| Pericarditis | 1 (5.5%) |
| Pericardial effusion | 0 (0%) |
| Pleural effusion | 0 (0%) |
| Wound dehiscence | 0 (0%) |
| Sepsis | 0 (0%) |
| Diaphragmatic hemiparesis (phrenic nerve lesion) | 2 (11%)(*) |
CVVH: continuous veno-venous hemodiafiltration; IABP: intra-aortic balloon pump; ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; PM: pacemaker.
(*) One patient fully recovered following rehabilitation, the other remained mildly symptomatic.
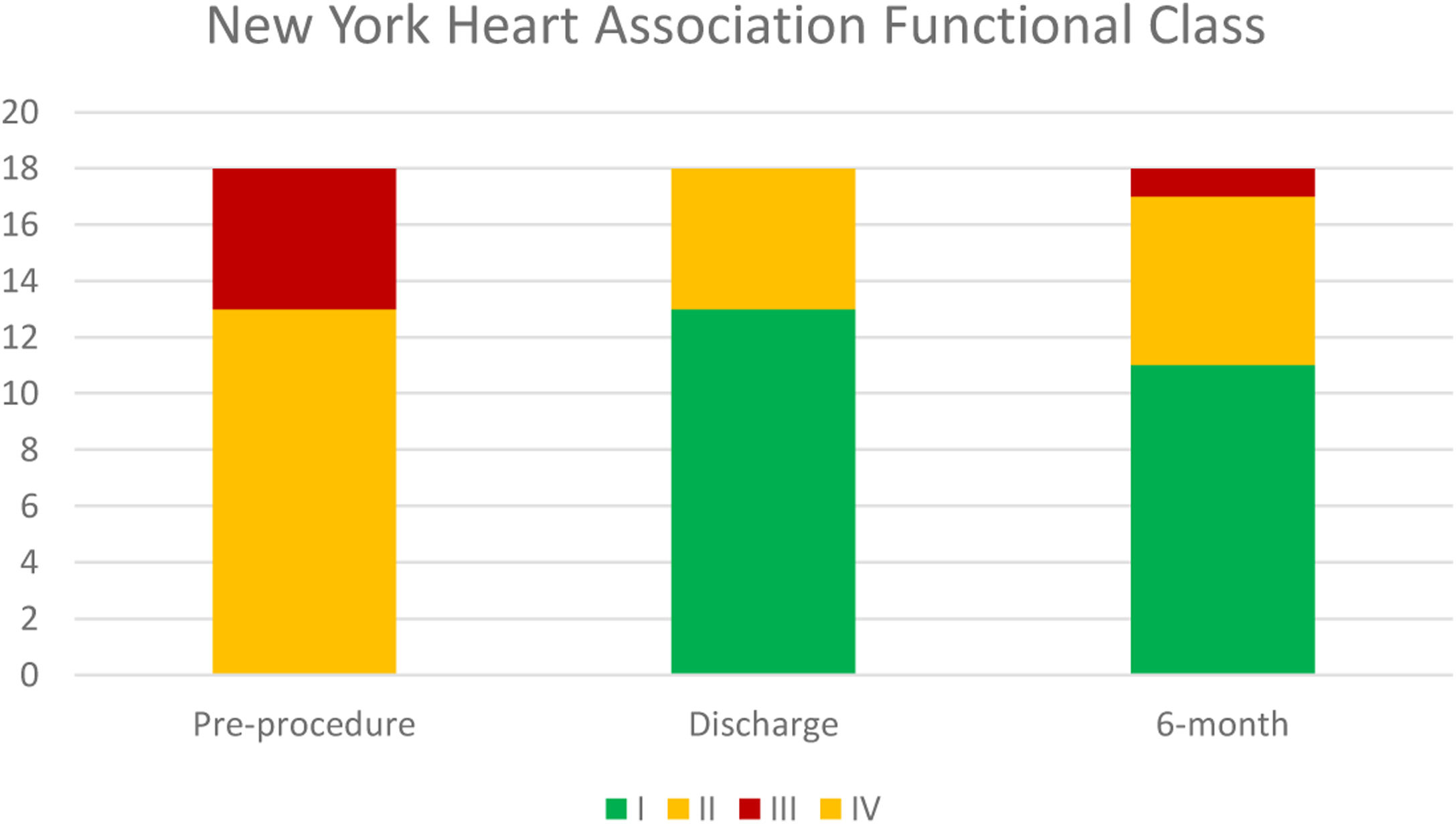
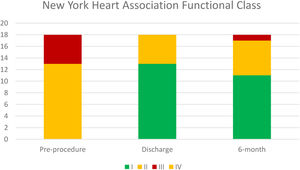
Median follow-up was 194 days. NYHA class was ≤II in 94.5% patients at six-month follow-up, which represented a significant improvement in symptomatic status (p=0.002). Temporal evolution of patient's functional class is presented in Figure 2.
MR was quantified as grade ≤2 in 88.2% (N=15) patients by the time of discharge. In follow-up, 72% of patients (N=13) had grade ≤2 MR. Three patients had grade 3MR, and two patients had grade 4 MR.
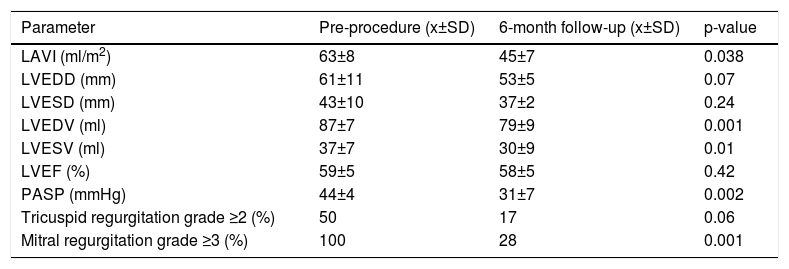
There was a significant reduction in mean indexed LA volume (63±7 mL/m2 pre-procedure, compared to 45±6 mL/m2 in F-UP, p=0.038), mean indexed LV end-diastolic volume (87±7 mL/m2 vs. 79±9 ml/m2, p=0.001), and pulmonary artery systolic pressure (PASP) (44±4 vs. 31±8 mmHg, p=0.002); with no significant differences regarding tricuspid regurgitation severity or LV systolic function. Table 3 details relevant echocardiographic parameter evolution over the follow-up period.
Echocardiographic variables before mitral valve repair with Neo-Chord DS1000 device and at six-month follow-up.
| Parameter | Pre-procedure (x±SD) | 6-month follow-up (x±SD) | p-value |
|---|---|---|---|
| LAVI (ml/m2) | 63±8 | 45±7 | 0.038 |
| LVEDD (mm) | 61±11 | 53±5 | 0.07 |
| LVESD (mm) | 43±10 | 37±2 | 0.24 |
| LVEDV (ml) | 87±7 | 79±9 | 0.001 |
| LVESV (ml) | 37±7 | 30±9 | 0.01 |
| LVEF (%) | 59±5 | 58±5 | 0.42 |
| PASP (mmHg) | 44±4 | 31±7 | 0.002 |
| Tricuspid regurgitation grade ≥2 (%) | 50 | 17 | 0.06 |
| Mitral regurgitation grade ≥3 (%) | 100 | 28 | 0.001 |
LAVI: left atrium volume index; LVEDD: left ventricle end-diastolic diameter; LVEDV: left ventricle end-diastolic volume; LVEF: left ventricle ejection fraction; LVESD: left ventricle end-systolic diameter; LVESV: left ventricle end-systolic volume; PASP: pulmonary artery systolic pressure.
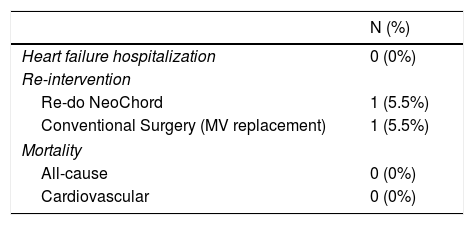
No re-hospitalizations for heart failure were observed in follow-up. No major adverse cardiac or cerebrovascular events were registered. Table 4 details major clinical safety outcomes.
The re-intervention rate was 11.1% (N=2), driven by recurrence of severe MR. In one patient, the mechanism of procedure failure was related to re-prolapse of a treated leaflet, thought to be a consequence of LV reverse remodeling causing relative elongation of implanted neochordae. This patient underwent a re-do NeoChordTM with chord re-tensioning. In another patient, a rupture of three neochords at the apical attachment site was documented, and the patient later underwent standard surgical MVr with adjunctive annuloplasty, with good outcome.
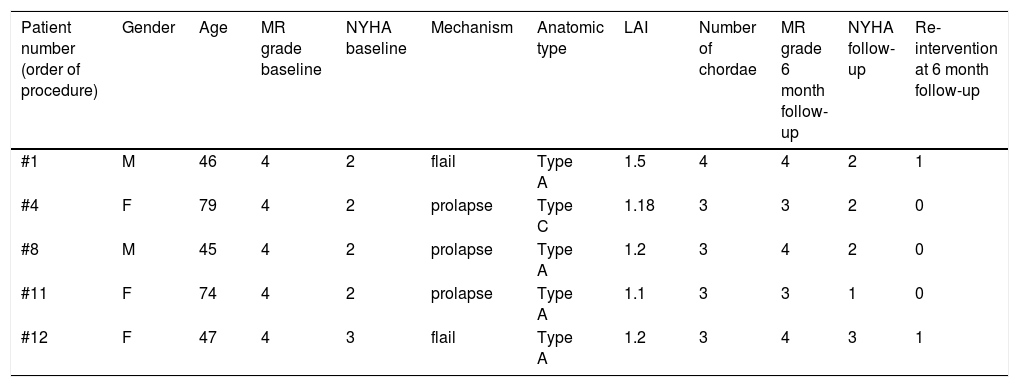
Table 5 summarizes the main characteristics of patients with recurrent MR grade ≥3 at six-month follow-up.
Characteristics of patients with recurrent MR grade ≥3 at six-month follow-up.
| Patient number (order of procedure) | Gender | Age | MR grade baseline | NYHA baseline | Mechanism | Anatomic type | LAI | Number of chordae | MR grade 6 month follow-up | NYHA follow-up | Re-intervention at 6 month follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | M | 46 | 4 | 2 | flail | Type A | 1.5 | 4 | 4 | 2 | 1 |
| #4 | F | 79 | 4 | 2 | prolapse | Type C | 1.18 | 3 | 3 | 2 | 0 |
| #8 | M | 45 | 4 | 2 | prolapse | Type A | 1.2 | 3 | 4 | 2 | 0 |
| #11 | F | 74 | 4 | 2 | prolapse | Type A | 1.1 | 3 | 3 | 1 | 0 |
| #12 | F | 47 | 4 | 3 | flail | Type A | 1.2 | 3 | 4 | 3 | 1 |
LAI: leaflet to annulus index; MR: mitral regurgitation; NYHA: New York Heart Association.
We describe an early single-center Portuguese experience of a transapical off-pump MVr technique. This series demonstrates the feasibility of a new less invasive cardiac procedure, with a minithoracotomy approach without cardiopulmonary bypass. These results strengthen the technique efficacy and safety profile in short-term follow-up. The successful placement of two or more neochordae was achieved in all patients and resulted in a significant peri-operative reduction of MR severity (grade ≤2). Acute procedural success rates above 85% have been consistently reported in previous series9,12-14.
The rate of conventional MV surgery (repair or replacement) for NeoChordTM failure at 30-day follow-up was 0%, lower than that reported by Colli A. et al. (8%)9 or in the TACT Trial (27%).5
We observed a significant clinical improvement after the procedure, assessed by NYHA functional class (NYHA class≥III was 28% pre-procedure vs. 0% in early follow-up, i.e., at 30 days).
Left heart chamber positive remodeling is expected following the correction of the volume-overload status. We observed a significant reduction in mean indexed LA volume and mean indexed LV end-diastolic volume in early follow-up. A similar analysis was conducted by Kurnicka K. et al., with comparable results.15
Pulmonary arterial systolic pressure reduction, although inconsistently reported in previous studies, is also a physiopathological, plausible finding. We observed a significant PASP reduction early on in follow-up.
Left ventricular ejection fraction is highly load-dependent, and severe MR correction may result in spuriously lower values early on in follow-up, related to a higher afterload status. Also, LV apex “stunning” after ventriculotomy may affect echocardiographic appreciation of global systolic function. Furthermore, patients deemed candidates for this procedure have mostly preserved LV systolic function. Together, these factors may lessen the positive influence of postprocedural LV remodeling on systolic function. Other series failed to document a significant improvement in this parameter,15 and our work replicated this finding.
A proportion of patients had recurrence of grade ≥3 MR between 30 day and 6 months (28%, N=5), but only a minority had indication for re-intervention (11%, N=2).
Importantly, in the case where MVr with NeoChord™ implantation was unsuccessful in early follow-up (30 days to 6 months after the procedure), the patient was safely managed with either a re-do procedure (N=1) or conventional on-pump surgery (N=1). This is also in accordance to previously published data.12,15 It is noteworthy that in none of our patients was re-intervention clinically driven; instead, echocardiographic parameters of MR severity prompted surgical indication, along with an oligosymptomatic status that was comparable with the pre-procedure condition. Furthermore, in the patient who underwent conventional cardiac surgery, a re-repair was possible, highlighting one of the advantages of the technique, as the mitral valve is barely touched in the first procedure.
No major cardiovascular and/or cerebrovascular adverse events were recorded in our series over the abovementioned period.
Several mechanisms have been proposed to explain early recurrence of grade ≥3 MR, and are mainly related to patient selection criteria, technical issues of the procedure (mainly related to native chordae injury, not necessarily accompanied by acute rupture), or the so-called “curling” phenomenon of posterior leaflet (which displays postoperative posterior annular displacement causing exaggerated posterior systolic motion, with resultant end-systolic prolapse and an eccentric anteriorly directed regurgitant jet). Interestingly, in a case series of patients that experienced recurrent regurgitation, only a minority was attributable to reverse remodeling of the LV causing relative Neochord™ elongation (1.9%).16 In the early days, it was thought this would become the Achilles heel of the technique.
A variety of factors may explain such mismatch between MR grade ≥3 recurrence and the minimal impact on clinical status, possibly including a strong treatment placebo effect, reverse atrial and ventricular remodeling (with better dynamic systolic and diastolic performance on exertion), and small but clinically significant reductions in PASP.
Several procedure refinements are being widely adopted to minimize recurrence of moderate to severe MR, and include (1) placement of one to two more neochordae than the number deemed necessary in order to avoid excess chord tension; (2) overtension of implanted neochordae to account for LV reverse remodeling; (3) exclusion of patients with inadequate coaptation depth (LAI<1.15); (4) more posterolateral access at the left ventricle apex and careful device navigation in LV, along the posterior wall axis, to minimize the risk of native chord entanglement and injury; (5) treat preferentially anatomic type A and B patients to ensure more predictable results; and (6) perform more anterior left ventricular access in patients with borderline LAI to bring the MV coaptation line more anteriorly.17
Our results are consistent with the largest series concerning medium-term (12-month) follow-up, which included 144 patients.18 The authors reported the safety and feasibility of this approach, together with restoration of surgical-like MV functional status in 70% of all cases, and significant ventricular and atrial reverse remodeling.
To date, there are limited data on medium-term outcomes, consisting of a small series of six patients originally enrolled in the TACT trial. At five years, three patients experienced procedural failure and underwent conventional surgery, and three patients remained with residual MR and no symptoms over this period.6
A recently published analysis addressed the learning curve effect in this subset: An initial “learning phase” (up to 20 procedures) may have a probability of failure as high as 25%, whereas in the so-called “expert phase”, it may decrease to 5%.19 In this series, an overall failure rate of 11% at one year follow-up was found, which is similar to our results, even though we are still in the first twenty procedures.
The minimally invasive nature of the procedure, with short in-hospital and intensive care unit length of stay, low procedural complication rates, and encouraging medium term results may set the stage for the technique to grow in the near future. Given that a proportion of patients with severe MR recurrence over follow-up may still be treated with either a re-do procedure or conventional on-pump surgical MV repair, provides additional safety.
As the therapeutic armamentarium of minimally invasive techniques for off-pump MVr rapidly grows (either through transapical or trans-septal routes), careful patient selection remains the key. Multiparametric echocardiographic evaluation is of outmost importance, in procedural planning and in real-time guidance,20 with protocol refinements and technological improvements rendering evermore safe and predictable results.21
There are some important limitations of this study to acknowledge. First, we describe a small case sample with limited follow-up, which can preclude solid conclusions about infrequent short and medium-term clinical and/or echocardiographic adverse events. Second, we cannot ignore the learning curve effect of the assisting heart team, as it could have mitigated some advantages of the technique driven by sub-optimal results in the very early experience. This includes a non-negligible MR ≥3 recurrence at six-month follow-up: 28% (n=5 pts), albeit without significant worsening in the clinical status. Third, we applied some pathophysiological and anatomical exclusion criteria widely used in this subset, but it prevents the authors from drawing conclusions about the most complex cases of degenerative MV disease, once again emphasizing the major challenge of proper patient selection as the procedure technique matures and is disseminated. According to some authors, adjunctive mitral ring annuloplasty is a key element to maintaining annular stability, improving leaflet coaptation and durability of repair, as in most instances there is concomitant annular enlargement or dysfunction. This shortcoming should be acknowledged when selecting patients for this procedure. Finally, we must keep in mind that transapical neochord implantation lacks clinical data on long-term follow-up, and further studies with a larger patient population and longer follow-up periods are needed before widespread clinical application of this technique. It must be highlighted that this treatment strategy, and presented results, only apply to the treatment of primary (degenerative) MR. At present, the technique must be regarded as an alternative to conventional MV repair (gold standard). Careful inclusion and exclusion criteria should be applied, and patients must be informed about the probability of short-to-medium-term rates of success, concerning MR recurrence.
ConclusionMinimally invasive MVr using the NeoChord™ system is safe, effective and reproducible in the short term. The technique may become a valuable treatment option for carefully selected patients with primary severe MR due to degenerative MV prolapse, more so in those with isolated leaflet disease. Early clinical and echocardiographic results suggest a significant symptom improvement, sustained MR grade decrease, and favorable left cardiac chamber remodeling, with low re-intervention rates. Owing to the minimal interference with valvular and subvalvular apparatus, re-doing MVr, either using minimally invasive or conventional surgery, is still possible in patients with suboptimal clinical and/or echocardiographic result. These results warrant further confirmation in larger cohorts, in longer period of follow-up.
Conflicts of interestThe authors have no conflicts of interest to declare