Acute myocardial infarction (AMI) in coronary heart disease is a leading cause of sudden death primarily due to malignant ventricular arrhythmias (VAs). Inflammatory cell infiltration and inflammation-induced overactivation of sympathetic nerves are the major cause of VAs in AMI pathophysiological processes. Type 2 macrophages play an anti-inflammatory role in AMI. Targeting macrophages may be a therapeutic strategy to prevent VAs post AMI. We found that gamma aminobutyric acid (GABA) promotes macrophages polarized to M2 and hypothesized that GABA might exert anti-inflammatory effects by promoting type 2 macrophage polarization in AMI. We aim to characterized GABAB receptor distribution, function, and mechanisms in M2 macrophage polarization and explored the functional aspect of GABAB receptor activation in sympathetic remodeling.
ResultsGamma aminobutyric acid B receptors were expressed on macrophage surface both in vitro and in vivo. GABAB receptor agonist baclofen, GABA promoted macrophage switch to M2. While GABAB receptor antagonist CGP52432 blocked a baclofen induced switch to M2 polarization. GABA and baclofen increased M2 macrophage percentage and CGP52432 blocked this process in vivo. Also, IL-10 and TGF-β1 released by M2 were increased in both AMI and baclofen/AMI group; Serum NE levels were decreased by baclofen. All the above effects were reversed by CGP52432 treatment. Baclofen decreased TH and GAP-43 staining while CGP52432 enhanced their expression post AMI indicating GABAB receptor activation inhibited sympathetic nerve sprouting and activity by reducing NE release.
ConclusionsGamma aminobutyric acid B receptor activation promoted M2 polarization and protested AMI heart by regulating sympathetic nerve remodeling.
O enfarte agudo do miocárdio (EAM) é uma importante causa de morte súbita, sobretudo por arritmias ventriculares (AV) malignas. A infiltração por células inflamatórias e a hiperativade simpática induzida pela inflamação são uma das principais causas de AV no EAM, no qual os macrófagos do tipo 2 podem desempenhar um papel anti-inflamatório. Os macrófagos podem, pois, ser o alvo de estratégias terapêuticas para prevenir as AV após EAM. Assim, este estudo pretende avaliar se o ácido gama-aminobutírico (GABA) poderá ter um papel anti-inflamatório ao promover a polarização dos macrófagos do tipo 2 no EAM.
MétodoCaracterizamos a distribuição, função e os mecanismos dos recetores GABAB na polarização de macrófagos M2 e avaliamos o impacto funcional da ativação dos recetores GABAB na remodelagem simpática.
ResultadosOs recetores GABAB são expressos na superfície dos macrófagos, tanto in vitro como in vivo. O agonista do recetor GABAB baclofeno e o GABA promoveram a mudança dos macrófagos para M2, enquanto o antagonista do recetor GABAB, CGP52432, bloqueou a mudança induzida pelo baclofeno para M2. O GABA e o baclofeno aumentaram a percentagem de macrófagos M2 e o CGP52432 bloqueou este processo in vivo. Além disso, os níveis de IL-10 e de TGF-β1, libertados pelo M2, aumentaram tanto no grupo EAM como no grupo baclofeno/EAM. A redução dos níveis séricos de NE pelo baclofeno demonstrou uma redução da atividade simpática. Todos os efeitos acima referidos foram revertidos pelo tratamento com CGP52432. O baclofeno diminuiu a expressão de TH e de GAP-43, enquanto o CGP52432 aumentou a sua expressão após o EAM, indicou que a ativação do recetor GABAB inibiu a atividade dos nervos simpáticos e reduz a libertação de NE.
ConclusãoA ativação do recetor GABAB promoveu a polarização de M2 e revelou efeitos protetores no EAM ao regular a remodelagem simpática.
Acute myocardial infarction (AMI) in coronary heart disease is a leading cause of sudden death primarily due to lethal arrhythmias, particularly ventricular arrhythmias (VAs). One mechanism of VAs post AMI is the sympathetic nerve sprouting and hyperinnervation caused by adverse cardiac remodeling post AM.1–3 A healing response coincides with a general inflammatory response to clear and replace injured myocardium with scar tissue and eventually resolve inflammation. However, excess inflammation leads to adverse ventricular and nerve remodeling, thereby promoting heart failure (HF) development and VAs.4 The injured myocardium attracts monocytes from circulating blood that infiltrate into the myocardium and differentiate into macrophages.5,6 Two well-established polarized macrophage phenotypes, M1 and M2, are involved in different stages post AMI, in which M1 macrophages dominate the cellular infiltrate, clear cellular debris,7 and secrete cytokines, chemokines and growth factors.4 Additionally, M2 macrophages coordinate the consequent phases of healing and tissue regeneration after inflammation.8 However, the prolonged presence of M1 macrophages causes infarct area expansion by creating a pro-inflammatory environment post AMI,9 and delayed transition to M2 macrophages leads to HF due to adverse remodeling of injured myocardium. Targeting M1 macrophages to diminish the duration of the inflammatory phase has been shown to improve functional cardiac output post MI,9,10 and macrophage polarization toward the M2 phenotype was shown to promote the resolution of inflammation and improve infarct healing post MI.11 Therefore, modulating macrophage polarization may beneficially influence healing following MI.10,12
Macrophage polarization depends on multiple processes, including interaction with other leucocytes and systemic factors such as T regulatory cells (Tregs), bacterial lipopolysaccharide (LPS), interferon-gamma (IFN-γ) and interleukins. Polarization to M2 macrophages can be achieved by Tregs (in vivo), IL-4, IL-10, IL-13 or transforming growth factor beta (TGFβ) (in vitro) stimulation. Preclinical animal studies have shown that a switch or stimulation toward M2-like macrophages ameliorate infarct healing and diminish the development of adverse cardiac remodeling post MI.4 Influencing the above factors in clinical setting has been suggested as a therapeutic strategy. For example, loaded nanoparticles to target specific cell types are regarded as a promising treatment technology.12,13 In this study, we discussed a new target in M2 macrophage polarization without interacting with these factors, which are easily delivered and effective in myocardial healing post MI: GABA and the GABAB receptors in macrophages.
Gamma aminobutyric acid, the major inhibitory neurotransmitter in the brain, is reported to be functionally expressed in immune cells of both rodents14,15 and humans16,17 by binding with GABAA or/and GABAB receptors on the cell surface. In macrophages, GABA may induce inward currents by electrophysiological study14 and inhibits LPS-induced cytokine production (IL-6 and IL-12) in mouse peritoneal macrophages18 through GABAA receptors, indicating the suppression effects of GABA on macrophages. However, less is known about GABA in macrophage polarization, which is most important in infarct cardiac tissue post MI. Thus, we investigated the role of GABA in macrophage polarization both in vitro and in vivo, and the pathophysiological significance in sympathetic remodeling post MI.
MethodsThis study was conducted in accordance with the guidelines approved by the Committee on Animal Care and Use of Qianfoshan Hospital, and in accordance with the National Institutes of Health Guide for Laboratory Animal Care and Use of Laboratory animals (NIH Publications No. 8023, revised 1978). A special technician in our laboratory provided good care for all the animals during the experiment in an SPF barrier system. Animals were sacrificed via an injected overdose of 3% sodium pentobarbital at the end of experiment.
Macrophage cultureCryopreserved RAW 264.7 macrophages (mouse, BNCC 337875, China) were recovered in 37°C water, and centrifuged at 1500 rpm for 5 min with 3 ml Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum (FBS, Gibco, 10099141, USA). Cells were seeded in 60 mm dishes with a density of 5000 cells/cm2 with or without coverslips and incubated the cells at 37°C in 5% CO2. The cell culture medium was replaced with fresh DMEM every two days. Cells were used for immunofluorescent staining and drugs treatment when cell density reached 80% or above.
Chemicals and antibodiesThe GABAB receptor agonist baclofen (10-7 mol/L, 1048200, Sigma) and antagonist CGP52432 (10-7 mol/L, HY103531, MedChem Express) were used to treat cells. For animal experiments: GABA (orally, 500 mg/kg·d), Baclofen (10 mg/kg·d),19 CGP52432 (3 mg/kg d)20,21 were administrated. Antibodies used for immunofluorescence staining: Anti-GABAB receptor-1 Ab (1:100, ab55051, Abcam), anti-GABAB receptor-2 Ab (1:100, ab52248, Abcam), anti-CD206 Ab (1:100, 18704-1-AP, Proteintech), anti-CD68 Ab (1:50, HM3029, Hycult), anti-tyrosine hydroxylase (TH) Ab (1:200, ab1542, Millipore) and anti-growth-associated protein 43 (GAP 43) Ab (1:200, ab16053, Abcam), Alexa 594-conjugated goat anti-rabbit Ab (1:600, R37117, Invitrogen), FITC-conjugated goat anti-mouse Ab (1:200, ab6785, Abcam), and FITC-conjugated rabbit anti-sheep Ab (1:200, ab6743, Abcam). Antibodies used in western blot: anti-p-ERK1/2 (1:1000, 4370, CST), anti-ERK1/2 (1:1000, 9102, CST), anti-GABAB receptor-1 Ab (1:100, ab55051, Abcam), anti-GABAB receptor-2 Ab (1:100, ab52248, Abcam).
Immunofluorescence stainingCultured macrophages and iced-tissue slices were fixed with cold acetone for 10 minutes at room temperature (RT). Then the slices were washed with PBS for three times followed by blocking with 5% BSA in PBS at RT. The following procedures were the same as the immunofluorescent protocol as described in our previous article.22 Images were acquired with Nikon Eclipse Ti2 confocal microscope and NIS software, and the Olympus X100 Imaging System. ImageJ software (version 1.38x; National Institutes of Health, Bethesda, MD) was used to analyze the fluorescent density of target proteins.
Western blottingRAW264.7 cells were collected after treatment with different drugs for whole cell or cell membrane protein extraction with RIPA lysis buffer (P0013B, P0033, Beyotime)/PMSF (10:1) for western blot as we have previously described.22 Images were obtained with FluorChem E Imager (Protein-Simple, Santa Clara, CA, USA) by adding ECL (Millipore, Billerica, MA, USA) on the membranes. ImageJ software was used to analyze the protein band density of target proteins.
Animal modelAdult healthy male Sprague-Dawley (SD) rats (8–10-week-old, 230-260 g) provided by Vital River (Beijing, China) were used in the present study. All the animals were anesthetized by intraperitoneal injection with 3% pentobarbital sodium (30 mg/kg) if not specifically notified. Myocardial infarction model was performed as previously described.22
All the experimental animals were randomly divided into five groups before surgery, sham, MI group, MI+GABA (orally, 500 mg/kg.d) group for observing GABA's function post MI, MI+Baclofen group (Baclofen, 10 mg/kg),19 MI+Baclofen+CGP52432 group (CGP52432, 3 mg/kg)20,21 for observing GABAB receptor function post MI.
Enzyme-linked immunosorbent assayThe blood samples at day seven of MI were collected for ELISA test.22 ELISA tests were performed according to the operating instructions provided by the ELISA kit. The OD value at 450 nm was used to determine protein concentrations by the end. ELISA kits used in this study: norepinephrine (NE) ELISA kit (JL13428, j&L Biology), IL-12β ELISA kit (JL15573, j&L Biology), TGF-β1 ELISA kit (JL12342, j&L Biology), IL-10 ELISA kit (JL13427, j&L Biology).
StatisticsSPSS 17.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis of data. All data was represented by means ± standard errors (SE). Differences among groups were analyzed by t test or analysis of variance followed by least significant difference testing. A value of p<0.05 was considered significant.
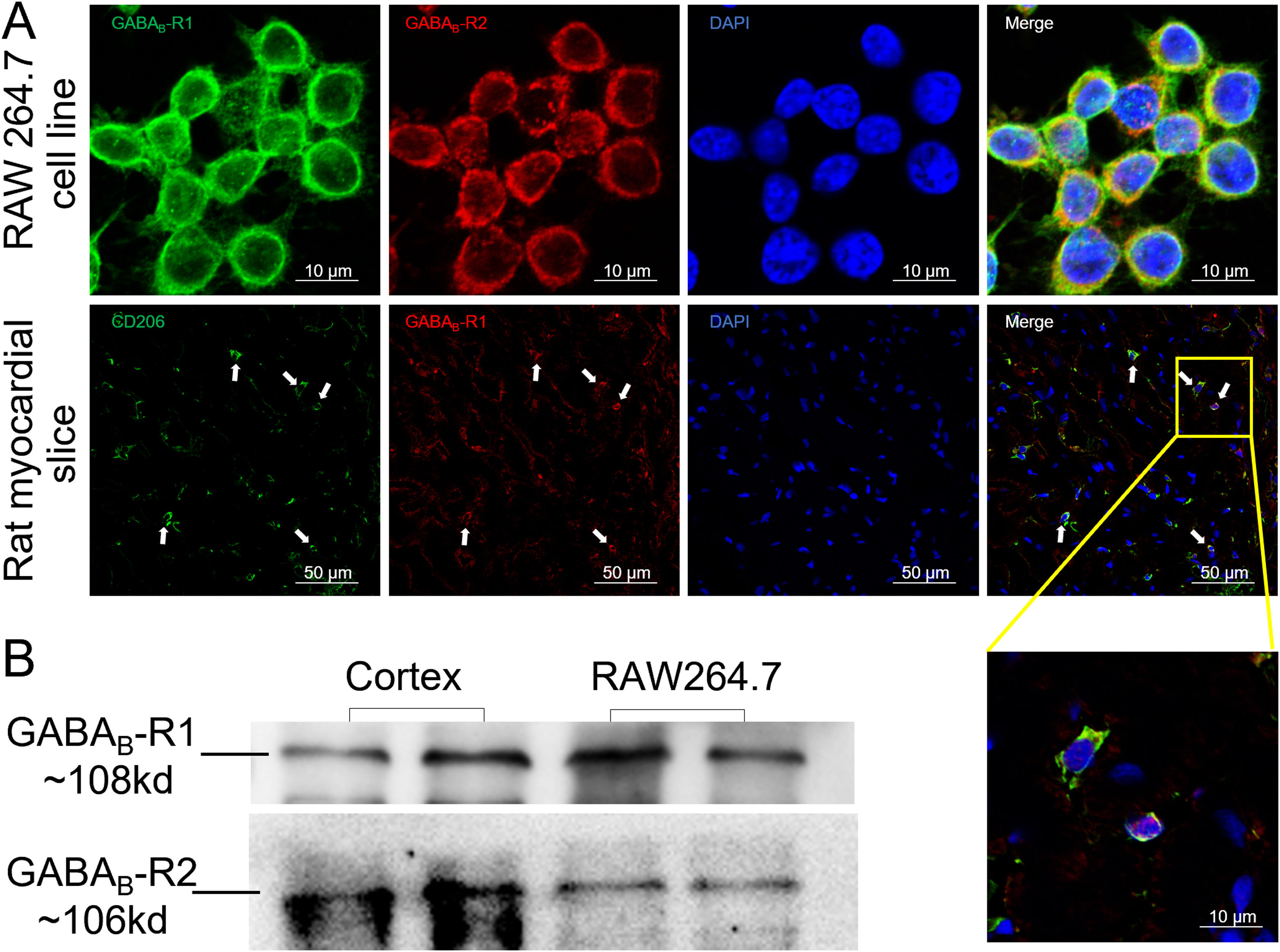
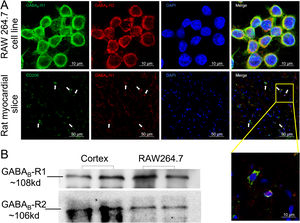
ResultsGamma aminobutyric acid B receptors were expressed on macrophagesTo determine whether GABAB receptors were distributed on macrophages in vitro and in vivo, we performed immunofluorescence staining on both macrophage cell lines (RAW264.7) and rat myocardial tissues. The data in Figure 1A showed that GABAB receptor R1 and R2 subunits were located on the cell surface of RAW264.7 (upper panel of Figure 1A). Meanwhile, myocardial slice showed a double staining of CD206, the M2 type macrophage marker, and GABAB receptor R1 subunit (Figure 1A, lower panel). We also detected the GABAB receptor R1 and R2 subunits bands from the membrane proteins of RAW264.7 by western blot (Figure 1B) indicating the expression of GABAB receptors on the macrophage membrane.
GABAB receptors expressing on macrophage surface.
A, GABAB receptor R1 (green) and R2 (red) subunits were positively stained in macrophage cell line RAW264.7 (upper panel). The GABAB receptor subunit R1 (red) colocalized with CD206-positive cells (green) in rat cardiac tissues (white arrow) (the lower panel). Cell nuclei were stained blue with DAPI. B, Western blot data showed GABAB receptor R1 (green) and R2 subunits were detected from RAW264.7 cell membrane. Rat cortex was used as positive control.
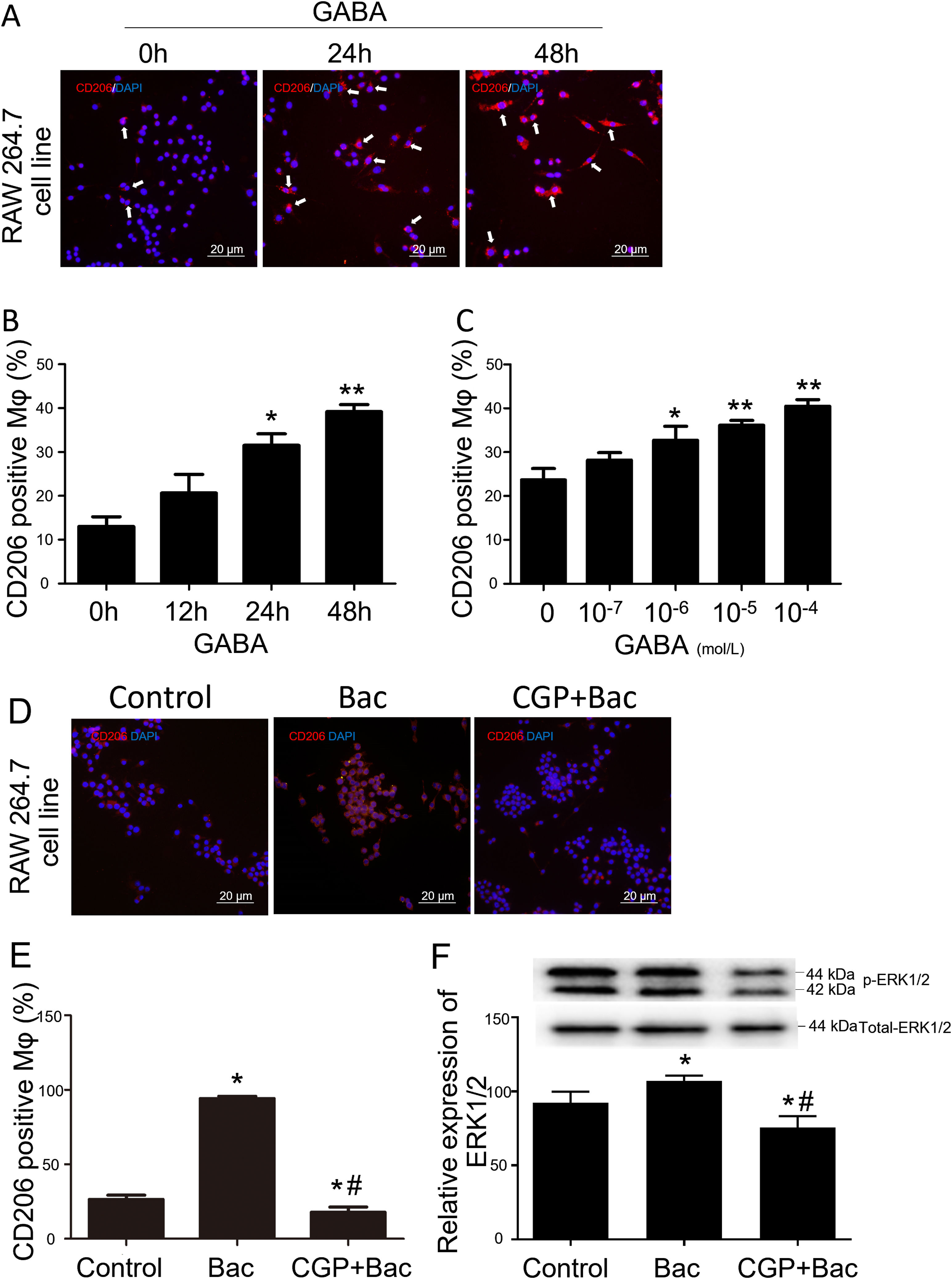
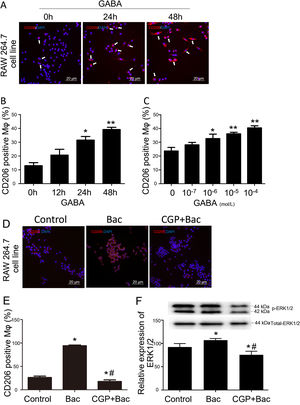
To test whether GABAB receptors on macrophage surface were involved in macrophage type switches or polarization, we assessed the percentage of CD206 positive cells to check our hypothesis. The M2 macrophage ratio is represented by CD206 positive cells/total cells for each observation scale. The results showed that CD206 positive cells were increased by 141% and 200% separately when treated with GABA for 24 and 48 hours compared with 0-hour group (Figure 2A and B). CD206 positive cells were increased by 38.21%, 52.68% and 71.04% in response to 10-6 M, 10-5 M and 10-4 M of GABA (Figure 2C) compared with control group (0-mol/L group) after 24-hours treatment. These are of significant difference compared with the control group (p<0.05).
GABAB receptor activation promote M2 macrophage polarization.
A, Representative graphs. GABA at 10-6 M increased CD206 positive cell (white arrow, stained red) percentage in RAW264.7 cell. B, GABA promoted expression of CD206 on macrophage surface in the time dependent manner. ‘*’: p<0.05, ‘**’: p<0.01, compared with 0-h group. C, GABA promoted expression of CD206 on macrophage surface in a dose dependent manner after 24 hours incubation with GABA. ‘*’: p<0.05, ‘**’: p<0.01, compared with control group (0 mol/L GABA). D, Representative graphs of cells treated with vehicle, baclofen, CGP52432+baclofen. E, Baclofen increased CD206 positive cells significantly and CGP52432 blocked baclofen induced elevation of CD206 positive cells. ‘*’: p<0.05, compared with control group.’#’: p<0.05, compared with baclofen group. F, Baclofen increased phospho-ERK1/2 which was blocked by CGP52432 significantly. The upper panel showed the representative bands of p-ERK1/2 to total ERK1/2. ‘*’: p<0.05, compared with control group.’#’: p<0.05, compared with baclofen group. Bac: baclofen group; CGP+Bac: CGP52432+baclofen group.
Baclofen and CGP52432, the selective GABAB receptor agonist and antagonist, respectively, were used to test whether M2 macrophage polarization was mediated by GABAB receptor activation. In vitro study on macrophage cell line (RAW264.7), the results turned out to be M2 type dominant (256% elevation compared with control group, p<0.01) when cells were treated with baclofen, while CGP52432 blocked the increased expression of CD206 by 81% on macrophages compared with baclofen group (Figure 2D and E).
To disclose the mechanism underlying how GABAB receptors are involved in M2 macrophage polarization, we tested the MAPK/ERK pathway by evaluating phosphorylation of ERK1/2. Additionally, the results showed a significant increase in the p-ERK1/2 level in the baclofen group compared with that of the control group (p<0.05; Figure 2F), while CGP52432 reversed the effect caused by baclofen significantly (p<0.05; Figure 2F) indicating GABAB receptor mediates baclofen-induced M2 macrophage polarization.
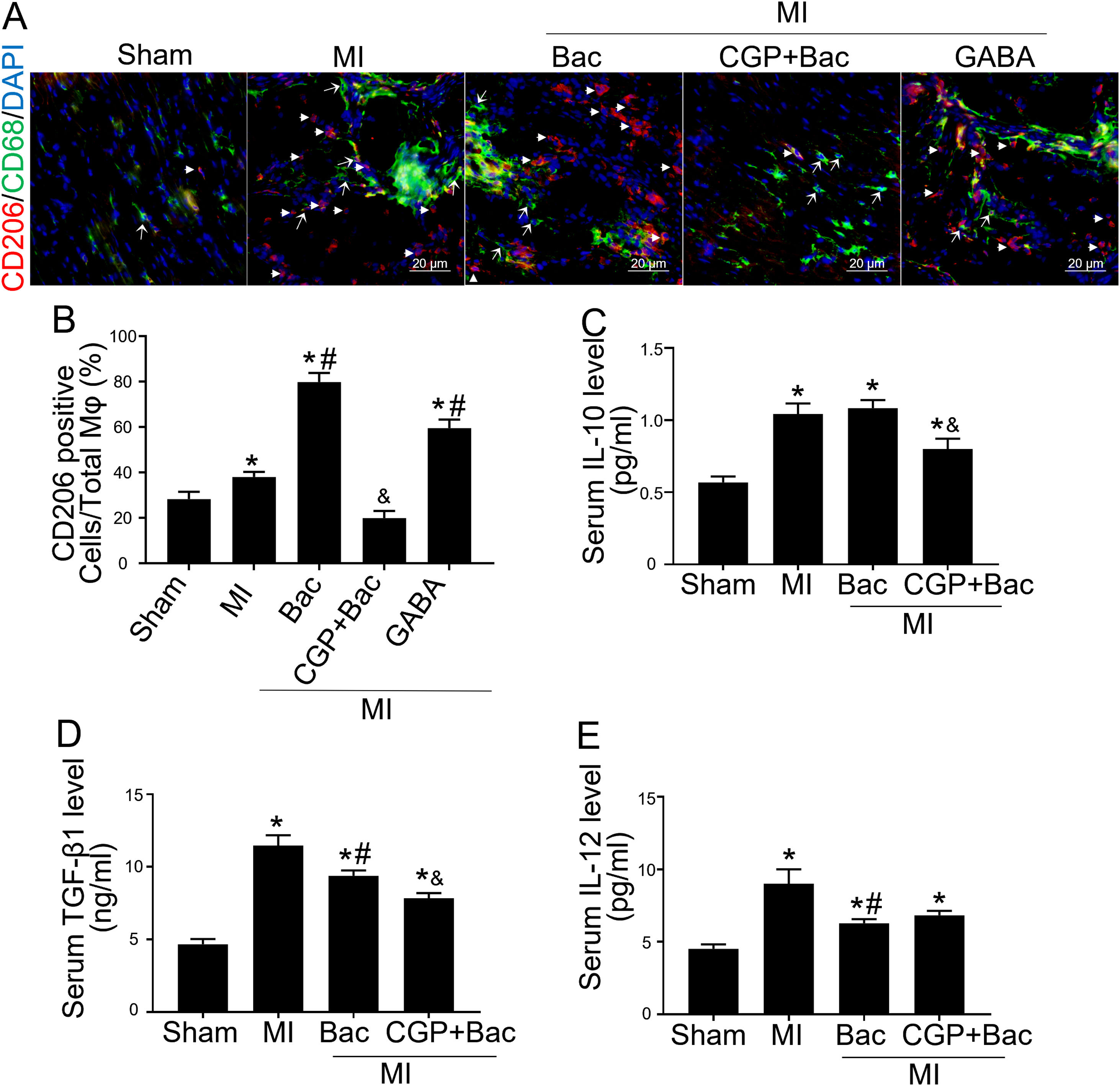
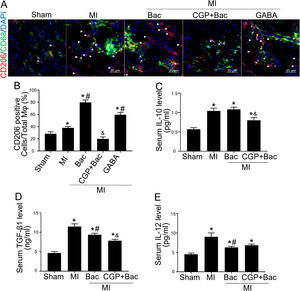
Gamma aminobutyric acid B receptor agonist promotes M2 macrophage polarization in vivo and protects the heart from myocardial ischemia injuryTo investigate the in vivo effect of GABAB receptor activation, we performed rat myocardial infraction models to test inflammatory state and cardiac sympathetic regeneration in response to GABAB receptor agonist and antagonist at day 7 post MI. Immunofluorescent results showed that CD206 positive cells were significantly increased in baclofen and GABA groups (p<0.05 compared with MI group) (Figure 3A and B). While the GABAB receptor antagonist, CGP52432 reversed the elevation of CD206 positive cells (Figure 3A and B) indicating GABAB receptor activation was involved in M2 macrophage polarization. Serum IL-10 and TGF-β1 were significantly increased in both MI and baclofen groups compared with the sham group (p<0.05), while CGP52432 partially blocked the elevated IL-10 and TGF-β1 (p<0.05 compared with the baclofen group) (Figure 3C and D). Baclofen also decreased serum IL-12 level significantly after MI (p<0.05 compared with MI group), but CGP52432 did not alter serum IL-12 level compared with the baclofen group (Figure 3E). The increased M2 macrophage percentage decreased the level of IL-10 and TGF- β1 indicating that GABAB receptor activation promoted M2 macrophage polarization in vivo.
GABAB receptor activation promoted M2 macrophage polarization in vivo.
A, Representative graphs of CD206 positive cell staining (red, white arrow head) in cardiac tissue slice from MI rats of con, Bac, CGP+Bac and GABA groups. CD68 positive cells were stained green (white arrow). B, MI, Bac and GABA groups increased CD206 positive cell number significantly (‘*’: p<0.05, compared with MI group). Baclofen and GABA induced a higher CD206 positive cell percentage comparing with MI group (con in figure) (‘#’: p<0.05). CGP52432 decreased CD206 positive cell percentage comparing with Bac group (‘&’: p<0.05). C and D, Serum IL-10 and TGF-β1 levels were increased in all MI surgery groups (‘*’: p<0.05, compared with Sham group). CGP52432 decreased IL-10 and TGF-β1 level significantly comparing with Bac group (‘&’: p<0.05). Baclofen decreased TGF-β1 level comparing Con group (‘#’: p<0.05). E, Serum IL-12 level were increased in MI groups comparing sham group (‘*’: p<0.05). Baclofen inhibited IL-12 secretion significantly comparing Con group (‘#’: p<0.05). Sham: surgery without ligating LAD; MI: myocardial infarction group; Bac: baclofen group; CGP+Bac: CGP52432+baclofen group.
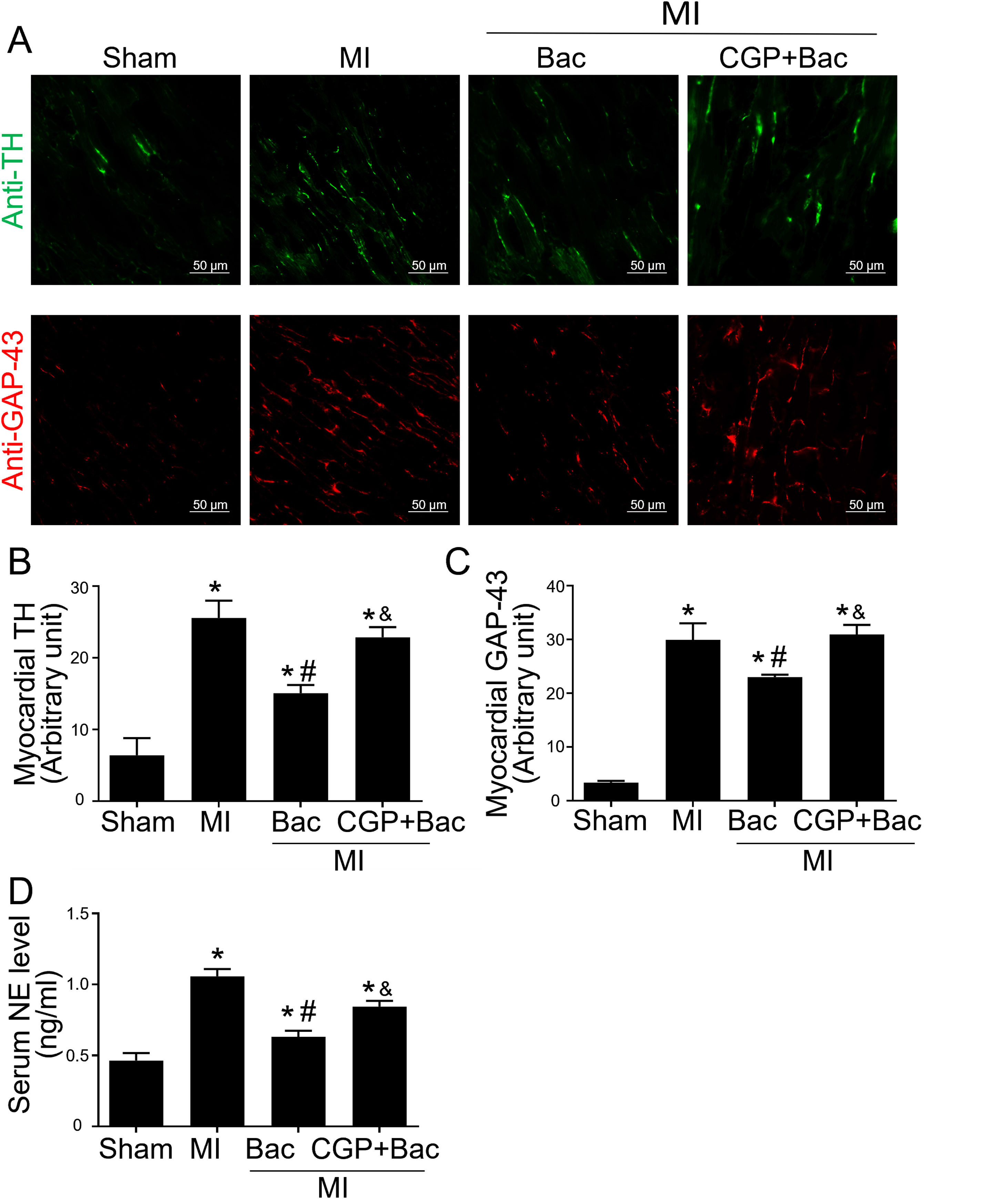
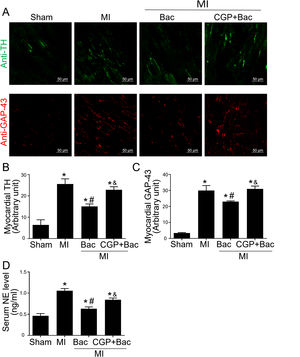
The above results indicated that GABAB receptor activation protected the infarct myocardium through M2 macrophage polarization. However, the functional consequence of macrophage GABAB receptor activation remains unclear. Therefore, we explored the effects of GABAB receptor activation on cardiac sympathetic nerve regeneration and sprouting whose adverse remodeling leads to VAs. TH was used to test the functional sympathetic nerves, and GAP-43 to detect the regeneration and spouting status of the sympathetic nerve in the para-infarct area. Immunofluorescence results showed increased TH and GAP-43 staining in the MI group, while there was a lower TH and GAP-43 positive staining in the baclofen group compared with sham group (Figure 4A-C). CGP52432 attenuated the decreased TH and GAP-43 expression induced by baclofen (Figure 4A-C). Meanwhile, we assessed the serum NE levels to determine the sympathetic activity. The results showed baclofen decreased NE levels significantly compared with MI group (p<0.05) and CGP52432 partially blocked baclofen-induced decrease of serum NE level when comparing baclofen and CGP52432+baclofen group (p<0.05) (Figure 4D) indicating that GABAB receptor activation reduced sympathetic nerve activity in vivo.
GABAB receptor activation inhibited cardiac sympathetic nerve regeneration post MI.
A, Representative graph of TH (green) and GAP-43 (red) staining in the four groups, Sham, MI, Bac/MI, CGP+Bac/MI. B and C, TH and GAP-43 immunofluorescent density were significantly increased in MI groups comparing with sham group (‘*’: p<0.05). Baclofen treated rats showed a decreased TH and GAP-43 staining comparing with Con group (‘#’: p<0.05). CGP52432 abolished baclofen included downregulation of expression of TH and GAP-43 (‘&’: p<0.05). D, Serum NE levels significantly increased in MI groups comparing with Sham group (‘*’: p<0.05). Baclofen decreased NE level significantly comparing with Con group (‘#’: p<0.05). CGP52432 abolished baclofen included downregulation of serum NE level (‘&’: p<0.05). Sham: surgery without ligating LAD; MI: myocardial infarction group; Bac: baclofen group: myocardial infarction animals received baclofen (baclofen, 10 mg/kg); CGP+Bac: CGP52432+baclofen group.
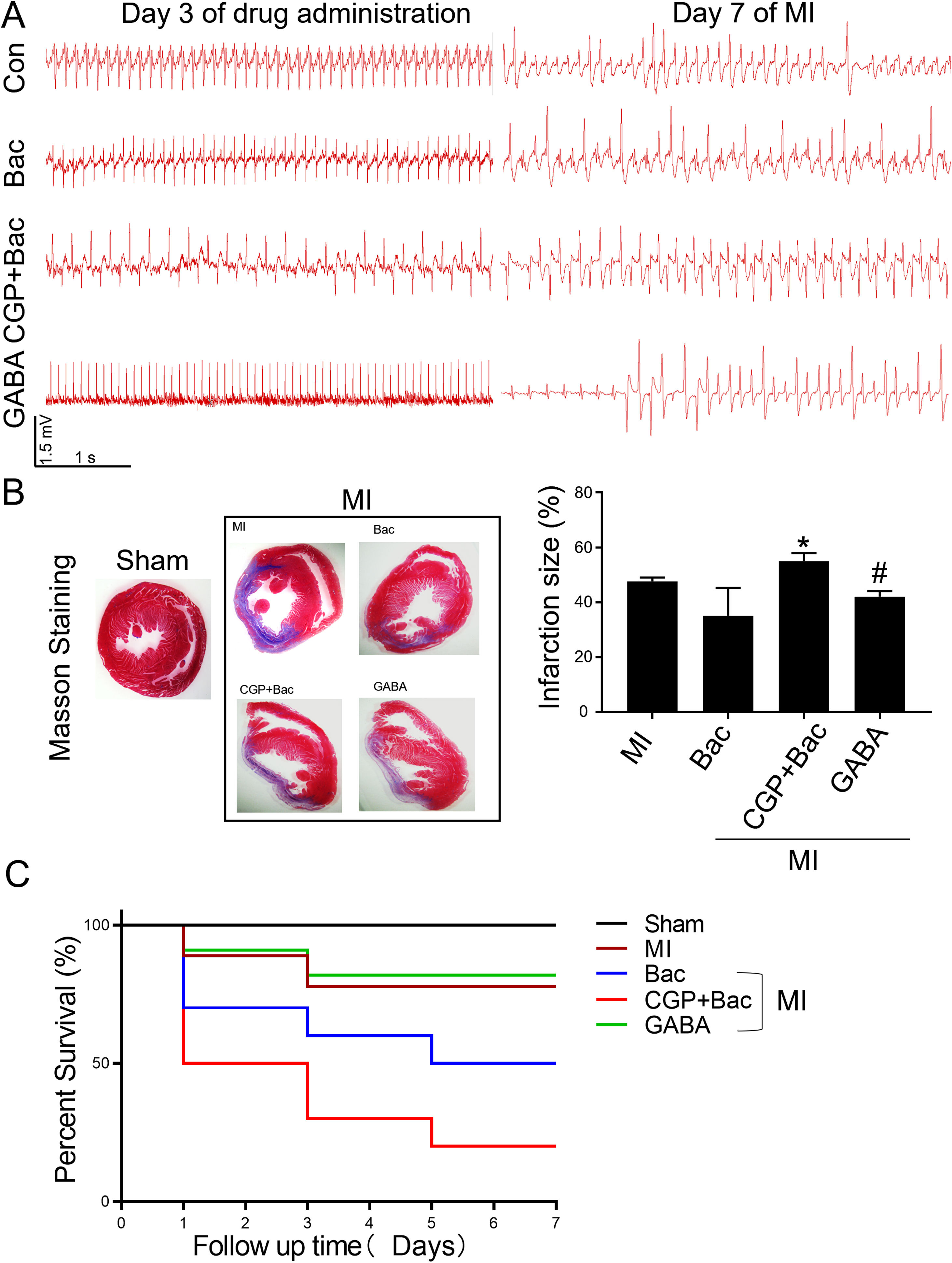
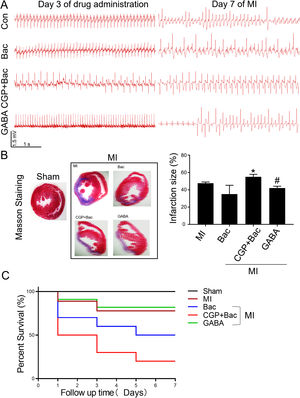
To test the protective effect of GABAB receptor activation on myocardial infarction injury, we recorded the ECGs before and seven days after MI surgery and assessed the myocardial infarct size in all the MI groups. The ECGs recorded before MI surgery showed a relatively normal rhythms except for the CGP52432 group for there were always Premature Ventricular Contractions (PVC) (8 in 10 rats) indicating GABAB receptor might physiologically regulate cardiac functions. At day 7 after MI, almost all the animals showed different kinds of arrhythmias, in which ventricular tachycardiac (VT) was the most common as shown in the represent traces in Figure 5A. The infarct size was decreased significantly in GABA/MI group compared with MI group (p<0.05), but not baclofen/MI group (Figure 5B). Meanwhile, the mortality of MI rats was significantly higher when GABAB receptors were blocked with CGP52432 compared with GABA/MI group (Figure 5C), implying the protective effect of GABAB receptor activation in vivo.
GABAB receptor activation on MI infarct size.
A, Representative Electrocardiogram (ECG)s of Con, Bac, CGP+Bac, and GABA groups 3 days after drug administration and 7 days after MI. B, Massion staining of myocardium in Sham and MI groups (MI, Bac, CGP+Bac, and GABA) (left figure). The bar graph showed CGP52432 increased infarct size comparing with Bac group (‘*’: p<0.05). GABA decreased the infarct size significantly comparing with MI group (‘#’: p<0.05). C, Kaplan-Meier survival curves showed survival rates and mortality. Mortality in CGP+Bac group was 80% and increased significantly (p<0.05) comparing with Bac group. Sham: surgery without ligating LAD; MI: myocardial infarction group; Bac: baclofen group; CGP+Bac: CGP52432+baclofen group.
Gamma aminobutyric acid B, the primary inhibitory neurotransmitter in the central nervous system, was reported to be present in resident macrophages14,16,18 and regulates mouse pulmonary macrophages using an autocrine GABA signaling system.23 One study in 201419 reported that GABAB receptor activation promoted nerve regeneration and reduced CD68 positive macrophages in coronal sections of the sciatic nerve. Consistently, we found GABAB receptor activation promoted M2 polarization characterized by increased CD206 positive cell in cell line, and decreased CD68 positive cells in tissue slice. But different from that study, baclofen inhibited sympathetic regeneration and spouting in our animal model. This is probably due to the diversity of GABAB receptor locations that helps distinguish the functional effects. The previous study19 showed GABAB mediated effects arise from direct and indirect effect on the axonal and Schwann cell compartments, respectively. In our study, we investigated GABAB receptor activation on macrophages polarization which modulated sympathetic nerve regeneration indirectly by cytokines released from inflammatory cells. Whether GABAB receptor is expressed on cardiac sympathetic nerve endings and modulates sympathetic nerve regeneration directly needs further investigation.
There were classically activated M1 macrophages and alternatively activated M2 macrophages, according to the eliciting cells such as Th1 and Th2 cells,24,25 and this process by which macrophages differentiate into M1 or M2 macrophages driven by different stimuli of the surrounding environment is called macrophage polarization. M1 macrophages are dominant in early-stage post MI, exerting an inflammatory effect of cleaning cellular debris, secreting cytokines, chemokines, growth factors and initiating tissue regeneration coordinated by M2 macrophages in the later stage. The M2 response, evoked by IL-4 and IL-13 treatment, is characterized by increased expression of CD206 in vitro.26 Studies have shown the augmentation of M2 macrophages in the early-stage prevented the process of sympathetic hyperinnervation post MI in rats.27,28 We used CD206 to mark M2 macrophages and assessed the corresponding cytokines, IL-10 and TGF-β1 released by M2 in this study, and found that increased M2 macrophage polarization in early-stage post MI reduced cardiac sympathetic hyperinnervation and nerve regeneration by immunofluorescence staining with TH and GAP-43 on day seven post MI. The mechanism of this process might be reduced nerve growth factor (NGF) secretion by M1 macrophages that is responsible for sympathetic hyperinnervation. However, the specific mechanisms need further exploration. In addition, M1/M2 macrophages are developed based on a conceptual framework representing two polar extremes of signals computed by macrophages24 which were ideal models in describing immune response in acute infections.29 Recent concept of human macrophage activation is much more complex with transcriptome-base network reflecting macrophage activation in response to kinds of stimuli29 physiologically or pathophysiologically. Our study only tested M2 macrophage polarization, which has limitations regarding the complexity of macrophage activation in response to stimuli.
Pharmacologically, we used the GABAB receptor selective agonist and antagonist baclofen and CGP52432, respectively, to test the specific functions of GABAB receptor activation in macrophage polarization, making the results more convincing. Interestingly, CD206 expression in M2 macrophages is IL-4 or IL-13/STAT6 signal pathway dependent according to previous reports.26,30 However, GABAB receptor-related M2 polarization showed IL-4/IL-13-independent CD206 expression, indicating another intrinsic GABAB receptor-dependent pathway of M2 activation, likely through the GABAB receptor/MAPK/ERK pathway elicited from our results, at least in vitro. Alternatively, GABAB receptor activation promoted CD206 expression indirectly by modulating STAT6 phosphorylation as reported in Jurkat T cells.31 Additionally, GABAB receptor blockade by CGP52432 inhibited ERK1/2 phosphorylation significantly compared with that in the control group (Figure 2F), indicating an endogenous GABAB receptor-initiated signaling pathway in the physiological condition. Meanwhile, systemic administration of CGP52432 induced an even lower M2 macrophage percentage (Figure 3A and B), indicating the endogenous GABAB receptor activation was indeed involved in M2 macrophage polarization.
Physiologically, GABA (360-420 nM) circulates in the human peripheral blood stream.32 Monocytes in blood flow are GABA pretreated before they become macrophages in tissues. However, in this study, GABA induced M2 macrophage polarization at 1 μm in vitro, indicating GABA did not alter macrophage polarization lower than 1 μm. In vivo, we used 500 mg/kg.d GABA to induce M2 polarization and alleviated sympathetic hyperinnervation. To exclude GABAA receptor effects on macrophage polarization, baclofen was used to activate only the GABAB receptor and M2 macrophage population was increased in myocardial tissue post MI. These data suggested that the GABAB receptor plays an important role in M2 macrophage polarization in vitro and in vivo. However, we did not analyze the expression levels of different GABAB receptor subunits during M2 macrophage polarization on GABAA receptors as reported previously.23 GABAB receptor subunits (2 subunits) show less tissue diversity than GABAA receptor subunits (19 subunits) and are functional dependent. Although we have established the functional protection effect after GABAB receptor activation post MI, as shown in Figures 3 and 4, baclofen alone could only partially attenuate sympathetic hyperinnervation. In this aspect, it is feasible that other mechanism(s) were coordinated with the GABAB receptor to facilitate sympathetic hyperinnervation post MI.
ConclusionsOur study uncovered a novel regulatory mechanism of macrophage phenotype switching physiologically via the GABAB receptor/MAPK/ERK signal pathway. These findings provide new targets to reduce the inflammation response during MI, protecting the heart from hyperinnervation of the sympathetic nerve caused by overwhelming inflammation.
Conflicts of interestThe authors have no conflicts of interest to declare.
The work was supported by the Technology Development Planning of Shandong Province (2016GSF201034), the National Natural Science Foundation of China (NSFC, 81570305), Science Foundation of Shandong Province (2017MH067), Shandong Provincial Health Planning Commission Science and Research Fund (2016WS0457) and the Shandong Taishan Scholarship (Suhua Yan), Medical and Health Technology Development Program in Shandong province (2017WS088), China Postdoctoral Science Foundation (2016M602154), Shandong Provincial Natural Science Foundation (ZR2020MH023).