The 2019 ESC guidelines on chronic coronary syndromes updated the method for estimating the pre-test probability (PTP) of obstructive coronary artery disease (CAD). We aimed to compare the performance of the new PTP method against the 2013 prediction model in patients with stable chest pain undergoing coronary computed tomography angiography (CCTA) for suspected CAD.
MethodsWe conducted a single-center cross-sectional study enrolling 320 consecutive patients undergoing CCTA for suspected CAD. Obstructive CAD was defined as any ≥50% luminal stenosis on CCTA. Whenever invasive coronary angiography was subsequently performed, patients were reclassified accordingly. The two PTP prediction models were assessed for calibration, discrimination and the ability to change the downstream diagnostic pathway.
ResultsThe observed prevalence of obstructive CAD was 16.3% (n=52). The 2013 prediction model significantly overestimated the likelihood of obstructive CAD (relative overestimation of 130%, p=0.005), while the updated 2019 method showed good calibration (relative underestimation of 6.5%, p=0.712). The two approaches showed similar discriminative power, with C-statistics of 0.73 (95% CI: 0.66-0.80) and 0.74 (95% CI: 0.66-0.81) for the 2013 and 2019 methods, respectively (p=0.933). Reclassification of PTP using the new method resulted in a net reclassification improvement of 0.10 (p=0.001).
ConclusionsThe updated 2019 prediction model provides a more accurate estimation of pre-test probabilities of obstructive CAD than the previous model. Adoption of this new score may improve disease prediction and influence the selection of non-invasive testing.
As novas guidelines da ESC de síndromes coronárias crónicas de 2019 atualizaram o método para estimar a probabilidade pré-teste (PPT) de doença arterial coronária (DAC) obstrutiva. O objetivo do nosso trabalho foi comparar o desempenho do novo método de estimativa da PPT com o modelo de 2013 em doentes com angina estável submetidos a angiotomografia das artérias coronárias por suspeita de DAC.
MétodosFoi realizado um estudo transversal de centro único envolvendo 320 doentes consecutivos submetidos a angiotomografia coronária por suspeita de DAC. DAC obstrutiva foi definida como qualquer estenose ≥50% na angiotomografia. Sempre que realizada angiografia coronária invasiva, os doentes foram reclassificados em conformidade. Os dois modelos de previsão da PPT foram avaliados quanto à calibração, discriminação e capacidade para alterar a marcha diagnóstica subsequente.
ResultadosA prevalência observada de DAC obstrutiva foi de 16,3% (n=52). O modelo de previsão de 2013 sobrestimou significativamente a probabilidade de DAC obstrutiva (sobrestimação relativa de 130%, valor-p 0,005), enquanto o método de 2019 mostrou uma boa calibração (subestimativa relativa de 6,5%, valor-p 0,712). As duas abordagens mostraram poder discriminativo semelhante, com estatística C de 0,73 (IC 95%: 0,66-0,80) e 0,74 (IC 95%: 0,66-0,81) para os métodos de 2013 e 2019, respetivamente (valor-p 0,933). A reclassificação da PPT usando o novo método resultou numa melhoria de reclassificação líquida de 0,10 (valor-p=0,001).
ConclusõesO modelo de previsão atualizado de 2019 permite uma estimativa mais precisa das probabilidades pré-teste de DAC obstrutiva do que o modelo anterior. A adoção deste novo método pode melhorar a previsão de doença e ter impacto na seleção dos testes diagnósticos não invasivos.
Estimating the pre-test probability (PTP) of obstructive coronary artery disease (CAD) is a crucial step in the clinical assessment of patients with suspected CAD. This determination directly influences the subsequent work-up, including the need for diagnostic tests, choice of test, and interpretation of the results.1–3 The previous 2013 European Society of Cardiology (ESC) guidelines on the management of stable CAD recommended a tabular method for estimating the PTP of obstructive CAD according to age, gender and the nature of symptoms,4 using a modified Diamond-Forrester (MDF) prediction model.5 Notably, the proposed model was derived from patients referred for invasive coronary angiography (ICA), rather than non-invasive tests. Furthermore, the MDF method does not account for important additional risk factors, such as hypertension, diabetes or smoking. To address these limitations, new scores have been developed6,7 since the publication of this document and recent data from contemporary, unselected, all-comer populations have been obtained,8 suggesting that the MDF method substantially overestimated the prevalence of obstructive CAD. Accordingly, in the 2019 ESC guidelines on the management of chronic coronary syndromes, this tabular method was revised by significantly downgrading absolute PTP values in both sexes and across all ages and angina characteristics.9
ObjectivesThe aim of this study was to compare the diagnostic performance of the new PTP model with the MDF method in patients with stable chest pain referred for coronary computed tomography angiography (CCTA) for suspected CAD. Both methods were assessed for calibration, discrimination and net reclassification, as well as for their ability to influence the downstream diagnostic pathway.
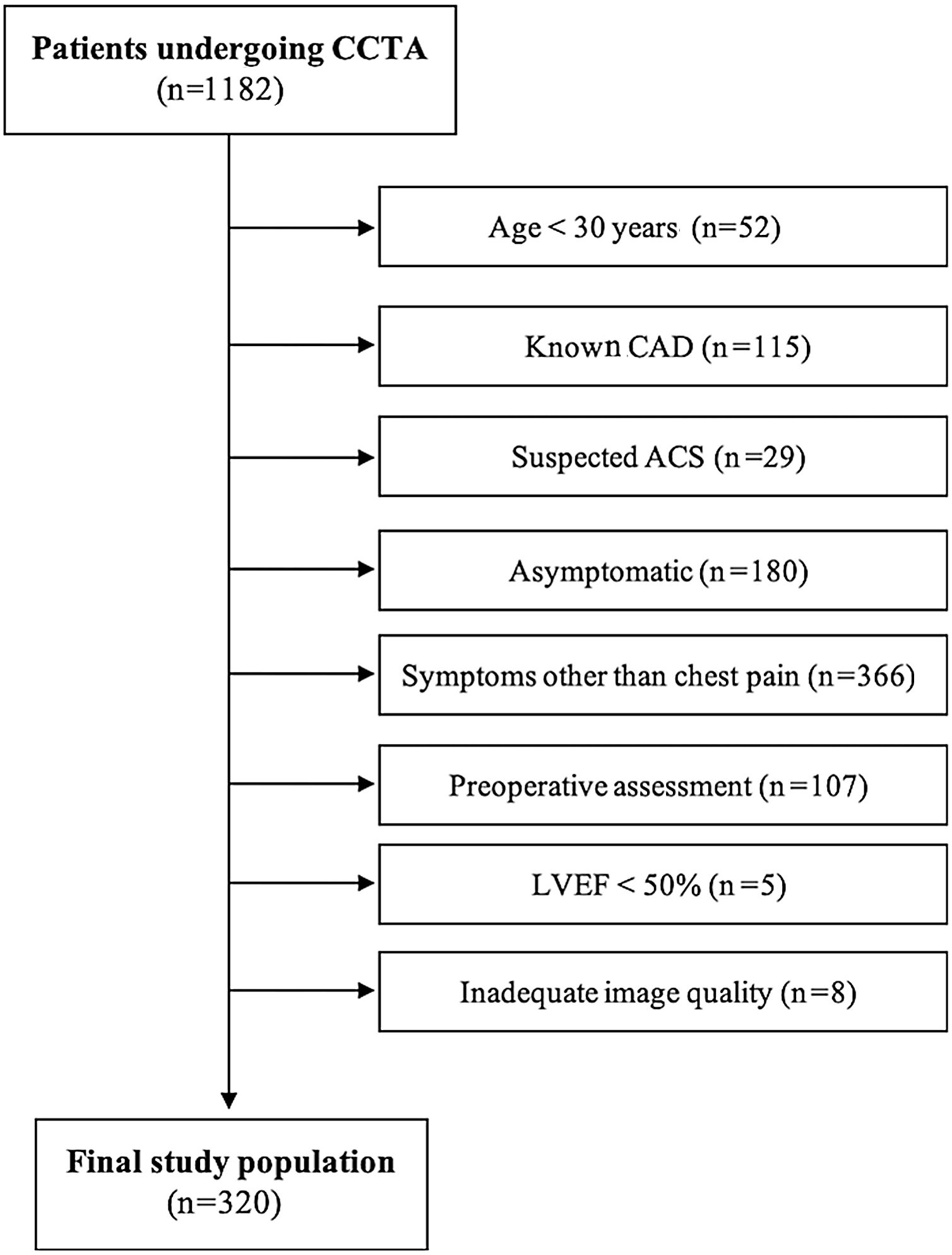
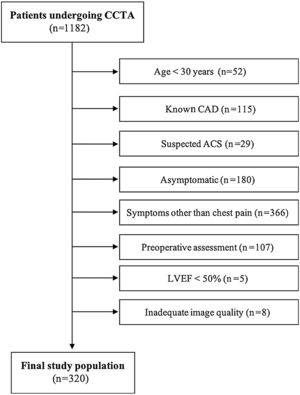
MethodsPopulationThis was a cross-sectional observational study performed at a single-center tertiary hospital in Lisbon, Portugal. The study population consisted of all consecutive patients referred for CCTA for suspected CAD between October 2016 and 2019. The following exclusion criteria were applied sequentially: age <30 years, known CAD, suspected acute coronary syndrome, asymptomatic, symptoms other than chest pain, preoperative assessment, known left ventricular ejection fraction <50% and inadequate image quality.
Study designClinical data were systematically collected from electronic medical records and included patient demographics and cardiovascular risk factors. Angina characteristics were assessed at the time of CCTA and classified according to the following three criteria: (1) substernal chest pain or discomfort; (2) caused by physical or psychological stress; and (3) relieved by rest and/or nitroglycerine. Chest pain was classified as typical if all three criteria were fulfilled, as atypical when two criteria were present, and as nonanginal if one or no criteria was reported.10 Hypertension was defined as a history of blood pressure ≥140/90 mmHg and/or treatment with antihypertensive medications.11 Diabetes was defined as fasting glucose of ≥126 mg/dl and/or use of hypoglycemic agents.12 Dyslipidemia was defined as a total cholesterol level ≥200 mg/dl or treatment with lipid-lowering medication.13 Current smoking status was defined as active smoking within three months of presentation. Family history of CAD was defined as myocardial infarction or cardiac death in a first-degree relative.14 From these data, the PTP of disease was calculated according to the new 2019 ESC PTP model and by the MDF method.
The 2013 ESC guidelines on the management of stable CAD recommended direct referral for ICA if PTP was >85%, non-invasive testing for PTP 15-85%, and no further testing for PTP <15%. The new 2019 ESC guidelines modified these recommendations: no specific threshold for choosing anatomical vs. functional testing is now recommended, and the threshold to withhold additional testing was lowered to PTP <5%. Considering this, in order to gauge how the method for estimating PTP might influence the diagnostic approach according to these new guidelines, we measured the proportion of patients who would be reclassified into a different risk category (low <15%; intermediate 15-85%; high >85%) when assessed by the new 2019 ESC PTP model instead of the MDF method. Additionally, we assessed the proportion of patients who would have no indication for further testing according to the old and new guidelines (MDF PTP <15% and 2019 ESC PTP <5%, respectively).
The study was approved by the local institutional review board and all patients gave written informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Scan protocol and image analysisAll scans were performed on a dual-source 64-slice scanner (SOMATOM Definition®, Siemens Healthineers, Erlangen, Germany) in accordance with the Society for Cardiovascular Computed Tomography guidelines valid at the time. A cardiologist and/or a radiologist with level III-equivalent experience analyzed all scans on an Aquarius® workstation (Terarecon Inc., USA) using axial images, multiplanar reconstructions and maximum intensity projections, as appropriate.
Obstructive CAD was defined as any ≥50% luminal stenosis on CCTA. Coronary atherosclerotic lesions were assessed by visual estimation and any disagreements were resolved by consensus. Patient records were also reviewed for downstream ICA performed during the subsequent six months. Whenever ICA was performed, patients were reclassified accordingly using the same ≥50% luminal stenosis threshold.
An Agatston score threshold of 400 was used as a general reference for withholding CCTA, but the ultimate decision was left to the discretion of the performing physician, taking into consideration the clinical context and the distribution of calcium in the coronary tree. Patients who had CCTA withheld due to high coronary artery calcium (CAC) were classified according to downstream testing if performed or, if not, were conservatively assumed to have obstructive CAD, since symptomatic patients with high CAC scores have a high likelihood of angiographically significant coronary stenosis.15
Statistical analysisCategorical data are presented as frequencies and percentages. Continuous data are presented as mean ± standard deviation (parametric) or as median with interquartile range (IQR) (nonparametric). Baseline characteristics were compared between the two groups with the chi-square test (categorical data) and the unpaired Student's t test or Mann-Whitney U test, as appropriate (continuous data). The related-samples Wilcoxon signed rank test was used to assess the difference in PTPs calculated with the different methods. The median PTPs of patients with and without obstructive CAD were compared using the independent samples Mann-Whitney U test.
Model calibration reflects how close predicted probabilities are to the observed prevalence of obstructive CAD and was assessed by calibration-in-the-large (which compares the mean observed frequency of obstructive CAD with the mean predicted probability) and discordance ([expected percentage-observed percentage]/observed percentage).
The discriminative ability of each model was expressed by the C-statistic, which, for binary outcomes, is identical to the area under the receiver operating characteristic (ROC) curve.16 The areas under the ROC curves were compared using the nonparametric method described by De Long et al.17
Net reclassification improvement, which compares the proportions of patients moving up or down in clinically meaningful PTP categories in cases versus controls, was used to ascertain the ability of the new 2019 ESC PTP model to reclassify risk.18
Statistical analyses were conducted using IBM SPSS version 25.0 (IBM SPSS® Inc., Chicago, IL, USA) and MedCalc® version 9.3.8.0 (Mariakerke, Belgium). A two-tailed p-value <0.05 was considered statistically significant.
ResultsDuring the study period, 1182 patients were referred for CCTA at our hospital. After application of the exclusion criteria (Figure 1), 320 patients were included in the analysis. Of these, 272 underwent CCTA and 48 had their exam withheld due to high CAC. Twenty-six patients (9.6%) had obstructive CAD on CCTA, however, after ICA five of them turned out to be false positives (all with 50-69% stenosis on CCTA). The distribution and patient characteristics according to the CAD-RADS classification19 are presented in Supplementary Table 1.
Among the 48 patients whose CCTA was canceled due to heavy coronary calcification, 29 underwent ICA, nine underwent noninvasive functional testing (six myocardial single-photon emission computed tomography and three stress cardiac magnetic resonance imaging), and 10 others did not undergo any downstream testing. Eighteen of the 29 patients who underwent ICA had obstructive CAD, and three of the nine who underwent functional testing had significant ischemia. The 10 patients without downstream examination were also conservatively assumed to have obstructive CAD, yielding a total of 31 patients with obstructive or presumed obstructive CAD among the 48 who did not undergo CCTA.
Overall, the observed prevalence of obstructive CAD was 16.3% (n=52). Patients with obstructive CAD were more often male, were significantly older and had higher prevalence of typical angina and cardiovascular risk factors (Table 1). CAC scores were also significantly higher in this group of patients (median 517, IQR 238-1210 vs. median 0, IQR 0-52, p<0.001). Among patients with obstructive CAD and known coronary anatomy, 64.3% (n=27) had single-vessel disease, 23.8% (n=10) had two-vessel disease, and 11.9% (n=5) had left main or three-vessel disease.
Baseline patient characteristics.
| Total population (n=320) | Obstructive CAD | p | ||
|---|---|---|---|---|
| No (n=268) | Yes (n=52) | |||
| Age, years | 63 (53-70) | 61 (51-69) | 67 (60-74) | <0.001 |
| Male | 132 (41.3%) | 101 (37.7%) | 31 (59.6%) | 0.003 |
| Hypertension | 220 (68.8%) | 179 (66.8%) | 41 (78.8%) | 0.086 |
| Diabetes | 55 (17.2%) | 41 (15.3%) | 14 (26.9%) | 0.042 |
| Dyslipidemia | 179 (55.9%) | 141 (52.6%) | 38 (73.1%) | 0.007 |
| Smoking | 107 (33.4%) | 82 (30.6%) | 25 (48.1%) | 0.014 |
| Family history of CAD | 118 (36.9%) | 100 (37.3%) | 18 (34.6%) | 0.712 |
| Symptom typicality | ||||
| Typical angina | 45 (14.0%) | 29 (10.8%) | 16 (30.8%) | <0.001 |
| Atypical angina | 153 (47.8%) | 129 (48.1%) | 24 (46.2%) | 0.794 |
| Nonanginal pain | 122 (38.1%) | 110 (41.0%) | 12 (23.1%) | 0.015 |
| PTP, % | ||||
| 2013 ESC MDF method | 37±21 | 34±20 | 53±23 | <0.001 |
| 2019 ESC new PTP method | 15±12 | 13±11 | 24±15 | <0.001 |
CAD: coronary artery disease; ESC: European Society of Cardiology; MDF: modified Diamond-Forrester; PTP: pre-test probability.
The mean PTP of disease was 37±21% using the MDF method, and 15±12% with the new 2019 ESC PTP model. The median PTP values were 34% (IQR 20-49) and 11% (IQR 6-22), respectively (p<0.001 for comparison). PTP was significantly higher in patients with obstructive CAD, independently of the method of estimation.
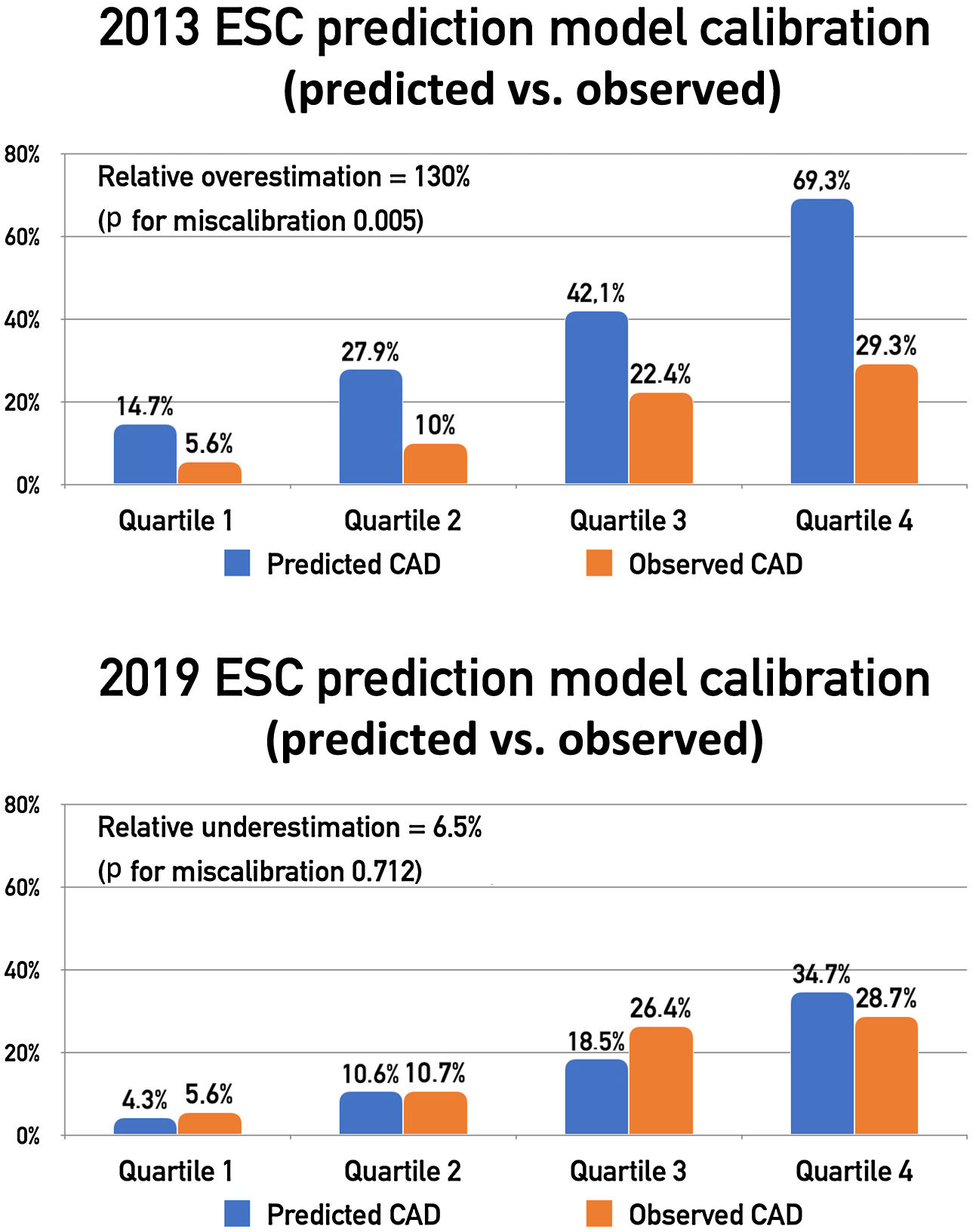
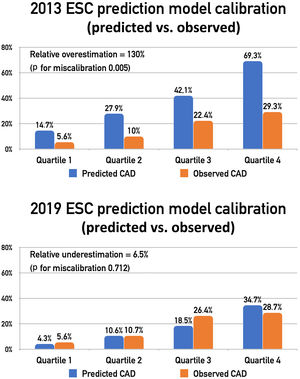
CalibrationOverall, the MDF method overestimated the likelihood of obstructive CAD by 130%, whereas the new 2019 ESC PTP model underestimated the probability of disease by 6.5%. The observed versus expected prevalence of obstructive CAD by quartiles of PTP can be observed in Figure 2. The MDF method overestimated the prevalence of obstructive CAD in all quartiles of PTP, with the calibration-in-the-large statistic indicating significant miscalibration (p for miscalibration 0.005). By contrast, the updated 2019 method showed good calibration for predicting the likelihood of obstructive CAD, with the calibration-in-the-large statistic supporting the validity of this model (p for miscalibration 0.712). Stratification by gender produced similar results. We also performed a sensitivity analysis excluding patients who were conservatively assumed to have obstructive CAD (n=10) and the results remained similar.
Comparison of observed vs. expected prevalence according to the two pre-test probability (PTP) prediction models. The 2013 European Society of Cardiology (ESC) prediction model significantly overestimated the observed prevalence of CAD across all quartiles of PTP, whereas the new 2019 ESC prediction model showed good calibration for predicting the likelihood of coronary artery disease. Abbreviations as in Figure 1.
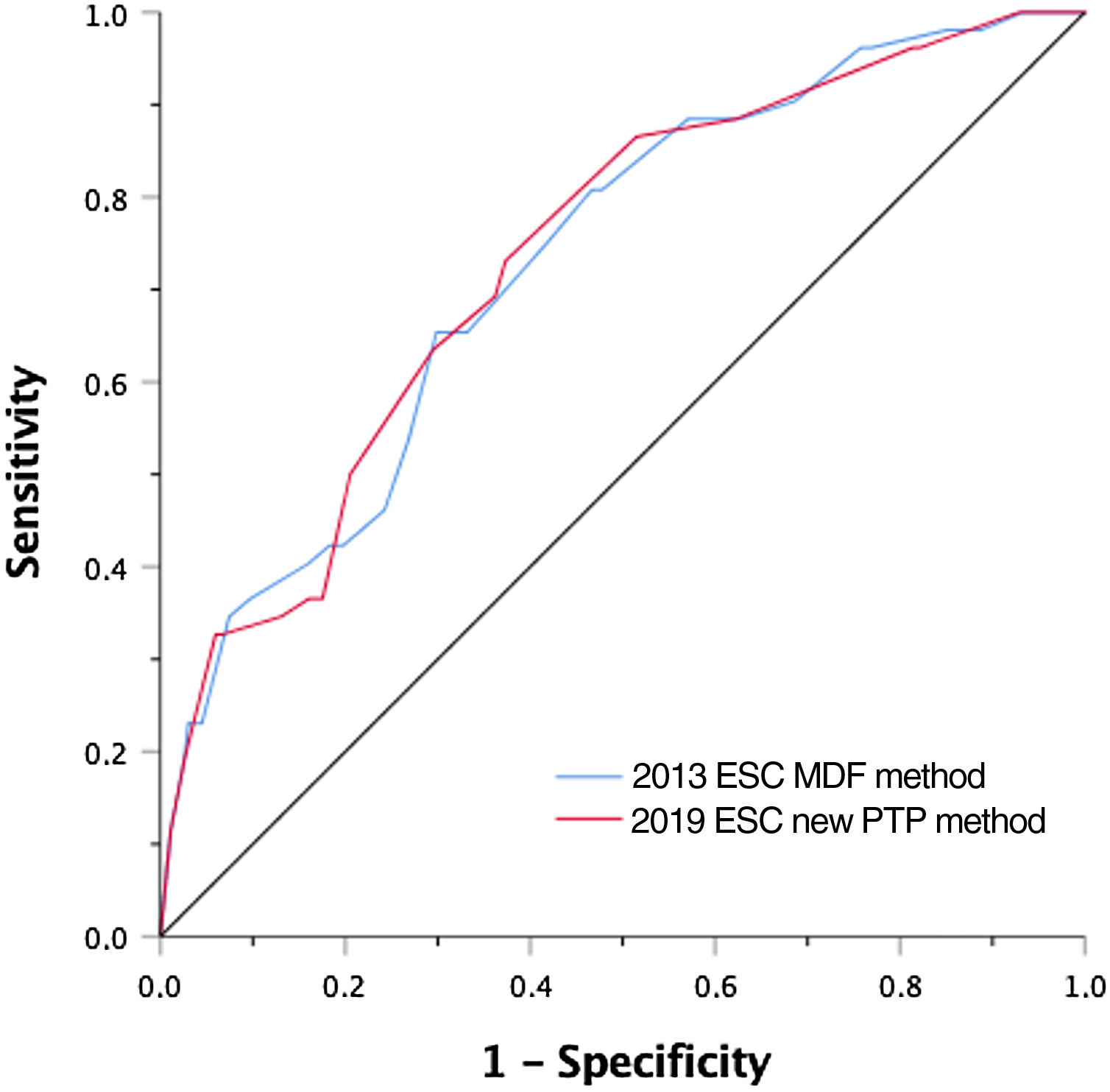
Regarding the ability of each model to differentiate patients with and without obstructive CAD, the two approaches showed similar discriminative power, with C-statistics of 0.730 (95% confidence interval [CI]: 0.658-0.802) and 0.735 (95% CI: 0.663-0.808) for the 2013 and 2019 methods, respectively (p for comparison 0.933) (Figure 3). These results were similar in men and women.
Receiver operating characteristic curves of the two models for the prediction of obstructive coronary artery disease on coronary computed tomography angiography. The two models showed similar discriminative power, with C-statistics of 0.730 (95% confidence interval [CI]: 0.658-0.802) and 0.735 (95% CI: 0.663-0.808) for the 2013 and 2019 methods, respectively (p for comparison 0.933). ESC: European Society of Cardiology; MDF: modified Diamond-Forrester model; PTP: pre-test probability.
As the calculation of PTP in the 2019 guidelines downgraded the tabular method presented in the previous document, the likelihood of disease assessed by the new method was significantly lower than the MDF estimate. On average, individual PTP was 22% lower in the new guidelines. According to the MDF method, most patients (84.4%, n=270) were classified as intermediate risk, while the 2019 ESC PTP model classified the majority of patients as low risk (58.4%, n=187). The reclassification across risk categories among patients with and without obstructive disease and the resulting net reclassification improvement are depicted in Table 2.
Risk reclassification across categories of pre-test probability (low <15%; intermediate 15-85%; high >85%) and resulting net reclassification improvement.
| 2013 ESC MDF method | Total | |||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| 2019 ESC new PTP method | ||||
| No obstructive CAD (n=268) | ||||
| Low | 40 | 131 | 0 | 171 |
| Intermediate | 0 | 94 | 3 | 97 |
| High | 0 | 0 | 0 | 0 |
| Total | 40 | 225 | 3 | 268 |
| Obstructive CAD (n=52) | ||||
| Low | 1 | 15 | 0 | 16 |
| Intermediate | 0 | 30 | 6 | 36 |
| High | 0 | 0 | 0 | 0 |
| Total | 1 | 45 | 6 | 52 |
No obstructive CAD NRI: 134/268=0.50.
Obstructive CAD NRI: -21/52=-0.40
Overall NRI: 0.10 (p=0.001)
NRI: net reclassification improvement; other abbreviations as in Table 1.
Overall, and assuming the same thresholds, the adoption of the new guidelines would reclassify 48.4% of patients (45.6% from intermediate-risk to low-risk [n=146], and 2.8% from high-risk to intermediate-risk [n=9]), possibly leading to a modification in the diagnostic pathway. As for the lower end of the spectrum, an MDF score-guided risk stratification would classify 12.8% of patients (n=41) as low-risk (PTP <15%), warranting no further testing. Of these, 2.4% (n=1) would have obstructive CAD. Using the new 2019 ESC PTP method and the new no-testing threshold (PTP <5%), 16.3% of our population (n=52) would not undergo further testing, 3.8% of whom (n=2) would have obstructive CAD.
DiscussionAssessment of the likelihood of obstructive CAD and of the need for and type of additional testing is a central component in the management of patients with suspected chronic coronary syndrome, previously known as stable CAD. Generally, this is achieved by applying prediction tools to estimate PTP. However, in order to be clinically useful, a prediction tool should be well calibrated across the risk spectrum and provide good discrimination between patients with and without the outcome of interest. Overestimation of PTP of obstructive CAD could expose patients to unnecessary procedures and costs, while underestimation could preclude the appropriate treatment of the disease.
Several studies and real-world data suggest that the selection of patients for additional testing has been largely ineffective.20–22 The prediction tool adopted by the 2013 ESC guidelines on the management of stable CAD, the MDF method, significantly overestimated the prevalence of obstructive CAD in contemporary cohorts, including our own. This considerable overestimation of risk may result from a change in epidemiological factors leading to an overall decrease in the prevalence of disease, but also from a gradual change in referral patterns for non-invasive testing, including a lower threshold for testing. To address this risk overestimation, the 2019 ESC guidelines updated the previous recommendations with a major downgrade of PTP. Importantly, this new PTP method retained the same tabular method, using only age, gender and symptom typicality, and not accounting for additional cardiovascular risk factors such as hypertension, diabetes or smoking, as proposed by other validated PTP scores such as CAD Consortium 2 and CONFIRM.6,7 The inclusion of these risk factors could improve the diagnostic performance of the method, but would inevitably increase its complexity and perhaps compromise its use in clinical practice.
To the best of our knowledge, the present study is one of the first to compare the performance of the two sets of guidelines, and to provide external validation for the proposed new tabular method. In line with previous studies, our results suggest that the previously recommended MDF method significantly overestimates the likelihood of disease among patients undergoing CCTA, adding that the new PTP method has good calibration for predicting the probability of obstructive CAD in this population. These results were not surprising since, unlike the MDF method, the updated 2019 ESC PTP method was derived from studies of patients referred for CCTA and ICA, as well as all-comers from the general population. Nevertheless, the two approaches showed similar discriminative power. The use of the same tabular method, including identical variables, probably explains these results. Likewise, in agreement with other reports assessing patients referred for non-invasive imaging, the prevalence of obstructive CAD in our study population of patients referred for CCTA was generally low.23,24
Reclassification of risk using the new PTP method instead of MDF could change the diagnostic strategy in a significant proportion of patients. If the 15% PTP threshold for non-invasive testing had remained unchanged, most of these would not undergo further testing, which could result in a higher number of patients with obstructive CAD dismissed without further investigation. This issue seems to have been addressed by the new guidelines, since they now consider testing in patients with a PTP of 5-15%, only withholding further testing for patients with PTP <5%.9 Our results suggest that, by simultaneously recalibrating PTP and lowering the threshold for testing, the new recommendations achieve the goal of keeping the false negatives of clinical triaging below 5%. At the other end of the scale, downgrading PTP may have important implications in the selection of further testing. Since CCTA seems particularly suitable for patients with a low to intermediate likelihood of CAD, it is possible that the reappraisal of PTP will contribute to a shift toward a more widespread use of this imaging technique, something that will warrant further investigation.
In conclusion, the adoption of the new PTP method, while retaining the same easy-to-use tabular method, offers significantly improved performance over the previous model and may lead to a shift in the selection of non-invasive testing.
LimitationsSome limitations of this study should be acknowledged. First, we studied a relatively low-risk population referred for CCTA, and so extrapolation of these findings to other subsets of patients should be performed with caution. Second, our definition of obstructive CAD was based on CCTA, which has a tendency to overestimate stenosis severity; nonetheless, in order to reduce the false positive rate, whenever ICA was subsequently performed patients were reclassified accordingly. Third, patients with dyspnea were excluded from the analysis, since the method proposed by the 2013 guidelines only applies to patients with some form of chest pain. Also, the presence of cardiovascular risk factors was determined based on patient self-reporting, CCTA requests by referring physicians, and electronic medical records, but not on specific blood tests or detailed medical history. Finally, clinical outcomes were not assessed or correlated with the PTP methods, as this was beyond the scope of this study.
ConclusionIn patients with stable chest pain undergoing CCTA, the updated 2019 prediction model provides a more accurate estimation of the PTP of obstructive CAD than the previous model. Adoption of this new score may improve disease prediction and may lead to a shift in the selection of non-invasive testing toward anatomical rather than functional tests.
Conflicts of interestThe authors have no conflicts of interest to declare.
None to disclose.


![Receiver operating characteristic curves of the two models for the prediction of obstructive coronary artery disease on coronary computed tomography angiography. The two models showed similar discriminative power, with C-statistics of 0.730 (95% confidence interval [CI]: 0.658-0.802) and 0.735 (95% CI: 0.663-0.808) for the 2013 and 2019 methods, respectively (p for comparison 0.933). ESC: European Society of Cardiology; MDF: modified Diamond-Forrester model; PTP: pre-test probability. Receiver operating characteristic curves of the two models for the prediction of obstructive coronary artery disease on coronary computed tomography angiography. The two models showed similar discriminative power, with C-statistics of 0.730 (95% confidence interval [CI]: 0.658-0.802) and 0.735 (95% CI: 0.663-0.808) for the 2013 and 2019 methods, respectively (p for comparison 0.933). ESC: European Society of Cardiology; MDF: modified Diamond-Forrester model; PTP: pre-test probability.](https://static.elsevier.es/multimedia/08702551/0000004100000006/v3_202206160259/S0870255122000683/v3_202206160259/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)


