Epicardial adipose tissue serves as a source of inflammatory cytokines and mediators. Cytokine storm is an important cause of morbidity and mortality in coronavirus disease 2019 (COVID-19).
ObjectivesTo investigate the association between epicardial fat volume (EFV), inflammatory biomarkers and clinical severity of COVID-19.
MethodsThis retrospective study included 101 patients who were infected with COVID-19. Serum inflammatory biomarkers including C-reactive protein (CRP), interleukin-6 (IL-6), procalcitonin (PCT) and ferritin levels were measured. Computed tomography images were analyzed and semi-automated measurements for EFV were obtained. The primary composite endpoint was admission to the intensive care unit (ICU) or death.
ResultsThe primary composite endpoint occurred in 25.1% (n=26) of patients (mean age 64.8±14.8 years, 14 male). A total of 10 patients died. EFV, CRP, PCT, ferritin and IL-6 levels were significantly higher in ICU patients. Moreover, a positive correlation was determined between EFV and CRP (r: 0.494, p<0.001), PCT (r: 0.287, p=0.005), ferritin (r: 0.265, p=0.01) and IL-6 (r: 0.311, p=0.005). On receiver operating characteristic analysis, patients with EFV >102 cm3 were more likely to have severe complications. In multivariate logistic regression analysis, EFV independently predicted admission to the ICU at a significant level (OR: 1.02, 95% CI: 1.01-1.03, p=0.025).
ConclusionEFV and serum CRP, IL-6, PCT and ferritin levels can effectively assess disease severity and predict the outcome in patients with COVID-19. EFV is an independent predictor of admission to the ICU in hospitalized COVID-19 patients.
O tecido adiposo epicárdico é fonte de citocinas inflamatórias e mediadores. A tempestade de citocinas é uma importante causa de morbilidade e mortalidade na doença coronavírus 2019 (COVID-19).
ObjetivosInvestigar a associação entre volume adiposo epicárdico (VAE), biomarcadores inflamatórios e gravidade clínica da COVID-19.
MétodosEste estudo retrospetivo incluiu 101 doentes infetados com COVID-19. Foram avaliados biomarcadores inflamatórios séricos, incluindo os níveis de proteína C-reativa (PCR), de interleucina-6 (IL-6), de procalcitonina (PCT) e de ferritina. Foram analisadas imagens de tomografia computorizada (TC) e foram obtidas medições semi-automáticas do VAE. O endpoint primário composto foi a admissão na unidade de cuidados intensivos (UCI) ou morte.
ResultadosO endpoint primário ocorreu em 25,1% (n=26) dos doentes (idade média 64,8±14,8 anos, 14 homens). Um total de 10 doentes morreu. Os níveis de VAE, PCR, PCT, ferritina e IL-6 foram significativamente superiores nos doentes internados na UCI. Além disso, verificou-se uma correlação positiva entre o VAE e a PCR (r: 0,494, p<0,001), PCT (r: 0,287, p=0,005), ferritina (r: 0,265, p=0,01) e IL-6 (r: 0,311, p=0,005). Na análise de regressão logistica multivariada, os doentes com VAE>102 cm3 tinham maior probabilidade de ter complicações graves.
ConclusãoO VAE e os níveis séricos de PCR, IL-6, PCT e ferritina podem avaliar a gravidade da doença e prever o resultado em doentes com COVID-19. O VAE constitui um fator preditivo na admissão dos doentes hospitalizados com COVID-19 numa UCI.
Coronavirus disease 2019 (COVID-19) is an ongoing pandemic that has already affected millions of people worldwide and is associated with significant morbidity and mortality. It is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and first occurred in Wuhan in December 2019.1 On January 30, 2020, the World Health Organization (WHO) declared COVID-19 a Public Health Emergency of International Concern and on March 11 declared it a pandemic. As of May 6, a total of 3 588 773 people in 208 countries were reported to have COVID-19, leading to more than 247 500 deaths. Early reports from China and Italy indicate that SARS-CoV-2 causes illness of varying degrees of severity.2,3 It has a wide clinical spectrum, including asymptomatic infection, mild respiratory disease, severe pneumonia with acute respiratory failure and even death. An excessive inflammatory response to SARS-CoV-2 is thought to be one of the major causes of disease severity and death in patients with COVID-19.4 Evidence suggests that cytokine release syndrome (CRS) can play a major role in severe COVID-19.5 Inflammatory cytokines and chemokines including interleukin-6 (IL-6), interleukin-1 beta (IL-1β), induced protein 10 and monocyte chemoattractant protein-1 (MCP-1) are significantly elevated in COVID-19 patients, and some were more commonly seen in severe than in nonsevere patients.5
Epicardial adipose tissue (EAT) is a fat storage tissue located beneath the pericardium, representing approximately 15% of the cardiac weight.6,7 It is a visceral fat that secretes inflammatory cytokines and mediators like MCP-1, IL-6, and tumor necrosis factor alpha (TNF-α).8 It can be measured with different imaging techniques: transthoracic echocardiography, multislice computed tomography (MSCT) and magnetic resonance imaging.9,10 Computed tomography (CT) is a reliable and reproducible method for quantification of epicardial fat volume (EFV) independent of cardiac cycle phase.11,12 Chest CT is a cornerstone in the assessment of patients diagnosed with COVID-19. Among recent developments in non-invasive imaging methods, non-gated chest CT was shown to correlate with values measured by coronary CT angiography.2,13
C-reactive protein (CRP), procalcitonin (PCT), ferritin and IL-6 are known inflammatory biomarkers and their levels have previously been reported to correlate with disease severity in COVID-19 patients.14,15 However, there are no data about the association between EFV and disease severity, nor on the correlation with these inflammatory biomarkers. Recently, Zhao emphasized the role of epicardial fat in overweight patients, who have worse prognosis during COVID-19 infection.16
Early diagnosis of serious illness is critical to early classification and improvement of patients’ prognosis. Additionally, the early identification of patients who will become severely ill could facilitate the allocation of limited medical resources to patients in need of aggressive treatment. Therefore, further research is urgently needed on early diagnosis and prognosis. In this paper, we aimed to assess the association between EFV (measured by a 128-slice MSCT scanner), inflammatory biomarkers and prognosis of COVID-19 patients.
MethodsStudy design and participantsIn this retrospective, single-center study, a total of 101 consecutive patients were enrolled who were diagnosed with COVID-19. The hospital in which the study was conducted is a designated, large-volume hospital capable of receiving severe COVID-19 patients. The diagnosis of COVID-19 was made according to WHO interim guidance and confirmed by RNA detection of SARS-CoV-2 in an on-site clinical laboratory.17 In accordance with the report of the WHO-China Joint Mission on COVID-19,18 patients with COVID-19 were divided into mild (laboratory confirmed, without pneumonia), moderate (laboratory confirmed and with pneumonia), severe (dyspnea, respiratory frequency ≥30/min, blood oxygen saturation ≤93%, PaO2/FiO2 ratio <300, and/or lung infiltrates >50% of the lung field within 24-48 h) and critical (respiratory failure requiring mechanical ventilation, shock or other organ failure that requires intensive care). Severe and critical patients were admitted to the intensive care unit (ICU), whereas patients in the mild to moderate group were admitted to the ward.18 A combination of hydroxychloroquine (HCQ), azitromycin and favipiravir was used for the treatment of severe COVID-19 cases, whereas only HCQ was used for mild to moderate cases. The study was approved by the hospital's ethics committee, which waived the requirement for written informed consent.
Data collection and analysisA doctor and a nurse were responsible for data entry into a computerized database and tracked these data daily. The data were also reviewed by a team of physicians who treat patients with COVID-19. Demographic characteristics (gender and age), comorbidities (chronic obstructive pulmonary disease [COPD], hypertension, diabetes, coronary artery disease [CAD], cerebrovascular disease and chronic kidney disease), clinical manifestations, laboratory findings, treatment and outcomes (discharge/death) were extracted from electronic medical records using a standardized data collection form.
Routine blood examinations included complete blood count (CBC), coagulation profile, serum biochemical tests (including renal and liver function, creatine kinase, lactate dehydrogenase and electrolytes), myocardial enzymes, CRP, PCT, serum ferritin, IL-6, D-dimer and arterial blood gases (lactate and PaO2/FiO2 ratio) were collected at admission. Peripheral venous blood samples were obtained from a large antecubital vein on admission. Total CBC (Sysmex K-1000, Sysmex Corporation, Kobe, Japan) and blood chemistry parameters (Roche Diagnostics, Tokyo, Japan) were determined at the hospital's biochemistry laboratory. Blood samples were placed in standardized EDTA-containing tubes for total CBC tests and measurements were performed immediately after blood sampling. Serum CRP levels were measured by immune nephelometry (NFL BN-II; Siemens Dade Behring). PCT was determined using a Biomérieux Mini VIDAS automatic fluorescence immunoanalyzer. Serum ferritin levels were detected by electrochemiluminescence (Cobas E601, Roche), as was IL-6, using the corresponding reagent. D-dimer was quantitatively determined using a Sysmax CS-5100 coagulation analyzer. Chest radiographs and CT scans were also performed for all inpatients.
Data on all treatment measures, including antibiotic, antivirus, glucocorticoid and intravenous immunoglobulin therapy and respiratory support, were acquired during hospitalization. Throat swab samples were collected from all suspected patients at admission and laboratory confirmation of SARS-CoV-2 was performed using real-time reverse transcription polymerase chain reaction according to the manufacturer's protocol (Beijing Genomics Institute and GeneoDx Biotechnology Co. Ltd.). Repeated tests for SARS-CoV-2 were performed in confirmed patients to verify viral clearance before hospital discharge. The criteria for discharge were absence of fever for at least three days, clinical improvement of respiratory symptoms and two throat swab samples negative for SARS-CoV-2 RNA obtained at least 24 h apart.
Analysis of epicardial fat volume by computed tomographyFor patients referred to the hospital with suspected COVID-19, MSCT examination was carried out at the time of admission. All imaging was performed using a 128-slice scanner (GE Revolution Evo, USA) in supine position during end-inspiration. A low-dose CT protocol was applied with the following scanning parameters: gantry rotation time 0.4 s, pitch 0.98, table speed 39.3 mm/rotation, 20 mA, 120 kVp, and a 300×300 matrix. CARE Dose 4D and CARE kV scanning parameters were off. A 1.25 mm slice thickness and 1.2 mm reconstruction interval were used for sagittal and coronal image reconstruction. After each CT, passive air ventilation was performed for at least 30 min and machine surfaces were disinfected with ethanol and didecyldimethylammonium chloride.
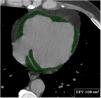
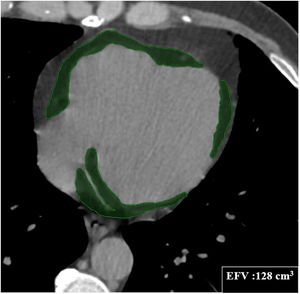
DICOM data were transferred to a picture archiving and communicating system. Then, two expert radiologists independently interpreted the images and a final decision was reached by consensus. Both radiologists were blinded to laboratory data, clinical features, and patients’ diagnosis. In the event of disagreement, the opinion of a third radiologist was used. All CT images were viewed in axial, sagittal and coronal planes. EFV was measured with a quantitative semi-automated procedure using the postprocessing software Advantage Workstation 4.7 (GE Healthcare Revolution, USA). Epicardial boundaries were manually traced to segment fat in the epicardium on axial images obtained from the pulmonary artery bifurcation to the lowest slice with a detectable pericardium (Figure 1). A CT attenuation threshold between -200 and -30 Hounsfield units was used to isolate epicardial fat.
Statistical analysisAll analyses were carried out using IBM SPSS Statistics for Macintosh, version 24.0 (IBM Corp., Armonk, New York, USA). The one-sample Kolmogorov-Smirnov test was used to assess the distribution of numerical variables. Categorical data were presented as counts (percentages), while continuous data were presented as mean ± standard deviation and median (interquartile range). The Student's t test was applied to numerical data that conformed to a normal distribution, and the Mann-Whitney U test was applied for skewed distributed variables. The chi-square test was used for categorical variables. For correlation analyses regarding EFV, Pearson's and Spearman's correlation analysis was used for data with normal and skewed distribution, respectively. Receiver operating characteristic (ROC) curve analysis was conducted to determine the cut-off values for the sensitivity and specificity of EFV in predicting ICU admission. Independent predictors of clinically severe COVID-19 were determined using logistic regression analysis. Variables that might be confounding factors or established risk factors for ICU admission in patients with COVID-19 such as diabetes, HT, CAD, COPD and EFV were included in logistic regression analyses. The effect of each variable on ICU admission was measured in univariate and multivariate analyses. A two-tailed p value of <0.05 was defined as statistically significant.
ResultsBaseline characteristicsA total of 101 patients infected with SARS-CoV-2 were retrospectively enrolled in our study. The primary composite endpoint (admission to the ICU or death) was reached in 26 (25.1%) patients. Patients’ demographic and baseline clinical features are presented in Table 1. According to these features, participants were divided into two groups: those admitted to the ICU and those admitted to the ward. Patients in the ICU were significantly older (64.8±14.8 vs. 50.6±15.8 years, p<0.001) and had more comorbidities – hypertension (46% vs. 20%, p=0.009), CAD (23% vs. 5%, p=0.009) and COPD (19% vs. 3%, p=0.004) – than those admitted to the ward. Other characteristics including gender, body mass index, smoking and diabetes showed no significant differences between the two groups. A total of 10 (38%) deaths occurred among the ICU patients. The mean age of patients who died was 71.9±14.3 years (range 44-90 years) and most were male (n=6). However, death was not observed among the 75 patients admitted to the ward. In the ICU, noninvasive ventilation was used in 15 patients (57%) and 11 patients (42%) were intubated.
Comparison of baseline characteristics of COVID-19 patients admitted to the ward and to the intensive care unit.
| Variable | Ward (n=75) | ICU (n=26) | p |
|---|---|---|---|
| Age, years | 50.6±15.8 | 64.8±14.8 | <0.001 |
| Male, n (%) | 45 (60) | 14 (54) | 0.583 |
| BMI, kg/m2 | 27.1 | 26.7 | 0.810 |
| Diabetes, n (%) | 17 (23) | 7 (27) | 0.660 |
| Hypertension, n (%) | 15 (20.0) | 12 (46) | 0.009 |
| CAD, n (%) | 4 (5) | 6 (23) | 0.009 |
| COPD, n (%) | 2 (3) | 5 (19) | 0.004 |
| Smoking n (%) | 6 (8) | 1 (4) | 0.472 |
| ICU stay, days | - | 8.5 (5-13) | - |
| Discharge time, days | 12 (9-15) | 17.5 (12-25) | <0.001 |
| Intubated, n (%) | 0 (0) | 11 (42) | <0.001 |
| Mortality, n (%) | 0 (0) | 9 (36) | <0.001 |
BMI: body mass index; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; ICU: intensive care unit.
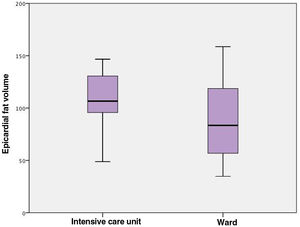
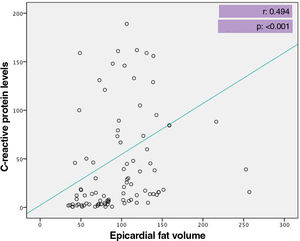
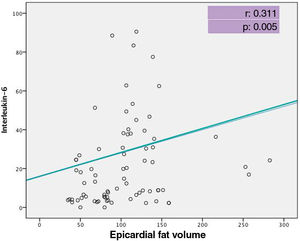
At admission, patients admitted to the ICU had significantly lower levels of total protein and albumin, hematocrit and lymphocyte count, but higher glucose, urea, aspartate aminotransferase, troponin I, D-dimer levels and white blood cell and neutrophil count compared with those admitted to the ward (Table 2). When inflammatory biomarkers were analyzed, CRP (133 vs. 12.4 g/l, p<0.001), IL-6 (45.4 vs. 8.8 pg/ml, p<0.001), PCT (0.14 vs. 0.05 μg/l, p=0.001) and ferritin (432 vs. 172 μg/l, p<0.001) levels were also significantly higher in patients admitted to the ICU than those in the ward. Moreover, patients admitted to the ICU had significantly larger EFV (115.1±44.0 vs. 94.3±45.5 cm3, p=0.037) than those admitted to the ward (Figure 2). Table 3 shows the correlation between EFV and inflammatory biomarkers including CRP, PCT, ferritin and IL-6 in all COVID-19 patients. A statistically significant positive correlation was detected between EFV and CRP (r: 0.494, p<0.001) (Figure 3), IL-6 (r: 0.311, p=0.005) (Figure 4), PCT (r: 0.287, p=0.05) and ferritin (r: 0.265, p=0.010).
Comparison of laboratory findings of COVID-19 patients admitted to the ward and to the intensive care unit.
| Variable | Ward (n=75) | ICU (n=26) | p |
|---|---|---|---|
| Glucose, mg/dl | 110±32 | 129±39 | 0.035 |
| Urea, mg/dl | 30 (24-37) | 37 (30-54) | 0.006 |
| Creatinine, mg/dl | 0.88 (0.75-1.04) | 1.01 (0.82-1.15) | 0.101 |
| AST, U/l | 25 (16-34) | 42(23-67) | <0.001 |
| ALT, U/l | 27 (19-38) | 28 (20-52) | 0.273 |
| Total protein, g/l | 7.0 (6.5-7.2) | 6.3 (5.8-6.8) | <0.001 |
| Albumin, g/l | 4.3±0.4 | 3.9±0.5 | <0.001 |
| Troponin I, ng/l | 3.0 (0-6.0) | 10.0 (6.0-22.5) | <0.001 |
| D-dimer, mg/l | 0.50 (0.29-0.82) | 1.01 (0.53-2.68) | <0.001 |
| WBC, ×109/l | 5.31(4.48-6.19) | 7.01 (5.29-9.34) | 0.002 |
| Neutrophils, ×109/l | 3.16 (2.65-4.26) | 4.87 (4.06-8.13) | <0.001 |
| Lymphocytes, ×109/l | 1.27 (0.93-1.62) | 0.78 (0.54-1.14) | <0.001 |
| Platelets, ×109/l | 211.0 (163.0-238.0) | 197.5 (168.7-262.7) | 0.741 |
| Monocytes, ×109/l | 0.33 (0.25-0.50) | 0.24 (0.18-0.46) | 0.085 |
| NLR | 2.52 (1.77-3.68) | 6.17 (4.27-15.0) | <0.001 |
| CRP, g/l | 12.4 (3.1-37.6) | 133 (59.8-189.0) | <0.001 |
| IL-6, pg/ml | 8.8 (4.0-24.1) | 45.4 (27.2-89.0) | <0.001 |
| Procalcitonin, μg/l | 0.05 (0.03-0.10) | 0.14 (0.07-0.89) | 0.001 |
| Ferritin, μg/l | 172 (60-296) | 432 (208-646) | <0.001 |
| EFV, cm3 | 94.3±45.5 | 115.1±44.0 | 0.037 |
Parameters are mean ± standard deviation or median (interquartile range).
ALT: alanine transaminase; AST: aspartate transaminase; CRP: C-reactive protein; EFV: epicardial fat volume; IL-6: interleukin-6; LMR: lymphocyte to monocyte ratio; NLR: neutrophil to lymphocyte ratio; WBC: white blood cells.
Correlation analysis between epicardial fat volume and inflammatory biomarkers in COVID-19 patients.
| Covariates | Epicardial fat volume | |
|---|---|---|
| r | p | |
| CRP | 0.494 | <0.001 |
| Il-6 | 0.311 | 0.005 |
| PCT | 0.287 | 0.005 |
| Ferritin | 0.265 | 0.010 |
| NLR | 0.181 | 0.070 |
CRP: C-reactive protein; Il-6: interleukin-6; NLR: neutrophil to lymphocyte ratio; PCT: procalcitonin.
ROC analysis was performed to assess clinical and laboratory biomarkers at the first visit for prediction of COVID-19 severity. This demonstrated that the area under the curve (AUC) of CRP, IL-6, PCT, ferritin and EFV for predicting severe COVID-19 was 0.90 (95% confidence interval [CI]: 0.84-0.96), 0.73 (95% CI: 0.60-0.87), 0.74 (95% CI: 0.63-0.85), 0.85 (95% CI: 0.74-0.96) and 0.86 (95% CI: 0.74-0.96), respectively (Table 4). ROC analysis also demonstrated that at a cut-off of 46 mg/l, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of CRP was 82%, 81%, 57%, and 93%, respectively; at a cut-off of 25 pg/ml, the sensitivity, specificity, PPV, and NPV of IL-6 were 83%, 80%, 55%, and 94%, respectively; and at a cut-off of 0.07 μg/l, the sensitivity, specificity, PPV, and NPV of PCT were 73%, 67%, 41%, and 89%, respectively. Moreover, at a cut-off of 202 μg/l, the sensitivity, specificity, PPV, and NPV of ferritin were 80%, 60%, 42%, and 89% respectively. Lastly, at a cut-off of 102 cm3, the sensitivity, specificity, PPV and NPV of EFV were 82%, 80%, 58%, and 94% respectively. ROC analysis also showed that the AUC of CRP and EFV to predict disease severity was higher than that of other inflammatory biomarkers, which demonstrated the excellent predictive power of these parameters for severity of COVID-19.
Receiver operating characteristic curves for severity in COVID-19 patients.
| Variable | Cut-off | AUC (95% CI) | Sensitivity, % | Specificity, % | PPV, % | NPV, % | PLR | NLR |
|---|---|---|---|---|---|---|---|---|
| CRP | 46.0 | 0.904 (0.843-0.965) | 82.6 | 81.1 | 57.5 | 93.7 | 4.37 | 0.21 |
| IL-6 | 25.65 | 0.857 (0.745-0.969) | 83.3 | 80.3 | 55.5 | 94.3 | 4.22 | 0.21 |
| PCT | 0.075 | 0.739 (0.606-0.872) | 73.9 | 67.1 | 41.4 | 89.09 | 2.24 | 0.39 |
| Ferritin | 202.5 | 0.740 (0.630-0.851) | 80.0 | 60.9 | 42.5 | 89.3 | 2.04 | 0.33 |
| EFV | 102.1 | 0.864 (0.742-0.967) | 82.2 | 80.6 | 58.5 | 93.9 | 4.27 | 0.21 |
AUC: area under the curve; CI: confidence interval; CRP: C-reactive protein; EFV: epicardial fat volume; IL-6: interleukin-6; NLR: negative likelihood ratio; NPV: negative predictive value; PCT: procalcitonin; PLR: positive likelihood ratio; PPV: positive predictive value.
Univariate regression analysis was performed in order to assess the relationship between clinical characteristics and ICU admission (Table 5), which showed that age (odds ratio [OR]: 1.07, 95% CI: 1.03-1.11, p<0.001), hypertension (OR: 2.90, 95% CI: 1.13-7.42, p=0.026) and EFV (OR: 1.03, 95% CI: 1.01-1.04, p<0.001) were associated with ICU admission. Multivariate regression analysis was then performed to detect independent predictors of admission to the ICU. Both age and EFV independently predicted ICU admission at a significant level (OR: 1.05, 95% CI: 1.01-1.10, p=0.013 and OR: 1.02, 95% CI: 1.01-1.03, p=0.025, respectively).
Independent predictors of admission to the intensive care unit in COVID-19 patients by logistic regression analysis.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.07 (1.03-1.11) | <0.001 | 1.05 (1.01-1.10) | 0.013 |
| Gender | 1.07 (0.44-2.60) | 0.872 | . | .. |
| Diabetes | 0.62 (0.21-1.86) | 0.391 | .. | .. |
| Hypertension | 2.90 (1.13-7.42) | 0.026 | 0.99 (0.29-3.34) | 0.987 |
| CAD | 2.96 (0.78-11.1) | 0.109 | .. | .. |
| COPD | 0.48 (0.10-2.31) | 0.362 | .. | .. |
| EFV | 1.03 (1.01-1.04) | 0.001 | 1.02 (1.01-1.03) | 0.025 |
Multivariate logistic regression model's Nagelkerke R square=0.338; -2 log likelihood=92.2; p<0.001.
Hosmer-Lemeshow test's chi-square value=9.1; p=0.331.
CAD: coronary artery disease; CI: confidence interval; COPD: chronic obstructive pulmonary disease; EFV: epicardial fat volume; OR: odds ratio.
To date, the global COVID-19 pandemic has imposed severe burdens on medical systems. It is critical to identify COVID-19 patients who may become severely ill at the first hospital admission in order to facilitate control of the disease and to improve patients’ prognosis in a context of limited medical resources. To our knowledge this is the first study to report an association between EFV and clinical severity of COVID-19. This retrospective cohort study identified several predictors of poor prognosis in adults hospitalized with COVID-19. In particular, older age, presence of comorbid diseases and elevated levels of inflammatory biomarkers including CRP, PCT, ferritin and IL-6 on admission were more commonly seen in severe COVID-19 illness. Furthermore, EFV as measured by MSCT was also demonstrated to be related to the clinical severity of these patients and was also positively correlated with the above-mentioned inflammatory biomarkers. Moreover, in multivariate logistic regression analysis both age and EFV independently predicted admission to the ICU.
EAT is a visceral fat deposit and as such has the capacity to secrete a number of cytokines and chemokines, together known as adipokines. These are biologically active molecules that are secreted by mature adipocytes themselves as well as stromal preadipocytes, macrophages, fibroblasts, mast cells and lymphocytes that are located within the adipose tissue.19 IL-6, -8, -10 and IL-1β, TNF-α, MCP-1, adiponectin, leptin and plasminogen activator inhibitor 1 are all examples of adipokines.20 In normal quantities, EAT has various putative physiological functions. However, many changes occur as EAT volumes increase. These changes are often characterized by hypertrophy, failure to store triglycerides, increased lipolysis and inflammation.21 As EAT expands, it becomes hypoxic and dysfunctional and is invaded by increasing numbers of macrophages and T lymphocytes, resulting in a shift in its metabolic profile.22 The result is increased secretion of proinflammatory cytokines such as IL-6, TNF-α, MCP-1 and many others that contribute to the inflammatory environment.8 In this setting, the beneficial paracrine effects of EAT are lost. Shimabukuro et al. showed that EFV was positively correlated with the expression of inflammatory cytokines in EAT, but was negatively correlated with adiponectin expression.23 Malavazos et al. studied the association between EAT thickness and plasma cytokine levels in severe patients with obesity. They reported that EAT thickness was positively correlated with cytokine levels (i.e. MCP-1 and the serum IL-6/IL-6 complex).24
The inflammatory response plays a critical role in COVID-19, and inflammatory cytokine storm increases the severity of COVID-19.25,26 Wang et al. found that the inflammatory response is crucial to the progression of COVID-19 and cytokine storm can lead to severe complications and death.27 The fifth edition of the Chinese Diagnosis and Treatment Protocols for COVID-19 recommends monitoring cytokine levels to improve treatment efficacy and reduce mortality,28 while the seventh edition of this guideline points out that peripheral blood inflammatory factors such as IL-6 may increase during COVID-19 infection.29
Cytokine releasing syndrome (CRS) occurs in most patients with severe COVID-19 and is known to be an important cause of death. It is a systemic inflammatory response that can be caused by infectious diseases, certain drugs and other factors, and is characterized by a sharp increase in the level of several proinflammatory cytokines.30–32 SARS-CoV-2 binds to alveolar epithelial cells, activates the innate and adaptive immune systems and thus leads to the release of large quantities of cytokines, including IL-6, which is the key molecule involved in CRS. IL-6 is an important member of the cytokine network and plays a central role in acute inflammation.33 It has both anti-inflammatory and proinflammatory effects. Liu et al. showed an increase in inflammatory cytokines (IL-6, IL-10, IL-2 and interferon gamma) in the peripheral blood in severe COVID-19 cases.34 Zhang et al. also found a relationship between higher IL-6 and CRP levels and disease progression in hospitalized COVID-19 patients.35 The present study likewise showed increased IL-6 levels in severe COVID-19 patients. In addition to this finding, a statistically significant positive correlation was detected between EFV and IL-6 level in this study.
Besides direct attack from the virus, progressive inflammatory injury has been suggested as a possible mechanism in COVID-19.1 CRP is a downstream acute phase protein in the innate immune response.36 It is produced following the increased synthesis of proinflammatory cytokines to activate the immune response.37 Serum CRP level has therefore often been used as a laboratory marker of inflammation.36,37 Zhang et al. reported CRP as a predictor of the progression of COVID-19.35 Similarly, we found significantly higher CRP levels at admission in severe cases of COVID-19 compared to mild cases. Moreover, we also demonstrated a positive correlation between CRP level and EFV. Eslami et al. investigated the association of CT-measured EAT thickness and density with clinical outcome in 87 patients with COVID-19 and found that mean EAT density was markedly lower in deceased patients compared with survivors, although this significant difference was not observed across the two groups in terms of EAT thickness.38 In the present study, we measured EFV in COVID-19 patients, rather than EAT thickness and density. Higher EFV was observed in severe patients compared to milder cases. Wei et al. studied EFV as a potential risk factor for myocardial injury in 400 laboratory-confirmed COVID-19 patients and, similarly to our result, found an association between increased EFV on admission chest CT scan and increased myocardial injury and mortality.39 They therefore suggested that assessment of EFV by chest CT scan on admission may provide a potentially useful threshold for predicting cardiovascular complications of COVID-19.
PCT is a glycoprotein without hormonal activity that is the precursor of calcitonin.40,41 Serum PCT levels are usually low or undetectable42 but are increased by bacterial infections and relatively low in viral infections, and can therefore be used to distinguish between bacterial and viral infections.43 Higher PCT levels detected in patients with severe COVID-19 usually suggest the presence of concomitant bacterial infection. Liu et al.44 recently studied PCT, CRP and IL-6 levels in COVID-19 patients, dividing them into two groups: with severe disease or with mild disease. They found significantly higher PCT, CRP and IL-6 levels in the severe group compared to the mild group. In addition, they identified all of these inflammatory biomarkers as independent factors predicting the severity of COVID-19. Our results also demonstrated higher levels of admission PCT, CRP and IL-6 levels in severe COVID-19 patients. Furthermore, we showed a positive correlation between these inflammatory biomarkers and EFV.
Serum ferritin can be elevated during infection and inflammation. High ferritin levels due to secondary hemophagocytic lymphohistiocytosis and CRS have been reported in severe COVID-19 patients. Velavan et al. recommended the use of serum ferritin, CRP and IL-6 levels in risk stratification of COVID-19 patients to predict severe and fatal COVID-19.45 In our study, we also showed significantly elevated ferritin levels in severe COVID-19 cases and a positive correlation of ferritin with EFV.
There are some limitations in this study. First of all, it is a single-center study with a relatively small sample size. Secondly, it is retrospective in nature, and the results need to be further verified by prospective studies. Thirdly, we aimed to analyze the risk factors that determine prognosis, but the poor prognosis group was small and we were therefore unable to analyze the differences in clinical characteristics of patients in this group. Fourthly, we did not include asymptomatic and mild cases managed at home, and hence our cohort may represent a more severe COVID-19 population. Fifthly, the treatment of these patients was clinically driven and did not follow a unified standard.
ConclusionEpicardial fat volume and serum CRP, IL-6, PCT and ferritin levels show a significant correlation with the severity of COVID-19. In hospitalized patients, clinicians should consider EFV in addition to serum levels of the above inflammatory biomarkers in risk stratification of patients to predict severe and fatal COVID-19. Our findings could help facilitate the early identification of severe COVID-19 patients and thus enable early treatment and intervention.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.