Although chemotherapy-induced cardiotoxicity is an emerging problem, limited information is available on the effects of chemotherapy on left ventricular (LV) mechanical functions in patients with non-small cell lung cancer (NSCLC).
ObjectiveWe aimed to explore chemotherapy-induced alterations in cardiac mechanical functions in patients with NSCLC using speckle tracking echocardiography (STE).
MethodsSeventy-one patients with NSCLC and 34 age and sex matched control subjects were consecutively included. Based on their good performance status (Eastern Cooperative Oncology Group performance status), 39 patients were treated with paclitaxel plus carboplatin (PC) regimen and 32 patients were treated with vinorelbine plus cisplatin (VC) regimen. All patients and controls underwent conventional two-dimensional echocardiography and STE at baseline to assess their LV functions. The echocardiographic examinations of NSCLC patients were repeated after the chemotherapy regimens.
ResultsNone of the NSCLC patients developed any signs or symptoms of clinical heart failure during or after the chemotherapy. There were not any significant differences in LV ejection fraction between NSCLC patients and controls before and after chemotherapy. There were not any significant differences in baseline LV global longitudinal strain (GLS), radial strain (RS), and circumferential strain (CS) between NSCLC patients and controls. However, all LV GLS, RS and CS significantly decreased in patients treated with the PC regimen resulting in a significant difference compared to both VC group and controls while no significant decreases were observed in strain measures in VC group.
ConclusionPaclitaxel plus carboplatin, but not VC, may induce subclinical cardiotoxicity in patients with NSCLC, which may be detected by STE.
Embora a cardiotoxicidade induzida pela quimioterapia seja um problema emergente, existe informação limitada sobre os efeitos da quimioterapia na função do ventrículo esquerdo (VE) em doentes com cancro do pulmão não de pequenas células (CPNPC).
ObjetivoAvaliar alterações induzidas pela quimioterapia na função cardíaca em doentes com CPNPC utilizando a ecocardiografia de speckle tracking (EST).
MétodosForam incluídos consecutivamente 71 doentes com CPNPC e 34 indivíduos caso controlo emparelhados por idade e género. Com base no seu desempenho (Eastern Cooperative Oncology Group performance status), 39 doentes foram tratados com regime de paclitaxel e carboplatina (PC) e 32 doentes foram tratados com regime de vinorelbina e cisplatina (VC). Todos os doentes e casos controlo foram submetidos a ecocardiografia bidimensional convencional (2DE) e a EST na fase basal para avaliar a função VE. As avaliações ecocardiográficas dos doentes com CPNPC foram repetidas após quimioterapia.
ResultadosNenhum dos doentes com CPNPC desenvolveu qualquer sinal ou sintoma clínico de insuficiência cardíaca durante ou após a quimioterapia. Não houve diferenças significativas na fração de ejeção do VE entre os doentes com CPNPC e os casos controlo antes e depois da quimioterapia. Não houve diferenças significativas nos valores basais da deformação longitudinal global do VE (GLS), deformação radial (RS) e deformação circunferencial (CS) do VE entre os doentes com CPNPC e os casos controlo. No entanto, todos os valores de deformação miocárdica VE diminuíram significativamente nos doentes tratados com o regime CP, resultando numa diferença significativa em comparação tanto com o grupo CV como com os casos controlo, embora não se tenham observado diminuições significativas nas medidas de strain no grupo VC.
ConclusãoApenas a PC, mas não a VC, pode induzir cardiotoxicidade subclínica em doentes com CPNPC, o que pode ser detetado por ecocardiografia de speckle tracking.
Today, lung cancer is the major cause of cancer-related mortality worldwide. Non-small cell lung cancer (NSCLC) is the most common form of lung cancer, accounting for more than eighty percent of all cases. Based on the current guidelines, surgical resection is the first choice in the management of the patients with early-stage NSCLC. Moreover, adjuvant platinum-based chemotherapy (paclitaxel plus carboplatin (PC) or vinorelbine plus cisplatin (VC)) is the standard option for treating completely resected NSCLC.1–4 Although, chemotherapy regimens have improved the survival of patients with cancer, these regimens may induce cardiotoxicity in some patients. Platinum-based chemotherapy may cause cardiotoxicity and increase in mortality leading to cessation of chemotherapy.5 Early detection of chemotherapy-induced cardiotoxicity is important for patient prognosis.
With advances in ultrasound deformation imaging techniques, speckle tracking echocardiography (STE) has been proposed as an alternative to conventional echocardiographic measures, mainly left ventricular (LV) ejection fraction (EF) for detecting myocardial dysfunction.6 STE is less dependent on Doppler beam angle, which decreases intra- and interobserver variability. Moreover, it provides a global approach, including information on the three spatial dimensions of cardiac contraction, to myocardial mechanics.7
The aim of this study was to evaluate the effects of PC and VC chemotherapy regimens on LV mechanical functions in patients with NSCLC by STE.
MethodsStudy populationThe research complies with the principles outlined in the Declaration of Helsinki. The study was approved by the local Ethics Committee and written informed consent was obtained from all participants.
Patients with histologically or cytologically confirmed NSCLC, who were pathological stage IB-IIIA based on the 7th edition of the American Joint Committee on Cancer the tumor–node–metastasis cancer staging system and had completely dissected mediastinal lymph nodes were invited. After the exclusion of the patients who had received neoadjuvant chemotherapy, known heart failure (HF) or LV systolic dysfunction (EF<50%), coronary artery disease, chronic kidney disease, diabetes mellitus, severe valvular heart disease or cardiomyopathy, 77 NSCLC patients who underwent surgical resection and would receive a platinum-based adjuvant chemotherapy were consecutively recruited. Six patients were subsequently excluded due to poor echogenicity for echocardiographic analysis and the remaining 71 patients were included in our study.
According to the American Society of Clinical Oncology (ASCO) treatment guideline 2017 update, 39 patients were expected to receive PC chemotherapy regimen while the remaining 32 patients were expected to receive VC chemotherapy regimen based on the good Eastern Cooperative Oncology Group Performance Status.1 Thirty-four age and sex matched subjects were included as a control group. The control group comprised healthy individuals who had no cancer diagnosis and were not receiving chemotherapy.
Demographic data and clinicopathological parameters were recorded. All patients underwent careful physical examinations and were questioned for signs and symptoms of HF at each visit (every 3 weeks). Overall, the mean follow-up of the patients was 162±19 days. HF was diagnosed based on clinical symptoms (New York Heart Association, Class III-IV functional capacity), physical signs (peripheral edema, elevated venous pressure or rales), radiological evidence of pulmonary congestion, or increased level of pro-brain natriuretic peptide (pro-BNP) level. The diagnosis of new or worsening HF was determined by objective evidence consisting of at least two physical examination findings or at least one physical examination finding and one laboratory criterion.8 Based on European Society of Cardiology (ESC) position paper on cancer treatments and cardiovascular toxicity, HF due to chemotherapy was defined as an absolute decrease in LVEF >10% associated with a decline below its normal limit of 50% and as a >5% decrease in LVEF together with the presence of HF symptoms.9 The electrocardiographic assessment was performed at baseline and one month after the end of CT therapy.
Treatment scheduleAll NSCLC patients were treated with an adjuvant chemotherapy regimen according to the ASCO clinical practice guideline 2017 update, with either PC or VC. In the PC group, the chemotherapy combination was administered every three weeks and repeated for six cycles which took 126 days total. Fixed doses were administered for paclitaxel: 175 mg/m2. The carboplatin dose was calculated by area under curve-5 (mg/mL per min) formula based on age, sex, height, weight, creatinine, prior chemotherapy history. Patients were hydrated following the administration of chemotherapy (100 mL/h for a total of 500–1000 cm3 Ringer's lactate solution according the patient's body surface area). In the VC group, vinorelbine (at 25 mg/m2 on day 1 and 8) and cisplatin (at 80 mg/m2 on day 1) were administered. The treatment was repeated for up to four cycles every three weeks and repeated for six cycles which totaled 126 days.
Echocardiography measurementsThe two-dimensional (2D) echocardiographic evaluations were performed at baseline and one month after the end of the CT therapy with an ultrasound system (Philips Healthcare Medical Imaging System, Andover, MA, USA) with a 3.5-Hz probe in accordance with the American Society of Echocardiography guideline.10 Seventy-seven consecutive patients were recruited in the study; but six patients were excluded because of poor echogenicity for STE analysis and the remaining 71 patients were included in the study.
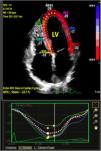
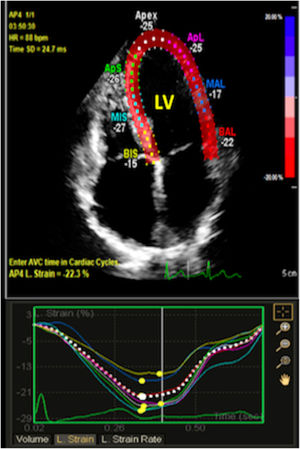
Two blinded cardiologist performed STE analysis using the QLAB Philips off-line software (Philips Healthcare Medical Imaging System, Andover, MA, USA). Three consecutive cardiac cycles were recorded in DICOM format for each view with a frame rate above 50 per second and myocardial contour was automatically traced after determination of baso-septal, baso-lateral and apical landmarks.11 The region of interest was adjusted to cover at least 90% of the myocardial wall thickness. If first tracking was thought to be suboptimal, myocardial contour was retraced manually or semi-automatically. LV longitudinal strain analysis (global longitudinal strain – GLS) was performed using the apical views (four-chamber, three-chamber, and two-chamber). Short-axis views at the basal, midpapillary, and apical levels were obtained for circumferential (CS) and radial strain (RS) analysis, as recommended (Figure 1).12–14
The STE analysis of 20 randomly selected patients was repeated one month later to determine intraobserver and interobserver variability, which were calculated as the average difference between the 20 measurements assessed by the same observer or a second independent observer, respectively. The intraobserver and interobserver variabilities represented as absolute differences in strain measurements were: 1.1% and 1.4% for LV GLS, 2.8% and 3.1% for LV CS, and 1.6% and 1.8% for LV RS.
Statistical analysisStatistical analyses were performed using the SPSS statistical software version 22.0 (SPSS Inc., for MAC, Chicago, IL, USA). The variables were investigated using visual (histograms, probability plots) and analytic methods (Kolmogorov–Smirnov test) to determine whether or not they were normally distributed. In sample size calculation, 39 patients were treated with PC regimen, 32 patients were treated with VC regimen and 34 healthy subjects in each group would be needed to detect a two-point difference in DAN scale, with a power of 80% and 1% of significance level. Categorical variables were presented as numbers and percentages and continuous data were expressed as mean ± standard deviation. Since all continuous variables were found to be normally distributed, statistical comparisons of quantitative data were performed by paired sample t test or one-way analysis of variance. When an overall significance was observed, pairwise post-hoc tests were performed using Tukey's test. Categorical variables were compared with the chi-square test. Kappa coefficients were calculated to estimate intraobserver and interobserver correlation in LV GLS analysis.15 A p value of lower than 0.05 was considered statistically significant result.
ResultsThe study included 71 consecutive patients with NSCLC (mean age: 63.1±6.0 years; 49 males (69.0%)) and 34 controls (mean age: 61.1±5.8 years; men, 19 male (55.8%)). Patients with NSCLC were divided into two groups based on their chemotherapy regimens: PC group (patients treated with the PC regimen) and VC group (patients treated with the VC regimen). The clinical and demographic characteristics of the patients are shown in Table 1. Clinical examination of the patients performed during and after the chemotherapy did not detect any major cardiac side effects, including the signs and symptoms of HF. 2D echocardiographic measurements of the patients with NSCLC and control subjects are shown in Table 2. E/é ratio was significantly increased in the PC group (6.4±2.7 vs. 7.0±3.1; p=0.02). However, no statistically significant difference was observed in conventional measurements of the NSCLC patients obtained before and after the chemotherapy with controls.
Clinical and demographic characteristics of patients with non-small cell lung cancer and healthy subjects.
| NSCLC patients receiving paclitaxel plus carboplatin (n=39) | NSCLC patients receiving vinorelbine plus cisplatin (n=32) | Control group (n=34) | p value | |
|---|---|---|---|---|
| Age (years) | 63.7±6.1 | 62.3±6.0 | 61.1±5.8 | 0.12* |
| Gender (male, %) | 25(64.1%) | 24(75.0%) | 19(55.8%) | 0.07** |
| Hypertension (n, %) | 9(23.1%) | 9(28.1%) | 10(29.4%) | 0.09** |
| Diabetes (n, %) | 8(20.5%) | 7(21.8%) | 8(23.5%) | 0.18** |
| Hyperlipidemia (n, %) | 7(17.9%) | 6(18.7%) | 7(20.5%) | 0.23** |
| Family history of CAD | 6(15.3%) | 4(12.5%) | 6(17.6%) | 0.15** |
| Smoking status | ||||
| Never smoker | 11(28.2%) | 11(34.3%) | 12(35.2%) | 0.20** |
| Ex-smoker | 16(41.0%) | 10(31.2%) | 11(32.3%) | 0.16** |
| Current smoker | 12(30.8%) | 12(37.5%) | 11(32.4%) | 0.21** |
| Histology | ||||
| Adenocarcinoma | 21(53.8%) | 19(59.4%) | ||
| Squamous cell carcinoma | 16(41.1%) | 11(34.3%) | ||
| Large cell neuroendocrine carcinoma | 2(5.1%) | 2(6.3%) | ||
| Disease stage | ||||
| Ib | 10(25.6%) | 8(25.0%) | ||
| IIa | 16(41.0%) | 13(40.6%) | ||
| IIb | 4(10.3%) | 3(9.4%) | ||
| IIIa | 9(23.1%) | 8(25.0%) | ||
| Surgical procedure | ||||
| Lobectomy | 35(89.7%) | 29(90.6%) | ||
| Pneumonectomy | 4(10.3%) | 3(9.4%) | ||
Data are presented as mean ± SD and percentile (%). CAD: coronary artery disease; NSCL: non-small cell lung cancer.
Conventional echocardiographic measurements of patients with non-small cell lung cancer and healthy subjects.
| NSCLC patients receiving paclitaxel plus carboplatin (n=39) | P1 | NSCLC patients receiving vinorelbine plus cisplatin (n=32) | P2 | Control group (n=34) | P3 | P4 | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After the chemotherapy* | Baseline | After the chemotherapy* | ||||||
| LV EDD (mm) | 43.5±3.5 | 44.9±3.8 | 0.66 | 44.2±3.8 | 44.7±3.4 | 0.53 | 43.4±3.5 | 0.24 | 0.29 |
| LV ESD (mm) | 29.6±2.5 | 27.7±2.1 | 0.35 | 28.3±2.0 | 27.4±2.3 | 0.42 | 30.4±2.1 | 0.21 | 0.25 |
| LV Ejection fraction (%) | 60.3±3.4 | 58.5±3.0 | 0.29 | 59.0±3.2 | 59.3±3.5 | 0.38 | 61.4±2.9 | 0.19 | 0.23 |
| IVS thickness (mm) | 7.3±1.5 | 7.6±1.7 | 0.37 | 7.1±1.6 | 7.3±1.5 | 0.40 | 7.0±1.3 | 0.29 | 0.26 |
| PW thickness (mm) | 7.4±1.2 | 7.9±1.8 | 0.26 | 7.2±1.5 | 7.5±1.8 | 0.33 | 7.3±1.4 | 0.19 | 0.21 |
| LV relative wall thickness | 0.34±0.04 | 0.35±0.0.5 | 0.34 | 0.32±0.06 | 0.33±0.05 | 0.29 | 0.33±0.03 | 0.24 | 0.26 |
| E/A ratio | 0.80±0.3 | 0.84±0.4 | 0.17 | 0.77±0.3 | 0.81±0.3 | 0.12 | 0.73±0.3 | 0.10 | 0.09 |
| E/é ratio | 6.3±2.7 | 7.1±3.1 | 0.03 | 6.3±2.8 | 6.6±2.9 | 0.09 | 6.0±2.1 | 0.21 | 0.14 |
| TAPSE (mm) | 18.1±4.8 | 17.2±4.9 | 0.21 | 18.9±5.7 | 18.6±5.9 | 0.27 | 19.7±3.6 | 0.19 | 0.12 |
| RV S′ (cm/s) | 10.9±3.0 | 11.3±3.4 | 0.34 | 11.2±3.3 | 11.0±3.2 | 0.30 | 12.3±2.9 | 0.25 | 0.28 |
P1 denotes statistical difference in the conventional echocardiographic measurements at baseline and after chemotherapy in non-small cell lung cancer (NSCLC) patients receiving paclitaxel plus carboplatin. Paired sample t test was performed.
P2 denotes statistical difference in the conventional echocardiographic measurements at baseline and after chemotherapy in NSCLC patients receiving vinorelbine plus cisplatin. Paired sample t test was performed.
P3 denotes statistical difference in the conventional echocardiographic measurements at baseline among the NSCLC patients and controls. One-way ANOVA was performed.
P4 denotes statistical difference in the conventional echocardiographic measurements after chemotherapy among the NSCLC patients and controls. One-way ANOVA was performed.
The echocardiographic measurements one month after the chemotherapy.
Data are presented as mean ± SD. Bold value indicate statistical significance.
CT: chemotherapy; E/A: transmitral peak E velocity/transmitral peak A velocity; EDD: end-diastolic diameter; EDS: end-systolic diameter; E/é: transmitral peak E velocity/transmitral peak é velocity; IVS: interventricular septum; LV: left ventricle; RV S′: right ventricular systolic velocity; TAPSE: tricuspid annular plane systolic excursion.
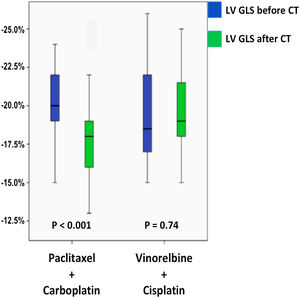
Speckle tracking echocardiography measurements for the patients with NSCLC and controls are shown in Table 3. There were not any significant differences in the baseline LV GLS, RS or CS between NSCLC patients and controls. LV GLS, LV RS, and LV CS decreased significantly in PC group after the PC regimen (Figure 2) while LV GLS, LV RS and LV CS showed insignificant decrease in VC group. The relative percentage reduction of LV GLS was 19.4% in PC group, which was a marker of early LV subclinical dysfunction based on ESC position paper on cancer treatments and cardiovascular toxicity. In PC group, 21 patients had a relative percentage reduction >15%.9,10 After the chemotherapy regimens, the PC group had significantly lower LV GLS, LV RS and LV CS compared to both VC group and controls.
Speckle tracking echocardiographic measurements of patients with non-small cell lung cancer and healthy subjects.
| NSCLC patients receiving paclitaxel plus carboplatin (n=39) | P1 | NSCLC patients receiving vinorelbine plus cisplatin (n=32) | P2 | Control group (n=34) | P3 | P4 | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | After the chemotherapy | Baseline | After the chemotherapy | ||||||
| LV global longitudinal strain (%) | 22.1±2.2 | 17.8±2.7 | <0.001 | 22.5±2.9 | 21.6±2.4 | 0.54 | 23.6±2.7 | 0.37 | <0.001 |
| LV radial strain (%) | 45.3±7.6 | 41.0±7.3 | <0.001 | 45.2±8.5 | 44.1±7.8 | 0.31 | 44.9±7.8 | 0.49 | <0.001 |
| LV circumferential strain (%) | 26.2±5.4 | 23.2±4.8 | <0.001 | 24.8±5.2 | 23.9±4.8 | 0.23 | 26.4±4.9 | 0.25 | <0.001 |
P1 denotes statistically difference in the speckle tracking echocardiographic measurements at baseline and after chemotherapy in non-small cell lung cancer (NSCLC) patients receiving paclitaxel plus carboplatin. Paired sample t test was performed.
P2 denotes statistically difference in the speckle tracking echocardiographic measurements at baseline and after chemotherapy in NSCLC patients receiving vinorelbine plus cisplatin. Paired sample t test was performed.
P3 denotes statistically difference in the speckle tracking echocardiographic measurements at baseline among the NSCLC patients and healthy. One-way ANOVA was performed.
P4 denotes statistically difference in the speckle tracking echocardiographic measurements after the chemotherapy among the NSCLC patients and healthy controls. One-way ANOVA was performed.
LV: left ventricle; NSCLC: non-small cell lung cancer.
Bold values indicate statistical significance p<0.05.
In the electrocardiography assessment, four patients had sinus bradycardia (10.2%), one patient had first-degree atrioventricular block (2.5%) after PC regimen therapy. One patient had sinus bradycardia (2.9%) and one patient had left bundle branch block (%2.9) after VC regimen therapy.
DiscussionAt present, the PC and VC combinations are the frequently used chemotherapy regimens for treating NSCLC. We conducted a prospective study to evaluate the potential cardiotoxicity of these chemotherapy regimens by performing conventional and advanced echocardiography techniques. While conventional 2D echocardiography did not detect any LV systolic dysfunction after chemotherapy, STE revealed significant decreases in LV GLS, RS, and CS in NSCLC patients receiving the PC regimen. This suggests that the PC regimen might induce subclinical cardiotoxicity in these patients. There were no significant decreases in strain measures after the VC regimen.
The ESC position paper on cancer treatments and cardiovascular toxicity indicated the importance of identifying cardiotoxicity in its early stage.9 Early identification of chemo-cardiotoxicity mainly relies on cardiac imaging which detects an impairment in LV systolic function without any apparent signs or symptoms of HF.16 LVEF is currently used to detect chemotherapy-induced cardiotoxicity. STE, on the other hand, enables accurate quantification of myocardial function and shows better agreement with cardiac magnetic resonance imaging (CMRI) measurements than conventional 2D echocardiographic measurements.17,18 Moreover, STE detects discrete and localized impairments in contractility that are deficient to affect the global LV systolic function but have potential diagnostic and prognostic implications.18–20 The ability of STE to detect subclinical LV dysfunction in the absence of a reduced LV EF has enhanced its value in recent chemo-cardiotoxicity studies. Previous studies detected a decrease in STE-measured GLS in the absence of a reduced LV EF in asymptomatic patients with breast cancer who received anthracycline chemotherapy.21,22 A meta-analysis assessing the early detection of cardiotoxicity in patients who had received cancer chemotherapy suggested that STE-measured LV GLS is a better measure than conventional 2D echocardiographic measurements and Doppler-derived indexes.23 The SUCCOUR study showed that a STE-guided strategy might prevent subclinical cardiotoxicity compared with current EF-guided management in patients receiving cardiotoxic chemotherapy.24 However, the recent guideline9 has not been updated and in our study, we did not revise the CT of the patients according to their strain measurements.
Cardiotoxicity is one of the most serious adverse effects of chemotherapy, associated with increased morbidity and mortality.9 Acute cardiotoxicity develops any time from the onset of treatment up to two to four weeks after the end of therapy. It is characterized by reversible arrhythmias, acute coronary syndrome, pericarditis, and LV systolic dysfunction.25,26 Chronic cardiotoxicity occurs four weeks after treatment and is divided into early cardiotoxicity, which appears within the first year after treatment, and late cardiotoxicity, which appears more than one year after chemotherapy. Chronic cardiotoxicity includes type I myocardial damage which means it is irreversible and cumulative dose-related (e.g., doxorubicin) and type II myocardial damage, which is reversible and not dose-related cardiac dysfunction (e.g., trastuzumab).27,28
Paclitaxel was discovered in 1962 as a mitotic spindle inhibitor. Since then, paclitaxel has showed efficacy in many type of cancers including breast, ovarian, gastric, and also non-small cell lung cancers.29,30 Current studies have showed that paclitaxel leads to congestive heart failure in 5–15% at conventional doses31,32. Del Mastro et al. showed cardiotoxicity in 9% of the patients with breast cancer after weekly paclitaxel treatment.33 The type of myocardial damage caused by paclitaxel has not yet been determined in large-scale clinical trials. Some studies have reported severe reductions in EF in 1–2% of patients.31,32 Osman et al. revealed a decrease in LVEF in patients with gynecological malignancy or breast cancer treated with paclitaxel after 30 months.34 PC regimen-induced subclinical cardiotoxicity has also been shown by STE in patients with gynecologic malignancy.35 To the best of our knowledge, the present study is the first to show a decrease in deformation imaging measurements (STE-measured LV GLS, RS, and CS) in patients with NSCLC who received the PC regimens.
Study limitationOur study has several limitations. First, our study was a single-center study involving a relatively small study sample. Evaluation of STE is a semiautomatic method, which requires manual determination of the myocardium border zone. Therefore, trained and experienced operators are needed for the appropriate evaluation and recognition of artifacts.36 We did not assess the LV functions of the patients with CMRI and real time three-dimensional echocardiography. Although several imaging modalities such as CMRI or multigated acquisition scans can be used in the assessment of cardiotoxicity as a gold standard, the benefit of echocardiography originates from its cost effectiveness, easy accessibility, ability to assess more than ventricular function, and absence of radiation exposure.37 In addition, we could not perform the RV strain analysis due to the fact that we did not have RV analysis software. The patients’ disease stage might have an effect on the responses to chemotherapy and on the strain measures. However, due to small sample size, we could not assess the effect of the disease stage. Whether the decrease in LV strain measures indicating the subclinical cardiotoxicity of PC was reversible or not could not be evaluated due to lack of follow-up data.
In addition, platinum-based chemotherapy may be associated with coronary vasospasm. Since coronary angiography was not performed in our study; the possibility of coronary vasospasm, which might affect the myocardial functions in these patients, could not be excluded. Due to a short follow-up period, no mortality was observed in our study and the association between strain measurements and mortality could not be evaluated. Long-term and large-scale outcome studies with clinical end points should be performed to identify the clinical significance of our findings.
ConclusionThe paclitaxel plus carboplatin regimen, but not VC regimen, may be associated with subclinical cardiotoxicity which is detected earlier and more successfully by STE in patients with NSCLC. Long term studies are needed to clarify the importance of strain measurements in the management of NSCLC patients.
Ethical approvalOur study was approved by the local Ethics Committee with regard to the Helsinki declaration and written informed consent was obtained from all participants.
Authors’ contributionsAll authors contributed to the study conception and design. Dr. Batur Gonenc Kanar was the primary investigator of the study. He was responsible for the design of the study, data collection, analysis of the data and drafting of the article. Dr. Beste Ozben, Dr. Kursat Tigen and Dr. Alğer Kepez were responsible for the critical revision of the article. Dr. Akın Ozturk, Dr. Bahar Dalkılıc and Dr. Dursun Akaslan were responsible for analysis of the data and drafting of the article. Dr. Erhan Ogur, Dr. Dursun Akaslan, Dr. Ayhan Küp and Dr. Kamil Gülşen were responsible for data collection and analysis of the data. The first draft of the manuscript was written by Dr. Batur Gonenc Kanar and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FundingNone declared.
Conflict of interestThe authors declare that they have no conflict of interest.