The MAGGIC risk score has been validated to predict mortality in patients with heart failure (HF).
ObjectivesTo assess the score ability to predict hospitalization and death and to compare with natriuretic peptides.
MethodsNinety-three consecutive patients (mean age 62±10 years) with chronic HF and left ventricular ejection fraction (EF) <50% were studied. The MAGGIC score was applied at baseline and the patients were followed for 219±86 days. MAGGIC score was compared with NT-proBNP in the prediction of events. The primary end point was the time to the first event, which was defined as cardiovascular death or hospitalization for HF.
ResultsThere were 23 (24.7%) events (3 deaths and 20 hospitalizations). The median score in patients with and without events was, respectively, 20 [interquartile range 14.2–22] vs. 15.5 [11/21], p=0.16. A ROC curve was performed and a cutoff point of 12 points showed a sensitivity of 87% and specificity of 37% with an area under the curve of 0.59 (95% CI 0.48–0.69) which was lower than that of NT-proBNP (AUC 0.67; 95% CI 0.56–0.76). The mean event-free survival time for patients above and below this cutpoint was 248.8±13 vs. 290±13.7 days (log rank test with p=0.044). Using the COX proportional hazard model, age (p=0.004), NT-proBNP >1000 pg/mL (p=0.014) and the MAGGIC score (p=0.025) were independently associated with the primary outcome.
ConclusionThe MAGGIC risk score was an independent predictor of events, including heart failure hospitalization. The addition of biomarkers improved the accuracy of the score.
O score de risco MAGGIC foi validado para predizer mortalidade em pacientes com insuficiência cardíaca (IC).
ObjetivosAvaliar a capacidade do score de predizer hospitalização e óbito e comparar com peptídeos natriuréticos.
MétodosForam estudados 93 pacientes consecutivos (idade média 62±10 anos) com IC crónica e fração de ejeção do ventrículo esquerdo (FE) <50%. O score MAGGIC foi aplicado no início do estudo e os pacientes foram acompanhados por 219±86 dias. O score MAGGIC foi comparado com o NT-proBNP na previsão de eventos. O desfecho primário foi o tempo até o primeiro evento, que foi definido como morte cardiovascular ou hospitalização por IC.
ResultadosOcorreram 23 (24,7%) eventos (3 óbitos e 20 hospitalizações). A pontuação média em pacientes com e sem eventos foi, respetivamente, 20 (intervalo interquartil 14,2-22) versus 15,5 (11/21), p=0,16. Curva ROC foi realizada e um ponto de corte de 12 pontos mostrou uma sensibilidade de 87% e especificidade de 37% com uma área sob a curva de 0,59 (IC 95% 0,48-0,69) que foi menor do que a do NT-proBNP (AUC 0,67; IC 95% 0,56-0,76). O tempo médio de sobrevida livre de eventos para pacientes acima e abaixo desse ponto de corte foi de 248,8±13 versus 290±13,7 dias (teste de log rank com p=0,044). Usando o modelo de risco proporcional COX, idade (p=0,004), NT-proBNP >1000 pg/mL (p=0,014) e o score MAGGIC (p=0,025) foram independentemente associados ao desfecho primário.
ConclusãoO score de risco MAGGIC foi um preditor independente de eventos, incluindo hospitalização por insuficiência cardíaca. A adição de biomarcadores melhorou sua acurácia.
Heart failure (HF) is a highly prevalent syndrome, with high morbidity and mortality,1–3 It affects 1–2% of the population, reaching 10% in individuals >70 years.1 The risk of an individual developing HF at 55 years of age is 35% in men and 28% in women.1 In the Brazilian population, the prevalence of HF was assessed in the city of Niterói in the DIGITALIS study,4 which appraised 633 individuals in the community and found a prevalence of symptomatic HF in 9.3% of individuals >45 years of age.
Most of the costs on the disease come from hospitalization, and therefore it is important to identify those at the highest risk not only of death but also of hospitalizations. In clinical practice, it is common to assess the stability of a patient with HF by determining the New York Heart Association (NYHA) functional class. However, this assessment is subjective and may be inaccurate. Patients in NYHA class II are believed to be stable patients. Nevertheless, the PARADIGM-HF study has shown that such patients still have room for improvement. In that study, approximately 75% of patients were in functional class I or II and would be considered stable. Even in these “stable” patients, the introduction of a new drug, sacubitril/valsartan, reduced the combined endpoint of mortality or hospitalization due to HF by 20%, compared to the standard medication, enalapril.5 This has led the medical community to question the “stability” of class I or II patients and new ways of assessing risk have been suggested.
There are several ways to assess the prognosis of patients with HF. Many clinical, laboratory and echocardiographic variables have been shown to be useful in this assessment.1–3 However, more refined methods can add information to the clinical evaluation. Ergospirometry is one of these methods, also used to indicate the need of heart transplantation.1–3 However, it has a relatively high cost and is not widely available in developing countries. Another useful test are the natriuretic peptides – the B-type natriuretic peptide (BNP) and the N-terminal pro-B-type natriuretic peptide (NT-proBNP).1–3 Natriuretic peptides, although a powerful tool in the management of HF, are also not available in all health systems in developing countries.
An objective and less expensive way to assess the risk in HF patients is the use of prediction scores. There are countless ones for evaluating patients with HF, among them the Heart Failure Survival Score (HFSS)6 and the Seattle Heart Failure Model (SHFM)7 are the most applied in clinical practice, but they seem to underestimate the patient's risk, especially in those hospitalized for decompensated HF.3 It is important to highlight that these scores have not been properly validated in a Brazilian population, which has particular characteristics.3 In addition, some of these scores are extensive or require variables that are not always available, making their wide application difficult.
In 2013, a new risk score was proposed; the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) study, analyzing 39372 patients included in 30 previous studies, created the MAGGIC Risk Score, using 13 simple variables usually measured in clinical practice.8 This score was validated in 2014, in a population of 51043 patients from the Swedish HF Registry, with good performance to estimate mortality in one and three years of follow-up.9 This score can be used for patients with HF with either reduced (HFrEF) or preserved ejection fraction (HFpEF). The application of the MAGGIC score in the population of the PARADIGM-HF study showed that despite the predominance of NYHA functional class II, the risk for some patients was not neglible.10
The derivation and validation studies of the score used mortality as an endpoint and its role to predict hospitalization due to HF has not been well established. Moreover, it is noteworthy that the score does not include natriuretic peptides in the calculation. Therefore, the aim of this study was to assess the value of the MAGGIC score in a real-world Brazilian population with stable HF in the prediction of cardiac death or HF hospitalization. Additionally, we sought to assess the performance of the score as compared with N-terminal pro-B-type natriuretic peptide (NT-proBNP).
MethodsStudy populationFrom July 2018 to July 2019, 93 consecutive outpatients with HFrEF and HF with mid-range ejection fraction (HFmrEF) from a HF outpatient clinic at an university hospital were included. Inclusion criteria were signs and symptoms of HF (at any stage of the evolution), transthoracic echocardiogram with left ventricular ejection fraction (LVEF) <50% and age greater than or equal to 18 years. People with terminal illnesses other than HF, with low life expectancy, were excluded from the study. At the time of inclusion, all patients had been followed-up for at least six months in the HF clinic and were on standard HF therapy as recommended in the HF guidelines.
The research complies with the principles outlined in the Declaration of Helsinki, and the Ethics Committee of our hospital approved the study protocol. Informed consent was requested and obtained from each patient before entry into the study.
Study designThis is a prospective, observational, longitudinal study. Patients were selected at the outpatient clinic, where clinical, laboratory, echocardiographic data were collected; the MAGGIC score was applied at the time of inclusion. LVEF was obtained with the Simpson's Rule, using the Cardiovascular system Siemens Acuson Cypress 20™ (Siemens, Erlangen, Germany). The exams were performed according to the American Society of Echocardiography and the European Association of Echocardiography recommendations for chamber quantification.
After the inclusion patients were seen at the HF clinic every three months, with a minimum follow-up of six months. Whenever necessary, the patients or their families were contacted by telephone. The primary endpoint was the time to first event, which was defined as a combination of cardiac death or HF hospitalization. The events were adjudicated by an external investigator. Hospitalization was defined as any unplanned admission to the hospital, which required an overnight stay. Hospitalizations were classified as caused by HF when they were associated with worsening symptoms of HF, with signs of fluid overload, requiring intravenous furosemide treatment.
MAGGIC score calculationThe MAGGIC risk score is composed of 13 variables used in routine clinical practice. The score is calculated using an online calculator (www.heartfailurerisk.org). After entering the variables into the calculator, a score is given and the expected mortality at one and three years is calculated. We anticipated that a low number of deaths might occur and the assessment of the score calibration might not be possible. Therefore, we used the integer score (the number of points calculated by the score) to assess the discriminatory power, not only for mortality prediction but for HF hospitalizations as well.
N-terminal pro-B-type natriuretic peptide measurementsThe measurement of NT-proBNP was performed using the immunofluorescence technique with an automatic analyzer, where the anti-BNP murine monoclonal antibody captures the NT-proBNP that is present in the sample. This complex is then linked by a second fluorescent compound labeled polyclonal antibody. The concentration of NT-proBNP is calculated by measuring fluorescence and expressed in pg/mL, starting from the standard curve of the device. Dosing was done within six hours after collection, using the Elecsys® system (Roche, Basel, Switzerland).
Statistical analysisA convenience sample of 93 patients was assessed. The normality of data distribution was appraised using the D’Agostino-Pearson test. In this assessment, the MAGGIC score had a non-normal distribution (p=0.042; symmetry coefficient (skewness) 0.2438, with p=0.31; Kurtosis coefficient −0.7746, p=0.0221). To compare numerical data, Student's t test for independent samples or the Mann-Whitney test was used. The homogeneity of the variance was tested by the Levene test. For comparisons of proportions (categorical data), the χ2 test or the Fisher's exact test was applied, when applicable. Receiver operating characteristic (ROC) curves were constructed to determine the best cut point for both the MAGGIC score and NT-proBNP to predict the primary outcome. Kaplan-Meier curves were also constructed. Multivariate analysis was performed to determine the independent relationship of variables with the primary outcome, using Cox proportional hazards model. The criterion for determining significance was the 5% level. The analysis was performed using MedCalc for Windows, version 19.5 (MedCalc Software, Ostend, Belgium).
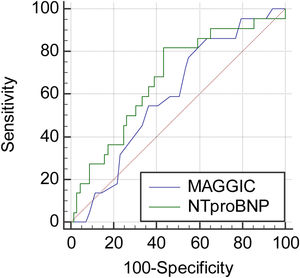
ResultsThe study comprised 93 patients with HFrEF and HFmrEF. Table 1 shows the baseline characteristics of the patients. As noted, there was a predominance of males, non-ischemic etiologies, and lower NYHA class (23.6% in class I and 58.2% in class II). Non-ischemic etiologies were hypertension (42%), alcoholic cardiomyopathy (20%), idiopathic dilated cardiomyopathy (10.8%), and diabetic cardiomyopathy (10%). Thirty-four (36.5%) patients had been hospitalized in the previous 12 months. Patients were adequately medicated, according to the recommendations of the medical guidelines. The median score of the MAGGIC calculation for the entire population was 16 (interquartile range (IQR) 11–21). This score would be equivalent to an estimate of mortality at one and three years of 7% and 17.5%, respectively. There were 23 (24.7%) events, with three deaths and 20 hospitalizations in the mean follow-up of 259±86.7 days. Median MAGGIC score in patients with and without events was, respectively, 20 (IQR 14.2–22) vs. 15.5 (11–21), p=0.16. The ROC analysis identified the cutoff of 12 points as the best cut point to discriminate the presence of events. The sensitivity for this cut point was good, but with very low specificity (sensitivity 87%, specificity 37.1%). The median NT-proBNP values in the groups >12 and ≤12 points were, respectively, 1746 (IR 565–3740) vs. 342 (IR 123.8–1255) pg/mL (p=0.002). The rate of events in patients above and below this cut point was 31.2% vs. 10.3% (p=0.019). The best cut point for NT-proBNP was 1028 pg/mL, with sensitivity of 81.8% and specificity of 56.5%. Figure 1 shows the comparison of the ROC curves between the MAGGIC score and the NT-proBNP. As observed, NT-proBNP was superior to the MAGGIC score, with the area under the curve (AUC) of 0.67 (95% confidence interval (CI) 0.56–0.76) vs. 0.59 (95% CI 0.48–0.69).
Baseline characteristics of the heart failure population (n=93).
| Characteristics | Results |
|---|---|
| Age (years) | 62±10 |
| Male sex | 56 (60.2%) |
| LVEDD (mm) | 61±18 |
| LVESD (mm) | 49±14 |
| LVEF (%) | 39±11 |
| Atrial fibrillationICD or CRTIschemic etiology | 17 (18.2%)3 (3.2%)16 (17.2%) |
| Hypertension | 63 (67.7%) |
| Diabetes mellitus | 24 (25.8%) |
| Current smoker | 19 (20.4%) |
| Alcoholism | 20 (21.5%) |
| COPD | 17 (18.2%) |
| NYHA class III or IV | 16 (17%) |
| Systolic blood pressure (mmHg) | 122.8±23 |
| Diastolic blood pressure (mmHg) | 75.7±11.8 |
| Heart rate (bpm) | 72.6±15.7 |
| Ascites | 14 (15%) |
| Hepatomegaly | 11 (12%) |
| Lower limb edema | 20 (21.5%) |
| Jugular venous distension | 17 (18.2%) |
| Body mass index (kg/m2) | 27±4 |
| Median MAGGIC score | 16 [11–21] |
| Hemoglobin (g/dL) | 12.8±2.4 |
| Fasting blood glucose (mg/dL) | 103.5±45.7 |
| Glycated hemoglobin | 6.3±1.4 |
| Blood urea (mg/dL) | 42.5 [31.8–56.5] |
| Creatinine (mg/dL) | 1.3±0.79 |
| Serum sodium (mEq/L) | 137±15.2 |
| Serum potassium (mEq/L) | 4.6±0.7 |
| Troponin T (ng/mL) | 0.125±0.38 |
| NT-proBNP (pg/mL) | 1140 (224.3–2810) |
| Digoxin | 8 (8.6%) |
| Sacubitril-valsartan | 17 (18.2%) |
| Enalapril or losartan | 74 (79.5%) |
| Spironolactone | 84 (90.3%) |
| Carvedilol or bisoprolol | 88 (94.6%) |
| Furosemide | 52 (56%) |
| Nitrate/hydralazine | 16 (17.2%) |
| Ivabradine | 9 (9.7%) |
COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronization therapy; ICD: implantable cardioverter defibrillator; LVEDD: left ventricular end diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end systolic diameter; NYHA: New York Heart Association.
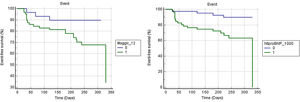
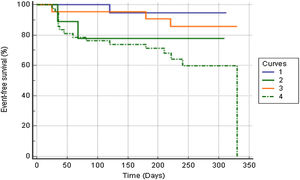
Figure 2 shows Kaplan-Meier curves for the MAGGIC score and NT-proBNP. NT-proBNP presented a survival curve with greater discrimination than the MAGGIC score curve. The mean event-free survival time for patients above and below the 12-cutpoint of the MAGGIC score was 248.8±13 vs. 290±13.7 days (p=0.045). The values for the cutoff >1000 and ≤1000 of NT-proBNP were 245.4±17.7 vs. 310.3±9.9 days (p=0.0029). As shown in Figure 3, patients with high NT-proBNP values had worse prognosis regardless of the MAGGIC score. Likewise, patients with low values of NT-proBNP had better prognosis regardless of high or low values on the MAGGIC score.
Comparison of Kaplan-Meier event-free survival curves for combined high and low values of the MAGGIC score and N-terminal pro-B-type natriuretic peptide (NT-proBNP) (p=0.018, log rank test).1=MAGGIC score ≤12 and NT-proBNP ≤1000 pg/mL; 2=MAGGIC score ≤12 and NT-proBNP >1000 pg/mL; 3=MAGGIC score >12 and NT-proBNP ≤1000 pg/mL; and 4=MAGGIC score >12 and NT-proBNP >1000 pg/mL.
Table 2 shows the Cox proportional hazards regression. Age, MAGGIC score and NT-proBNP correlated independently with the primary outcome. However, NT-proBNP outperformed the MAGGIC score. An increase of one point in the score increased the risk of the primary outcome by 10%. Patients with NT-proBNP >1000 pg/mL had a risk of death or hospitalization five times greater than below this cutoff.
Cox proportional hazards model to investigate the independent association with the primary endpoint.
| Variables | p value | HR | 95% CI |
|---|---|---|---|
| MAGGIC score | 0.025 | 1.10 | 1.01–1.2 |
| Age | 0.004 | 0.94 | 0.90–0.98 |
| Sex | 0.22 | 2.05 | 0.65–6.51 |
| Ejection fraction | 0.75 | 1.00 | 0.97–1.03 |
| Creatinine | 0.12 | 0.43 | 0.15–1.23 |
| NT-proBNP>1000 pg/mL | 0.014 | 5.36 | 1.39–20.5 |
CI: confidence interval; HR: hazard ratio; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
In the present study, the MAGGIC risk score was assessed in the real world, in an unselected population with chronic HF. The score demonstrated modest performance in predicting cardiac death or hospitalizations for HF. As natriuretic peptides are good predictors of risk and are not part of the MAGGIC score, a comparison of these tools was made, with better performance observed with natriuretic peptides. However, the score added information to the conventional variables, including NYHA class.
The derivation and validation of the MAGGIC score was performed in a European population, and there is a need for validation in other geographic and racial populations. Another issue is that the study used mortality as an outcome in an outpatient population. It is not known whether the score could predict hospitalizations or its performance in a population hospitalized for HF. To the best of our knowledge, this study is the first to assess this score in a Brazilian population and the first to assess hospitalizations as part of the outcomes in patients with HFrEF and HFmrEF. It was possible to demonstrate that, although the score seems to provide inferior prognostic information than natriuretic peptides, it was able to add information – even if modest – to the traditional evaluation. Another important aspect was that the score was able to predict hospitalizations (most of the combined events in our study were driven by HF hospitalizations). The MAGGIC score was designed to predict death at one and three years of follow-up. It is possible that if our follow-up had been longer, there could have been an improvement in the performance of the score, since a greater number of deaths would have occurred. On the other hand, the fact that a short six-month follow-up had some discriminatory power, suggests that in terms of hospitalizations, the score can be applied to predict events with less than one year of follow-up.
The MAGGIC score has already been validated in other populations, in studies in Japan,11 South Korea12 and the United States.13 These studies included recently populations hospitalized, where the score was applied at hospital discharge. Although such studies included hospitalized patients, the findings were similar to those in our study. In the study by Sawano et al., in a Japanese population,11 the score had a modest performance (discrimination by the C index of 0.71), which improved with the addition of biomarkers (C index 0.74). In spite of that, the score was well calibrated (R2 0.97). In the American study, the findings differed slightly from the previous ones.13 Two populations were studied – one outpatient and one hospitalized. The score performance in the hospitalized population was low and did not improve with the addition of natriuretic peptides. In the outpatient population, however, there was good a discrimination of the score, which improved significantly with the addition of biomarkers (C 0.78 and 0.82 statistics, respectively; p=0.002). Considered together, these studies suggest that, in general, the MAGGIC score has modest discrimination. As in the present study, the addition of biomarkers improved the accuracy of the score, something also observed in other clinical situations. For example, in diabetic patients, NT-proBNP alone was as accurate as a model formed by 20 clinical variables in predicting death and cardiovascular events and improved performance when added to clinical variables.14
The MAGGIC study has already been compared to other risk scores. In the PARADIGM-HF study, the MAGGIC score, and the EMPHASIS score showed similar performance.10 Using data from the European Society of Cardiology registry, four risk scores were compared.15 The MAGGIC score showed the best discrimination capacity for one-year mortality among the four scores, with an area under the curve for the MAGGIC, GISSI-HF, CHARM and SHFM scores of 0.743, 0.739, 0.729 and 0.714, respectively. Regarding the calibration, all the scores slightly overestimated mortality, except the SHFM. The observed-to-predicted survival ratios were, respectively, 1.03; 1.08; 1.10 and 0.98. The MECKI score has also been compared to the MAGGIC score. In this study, the MECKI score was superior to MAGGIC in a Dutch population, where the scores were assessed overtime, before and after cardiac rehabilitation.16
The MAGGIC score was derived and validated in populations with all ranges of ejection fraction, including HFrEF and HfpEF.8,9 However, besides validation in the Swedish Registry,9 it has also been validated in an exclusive study with HfpEF.17 In this scenario, the score performed well to predict not only death, but also hospitalizations. Regarding sex and race, it maintained a good performance regardless of sex and also in black individuals.18,19
The present study has some limitations. First, this is a single-center study with a small sample. As a consequence, the study may have lacked the power to predict events, especially mortality. Additionally, the generalization to other populations should be performed cautiously. Second, we were unable to assess the calibration of the score due to the small number of deaths and follow-up shorter than one year for most patients. It was, however, possible to assess the score discrimination in a real-world Brazilian population.
In conclusion, the MAGGIC score has an advantage over the other scores as it contains simple and readily available variables. However, the performance of the score is modest, as it is inferior to the isolated use of natriuretic peptides. In clinical practice, the addition of biomarkers, such as natriuretic peptides, improves the accuracy of the score.
Authors’ contributionsFelipe Mafort Rohen: investigation and writing-original draft.
Diane Xavier de Ávila: investigation and project administration.
Carolina Martins Cabrita Lemos: investigation.
Ricardo Santos: investigation.
Mário Ribeiro: investigation, echocardiograms.
Humberto Villacorta: conceptualization, project administration, data curation and writing-original draft.
Conflicts of interestH. Villacorta declares speaker honoraria from Roche Diagnostics. The remaining authors have no conflicts of interest to declare.