Compared to bare-metal stents (BMS), drug-eluting stents reduce stent restenosis and improve subsequent revascularization rates. The impact on patients’ survival has been the subject of debate.
ObjectiveTo assess the long-term (10-year) survival of patients undergoing percutaneous coronary intervention (PCI) with first-generation sirolimus-eluting stents (SES) in comparison with BMS.
MethodsIn a single-center registry, 600 consecutive patients who underwent successful PCI with SES between April 2002 and February 2003 were compared to 594 patients who underwent PCI with BMS between January 2002 and April 2002, just before the introduction of SES. Clinical and procedural data were collected at the time of intervention and 10-year survival status was assessed via the national life status database.
ResultsAll baseline characteristics were similar between groups except for smaller stent diameter (2.84±0.38 vs. 3.19±0.49 mm; p<0.001), greater stent length (18.50±8.2 vs. 15.96±6.10 mm; p<0.001) and higher number of stents per patient (1.95 vs. 1.46, p<0.001) in the SES group. Overall five- and 10-year all-cause mortality was 9.6% (n=110) and 22.7% (n=272), respectively. The adjusted HR for 10-year mortality in patients undergoing PCI with SES was 0.74 (95% CI 0.58-0.94; p=0.013), corresponding to a relative risk reduction of 19.8%. Other than PCI with BMS, older age, chronic kidney disease, chronic obstructive pulmonary disease and lower ejection fraction were independent predictors of 10-year mortality.
ConclusionTo date, this is the longest follow-up study ever showing a potential survival benefit of first-generation sirolimus-eluting stents versus bare-metal stents, supporting prior observations on their sustained efficacy and safety relative to contemporary BMS.
Em comparação com os stents de metal nulo (BMS), os stents com efeito de droga reduzem a restenose do stent e melhoram as taxas de revascularização subsequente. O impacto na sobrevivência dos pacientes tem sido objecto de debate.
ObjectivoAvaliar a sobrevivência a longo prazo (10 anos) de pacientes submetidos a intervenção coronária percutânea (ICP) com stents sirolimus-eluting de primeira geração (SES) em comparação com a BMS.
MétodosNum registo de um único centro, 600 pacientes consecutivos submetidos a ICP com SES entre Abril de 2002 e Fevereiro de 2003 foram comparados com 594 pacientes submetidos a ICP com BMS entre Janeiro de 2002 e Abril de 2002, imediatamente antes da introdução da SES. Os dados clínicos e processuais foram recolhidos no momento da intervenção e o estado de sobrevivência de 10 anos foi avaliado através da base de dados nacional do estado de vida.
ResultadosTodas as características de linha de base foram semelhantes entre os grupos, excepto para stents de menor diâmetro (2,84±0,38 vs. 3,19±0,49 mm; p<0,001), maior comprimento do stent (18,50±8,2 vs. 15,96±6,10 mm; p<0,001) e maior número de stents por paciente (1,95 vs. 1,46, p<0,001) no grupo SES. A mortalidade global de cinco e 10 anos por todas as causas foi de 9,6% (n=110) e 22,7% (n=272), respectivamente. A FC ajustada para mortalidade de 10 anos em pacientes submetidos a ICP com SES foi de 0,74 (95% CI 0,58-0,94; p=0,013), correspondendo a uma redução relativa do risco de 19,8%. Para além da ICP com SES, a idade avançada, doença renal crónica, doença pulmonar obstrutiva crónica e a fracção de ejecção inferior foram preditores independentes da mortalidade de 10 anos.
ConclusãoAté à data, este é o estudo de seguimento mais longo a demonstrar um potencial benefício de sobrevida dos stents farmacológicos de primeira geração versus stents metálicos, em linha com estudos prévios que demonstram a sua eficácia e segurança quando comparados com SC contemporâneos.
Drug-eluting stents (DES) are currently the mainstay of revascularization by percutaneous coronary intervention (PCI) for most patients and clinical settings. It is generally considered that DES reduce stent restenosis and improve subsequent revascularization rates, at the cost of increased risk of stent thrombosis compared with bare-metal stents (BMS), at least for first-generation DES.1 New-generation DES have been shown to be associated with lower rates of stent thrombosis than older DES and even contemporary BMS.2–4 Constant improvements in stent technology and optimized implantation techniques may be expected to translate into superior efficacy and safety and even reduced rates of death and myocardial infarction (MI).5–7
The first DES available for clinical use in Europe (2002) and shortly after in the US (2003) was the sirolimus-eluting Cypher™ stent (Cordis/Johnson & Johnson). Two large randomized clinical trials (RCTs), Randomized Comparison of a Sirolimus-eluting Stent With a Standard stent for Coronary Revascularization (RAVEL)8 and Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery (SIRIUS),9 showed an unequivocal benefit in stent restenosis with the Cypher™ stent. The superior efficacy of the new SES, and the growing enthusiasm around it, led to its being used increasingly in multiple clinical settings, sometimes even without supporting evidence (off-label use). However, based on observational data, concerns about the risk of late and very late stent thrombosis with SES and related mortality were steadily growing among interventional cardiologists. The controversy reached its peak at the 2006 European Society of Cardiology congress when two independent meta-analyses suggested that first-generation DES, particularly SES, were associated with increased mortality.10 This was known as ‘the ESC firestorm’, sowing deep suspicions concerning the safety of SES and leading to a halt in their widespread use.11 In the heat of the controversy, one of the first reactions was to prolong dual antiplatelet therapy for 12 months as standard treatment after PCI with SES, even though there was no reliable evidence to support such a measure at that time. Subsequently evidence from RCTs and meta-analyses have shown improvement in quality of life without greater mortality, despite a slight increase in very late stent thrombosis.10,12
The Cypher™ stent has been extensively studied in more than 20 RCTs comparing it with BMS in different clinical scenarios and lesion subsets. Despite extensive use (both within and outside clinical trials), very long-term clinical outcome data on this first-generation DES are still scarce. The five-year follow-up analysis of the SIRIUS trial showed a sustained benefit in clinical restenosis without significant differences in MI or death.13 All-cause mortality at 10 years associated with SES has recently been reported to be 20-24%.1,14 However, no comparison with BMS has been performed with such a long follow-up. With this analysis, we aimed to help clarify the effect of first-generation SES implantation on very late overall survival, in comparison with contemporary older BMS.
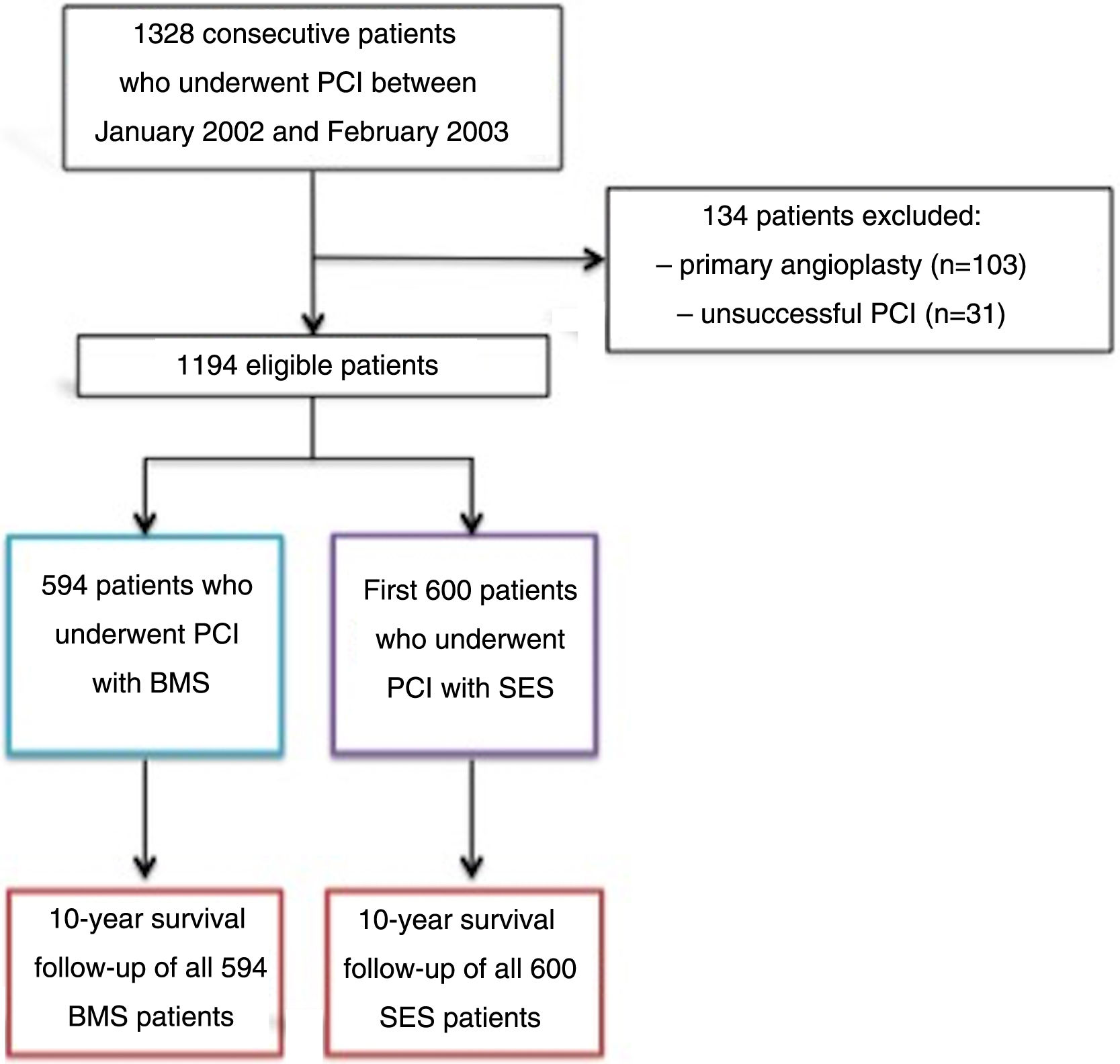
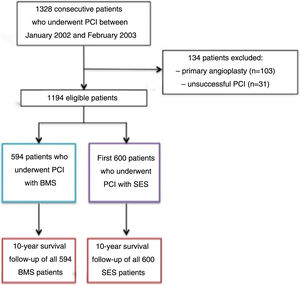
MethodsPatient selection and data collectionThis was a prospective registry,15 from a single tertiary center, of the first 600 patients who underwent successful PCI with SES between April 2002 and February 2003, who were compared to the previous 594 patients who underwent successful PCI with a BMS between January 2002 and April 2002, just before the introduction of SES into clinical practice. Exclusion criteria were as follows: primary PCI in the setting of ST-elevation myocardial infarction (STEMI), because this was considered an off-label indication for DES at that time; and an unsuccessful procedure, defined as greater than 30% residual stenosis, in-hospital clinical complications, such as periprocedural MI, need for urgent coronary artery bypass grafting (CABG), or death (Figure 1). At that time, for patients undergoing PCI with SES, dual antiplatelet therapy with aspirin and clopidogrel was used for eight weeks, in accordance with the protocol used in the RAVEL randomized trial.8
All data on demographic, clinical and interventional characteristics were prospectively entered in a study-specific database (Table 1). Follow-up data was limited to all-cause mortality. Information was collected from a nationwide life status database that records all mortality reports. Collection of other significant clinical events was not attempted due to complex challenges hampering all efforts to obtain accurate data.
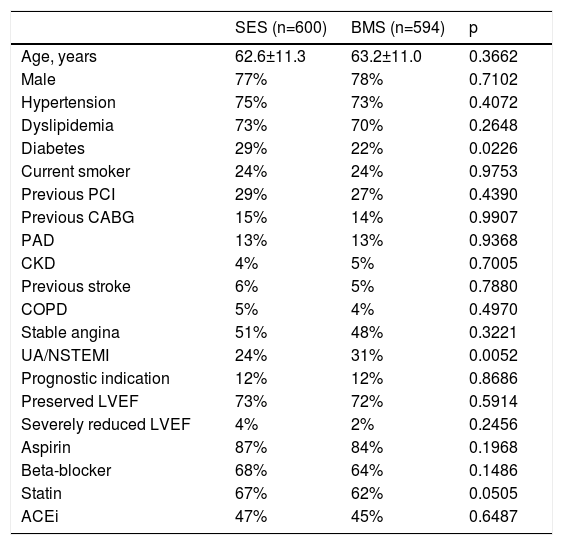
Baseline clinical characteristics of the study population.
| SES (n=600) | BMS (n=594) | p | |
|---|---|---|---|
| Age, years | 62.6±11.3 | 63.2±11.0 | 0.3662 |
| Male | 77% | 78% | 0.7102 |
| Hypertension | 75% | 73% | 0.4072 |
| Dyslipidemia | 73% | 70% | 0.2648 |
| Diabetes | 29% | 22% | 0.0226 |
| Current smoker | 24% | 24% | 0.9753 |
| Previous PCI | 29% | 27% | 0.4390 |
| Previous CABG | 15% | 14% | 0.9907 |
| PAD | 13% | 13% | 0.9368 |
| CKD | 4% | 5% | 0.7005 |
| Previous stroke | 6% | 5% | 0.7880 |
| COPD | 5% | 4% | 0.4970 |
| Stable angina | 51% | 48% | 0.3221 |
| UA/NSTEMI | 24% | 31% | 0.0052 |
| Prognostic indication | 12% | 12% | 0.8686 |
| Preserved LVEF | 73% | 72% | 0.5914 |
| Severely reduced LVEF | 4% | 2% | 0.2456 |
| Aspirin | 87% | 84% | 0.1968 |
| Beta-blocker | 68% | 64% | 0.1486 |
| Statin | 67% | 62% | 0.0505 |
| ACEi | 47% | 45% | 0.6487 |
ACEi: angiotensin-converting enzyme inhibitor; BMS: bare-metal stents; CABG: coronary artery bypass grafting; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; NSTEMI: non-ST-elevation myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; UA: unstable angina.
Continuous variables were expressed as means and standard deviation when normally distributed and as medians and interquartile range when not normally distributed. Normality was tested with the Kolmogorov-Smirnov test and/or visual assessment of Q-Q plots. Categorical variables were expressed as frequencies and percentages.
Baseline characteristics and outcomes were compared using the chi-square test or Fisher's exact test, as appropriate, for categorical variables and the Student's t test for continuous variables.
Baseline characteristics according to vital status at five and 10 years were compared using the beginning of follow-up as reference. No landmark analysis was performed.
Independent predictors of all-cause mortality at five and 10 years were determined by Cox regression. Event-free survival was computed using Kaplan-Meier analysis and the log-rank test was used for comparison.
All tests were two-sided and differences were considered statistically significant at a p-value of 0.05. The statistical analysis was performed with IBM SPSS 20.0 software (IBM SPSS Inc., Chicago, IL, USA).
ResultsThe baseline clinical, angiographic and procedural characteristics of the study population are shown in Tables 1 and 2.
Between January 2002 and February 2003, 1328 consecutive patients underwent coronary stenting at our center. Patients who had emergent (n=103) and unsuccessful (n=31) angioplasty (according to study criteria) were excluded. The remaining 1194 patients were included in the follow-up analysis.
Clinical characteristics were similar (Tables 1 and 2), except for a greater prevalence of diabetes and multivessel disease among patients who received SES and a higher rate of patients presenting with non-ST-elevation acute coronary syndromes (ACS) in the BMS group.
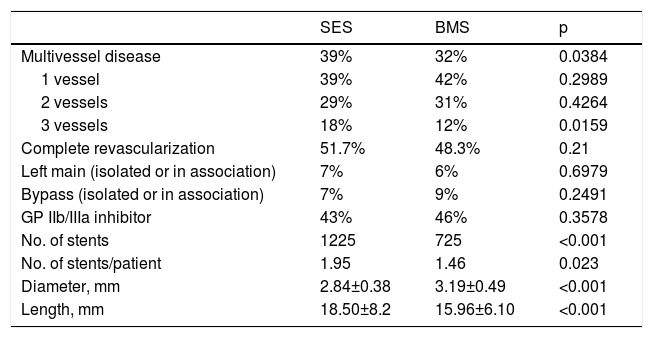
There was some heterogeneity in coronary anatomy and interventional characteristics between groups. Patients treated with SES had a higher incidence of multivessel disease (39% vs. 32%; p=0.0384) and a higher number of stents per patient (1.95 vs. 1.46; p<0.0001), and the implanted stents were both narrower (2.84±0.38 vs. 3.19±0.49 mm, p<0.0001) and longer (18.50±8.2 vs. 15.96±6.1 mm; p<0.0001) (Table 2). Despite these differences, the rates of complete revascularization were similar between the groups (51.7% in SES vs. 48.3% in BMS; p=0.21).
Baseline lesion characteristics.
| SES | BMS | p | |
|---|---|---|---|
| Multivessel disease | 39% | 32% | 0.0384 |
| 1 vessel | 39% | 42% | 0.2989 |
| 2 vessels | 29% | 31% | 0.4264 |
| 3 vessels | 18% | 12% | 0.0159 |
| Complete revascularization | 51.7% | 48.3% | 0.21 |
| Left main (isolated or in association) | 7% | 6% | 0.6979 |
| Bypass (isolated or in association) | 7% | 9% | 0.2491 |
| GP IIb/IIIa inhibitor | 43% | 46% | 0.3578 |
| No. of stents | 1225 | 725 | <0.001 |
| No. of stents/patient | 1.95 | 1.46 | 0.023 |
| Diameter, mm | 2.84±0.38 | 3.19±0.49 | <0.001 |
| Length, mm | 18.50±8.2 | 15.96±6.10 | <0.001 |
BMS: bare-metal stents; SES: sirolimus-eluting stents.
Vital status at 10 years was known for all patients included in the analysis.
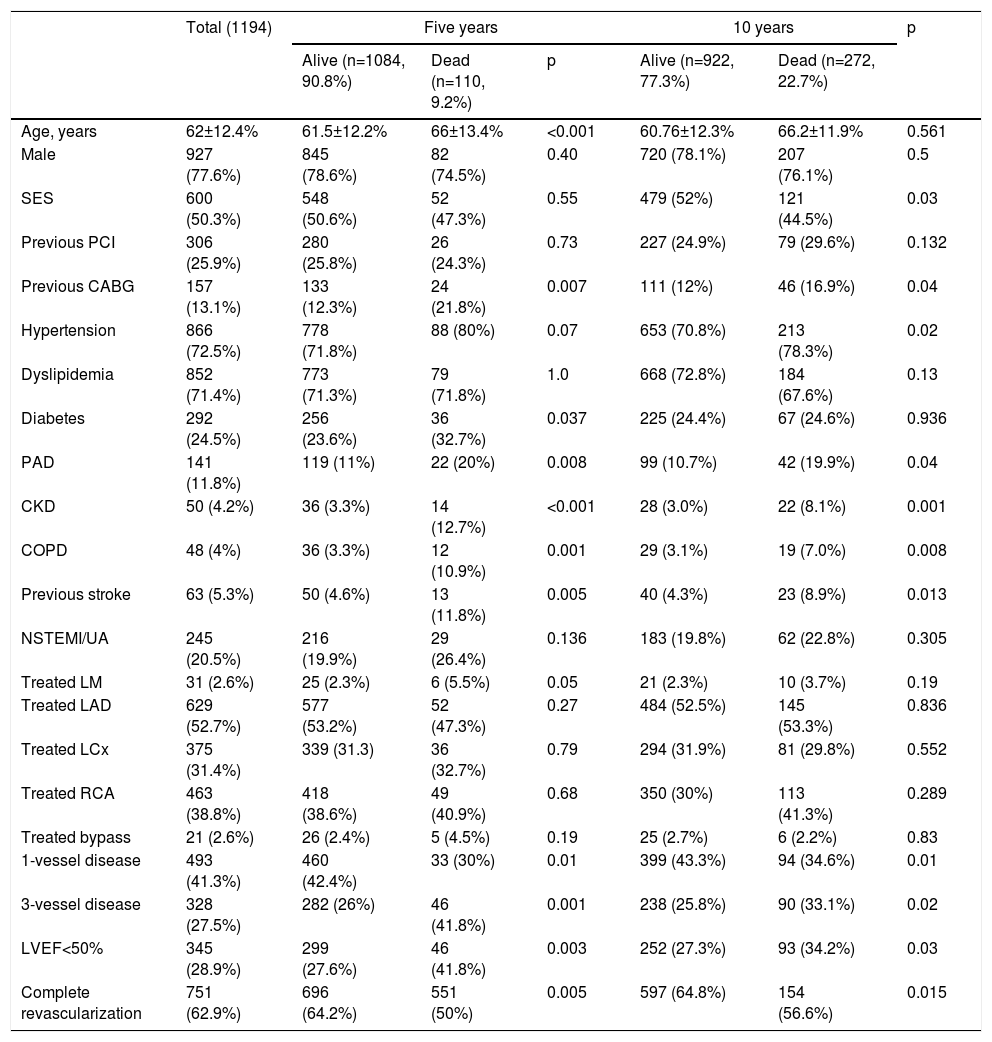
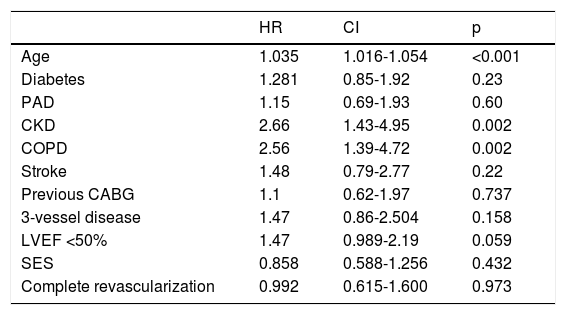
All-cause mortality at five yearsFive-year mortality was 9.2% in the overall patient cohort. Patients who died were older, had more comorbidities (including peripheral arterial disease [PAD], chronic kidney disease [CKD] and chronic obstructive pulmonary disease [COPD]), and were more likely to be diabetic and have a previous history of stroke. They also had more complex coronary artery disease – reflected by higher rates of previous CABG, multivessel and left main disease – and a lower ejection fraction at baseline (Table 3). After adjustment for confounders, increasing age, CKD and COPD were independent predictors of five-year mortality (Table 4). There was no statistically significant difference in five-year mortality between patients who received DES or BMS (47.3 vs. 52.7%; hazard ratio [HR] 0.86; 95% confidence interval [CI] 0.59-1.26; p=0.432).
Patient characteristics according to survival status at five and 10 years.
| Total (1194) | Five years | 10 years | p | ||||
|---|---|---|---|---|---|---|---|
| Alive (n=1084, 90.8%) | Dead (n=110, 9.2%) | p | Alive (n=922, 77.3%) | Dead (n=272, 22.7%) | |||
| Age, years | 62±12.4% | 61.5±12.2% | 66±13.4% | <0.001 | 60.76±12.3% | 66.2±11.9% | 0.561 |
| Male | 927 (77.6%) | 845 (78.6%) | 82 (74.5%) | 0.40 | 720 (78.1%) | 207 (76.1%) | 0.5 |
| SES | 600 (50.3%) | 548 (50.6%) | 52 (47.3%) | 0.55 | 479 (52%) | 121 (44.5%) | 0.03 |
| Previous PCI | 306 (25.9%) | 280 (25.8%) | 26 (24.3%) | 0.73 | 227 (24.9%) | 79 (29.6%) | 0.132 |
| Previous CABG | 157 (13.1%) | 133 (12.3%) | 24 (21.8%) | 0.007 | 111 (12%) | 46 (16.9%) | 0.04 |
| Hypertension | 866 (72.5%) | 778 (71.8%) | 88 (80%) | 0.07 | 653 (70.8%) | 213 (78.3%) | 0.02 |
| Dyslipidemia | 852 (71.4%) | 773 (71.3%) | 79 (71.8%) | 1.0 | 668 (72.8%) | 184 (67.6%) | 0.13 |
| Diabetes | 292 (24.5%) | 256 (23.6%) | 36 (32.7%) | 0.037 | 225 (24.4%) | 67 (24.6%) | 0.936 |
| PAD | 141 (11.8%) | 119 (11%) | 22 (20%) | 0.008 | 99 (10.7%) | 42 (19.9%) | 0.04 |
| CKD | 50 (4.2%) | 36 (3.3%) | 14 (12.7%) | <0.001 | 28 (3.0%) | 22 (8.1%) | 0.001 |
| COPD | 48 (4%) | 36 (3.3%) | 12 (10.9%) | 0.001 | 29 (3.1%) | 19 (7.0%) | 0.008 |
| Previous stroke | 63 (5.3%) | 50 (4.6%) | 13 (11.8%) | 0.005 | 40 (4.3%) | 23 (8.9%) | 0.013 |
| NSTEMI/UA | 245 (20.5%) | 216 (19.9%) | 29 (26.4%) | 0.136 | 183 (19.8%) | 62 (22.8%) | 0.305 |
| Treated LM | 31 (2.6%) | 25 (2.3%) | 6 (5.5%) | 0.05 | 21 (2.3%) | 10 (3.7%) | 0.19 |
| Treated LAD | 629 (52.7%) | 577 (53.2%) | 52 (47.3%) | 0.27 | 484 (52.5%) | 145 (53.3%) | 0.836 |
| Treated LCx | 375 (31.4%) | 339 (31.3) | 36 (32.7%) | 0.79 | 294 (31.9%) | 81 (29.8%) | 0.552 |
| Treated RCA | 463 (38.8%) | 418 (38.6%) | 49 (40.9%) | 0.68 | 350 (30%) | 113 (41.3%) | 0.289 |
| Treated bypass | 21 (2.6%) | 26 (2.4%) | 5 (4.5%) | 0.19 | 25 (2.7%) | 6 (2.2%) | 0.83 |
| 1-vessel disease | 493 (41.3%) | 460 (42.4%) | 33 (30%) | 0.01 | 399 (43.3%) | 94 (34.6%) | 0.01 |
| 3-vessel disease | 328 (27.5%) | 282 (26%) | 46 (41.8%) | 0.001 | 238 (25.8%) | 90 (33.1%) | 0.02 |
| LVEF<50% | 345 (28.9%) | 299 (27.6%) | 46 (41.8%) | 0.003 | 252 (27.3%) | 93 (34.2%) | 0.03 |
| Complete revascularization | 751 (62.9%) | 696 (64.2%) | 551 (50%) | 0.005 | 597 (64.8%) | 154 (56.6%) | 0.015 |
BMS: bare-metal stents; CABG: coronary artery bypass grafting; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; LAD: left anterior descending artery; LCx: circumflex artery; LM: left main; LVEF: left ventricular ejection fraction; NSTEMI: non-ST-elevation myocardial infarction; PAD: peripheral arterial disease; PCI: percutaneous coronary intervention; RCA: right coronary artery; SES: sirolimus-eluting stents; UA: unstable angina.
Independent predictors of all-cause mortality at five years (multivariate analysis).
| HR | CI | p | |
|---|---|---|---|
| Age | 1.035 | 1.016-1.054 | <0.001 |
| Diabetes | 1.281 | 0.85-1.92 | 0.23 |
| PAD | 1.15 | 0.69-1.93 | 0.60 |
| CKD | 2.66 | 1.43-4.95 | 0.002 |
| COPD | 2.56 | 1.39-4.72 | 0.002 |
| Stroke | 1.48 | 0.79-2.77 | 0.22 |
| Previous CABG | 1.1 | 0.62-1.97 | 0.737 |
| 3-vessel disease | 1.47 | 0.86-2.504 | 0.158 |
| LVEF <50% | 1.47 | 0.989-2.19 | 0.059 |
| SES | 0.858 | 0.588-1.256 | 0.432 |
| Complete revascularization | 0.992 | 0.615-1.600 | 0.973 |
CABG: coronary artery bypass grafting; CI: confidence interval; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; LVEF: left ventricular ejection fraction; PAD: peripheral arterial disease; SES: sirolimus-eluting stent.
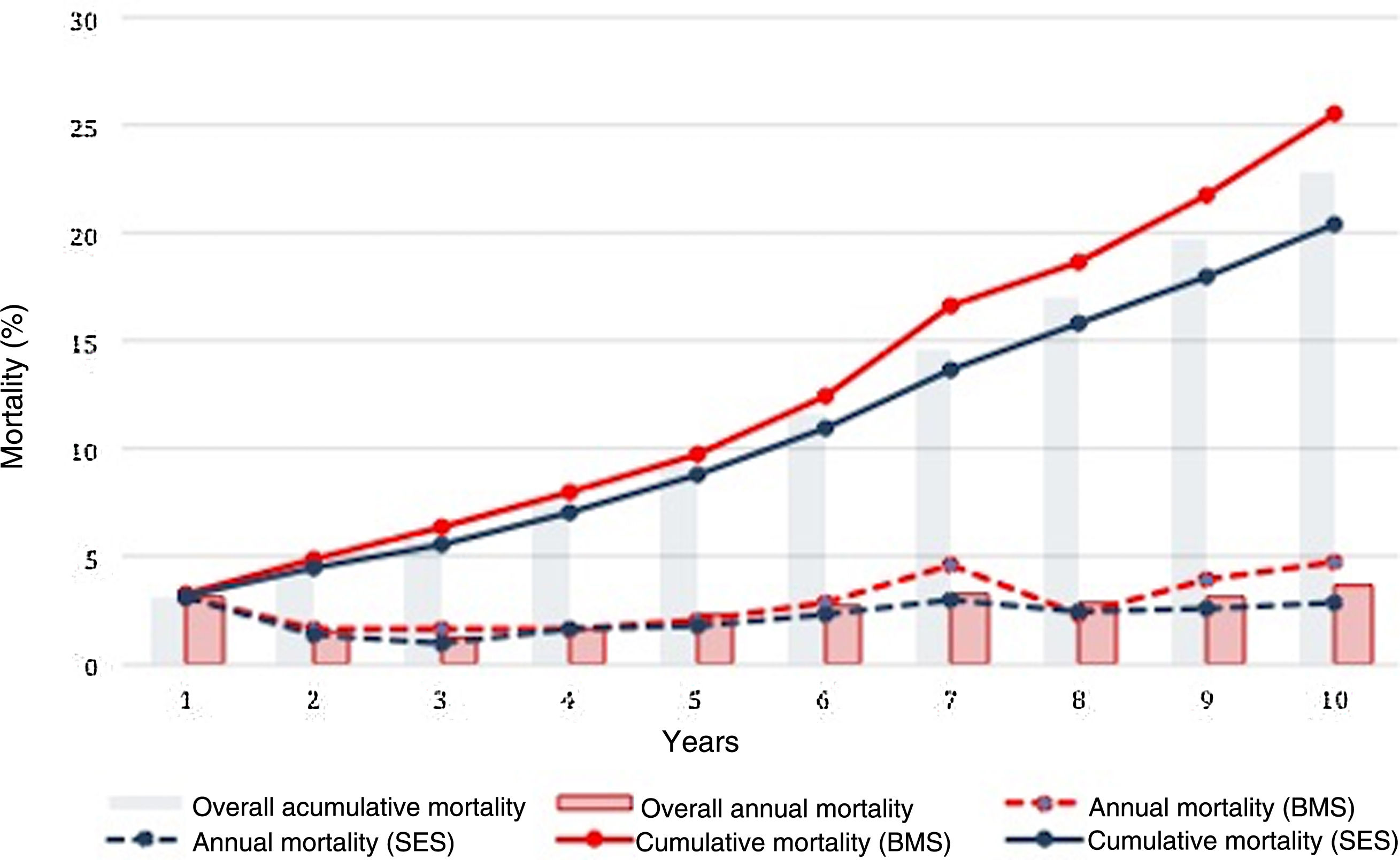
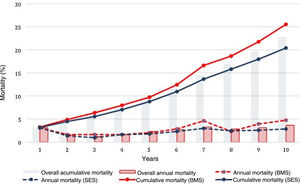
The cumulative rate of all-cause death at 10 years for the overall patient population was 22.7%. Mortality was associated with greater prevalence of hypertension, PAD, CKD, COPD, previous stroke, previous CABG, multivessel disease, left main disease, left ventricular ejection fraction (LVEF) <50%, incomplete revascularization and BMS use.
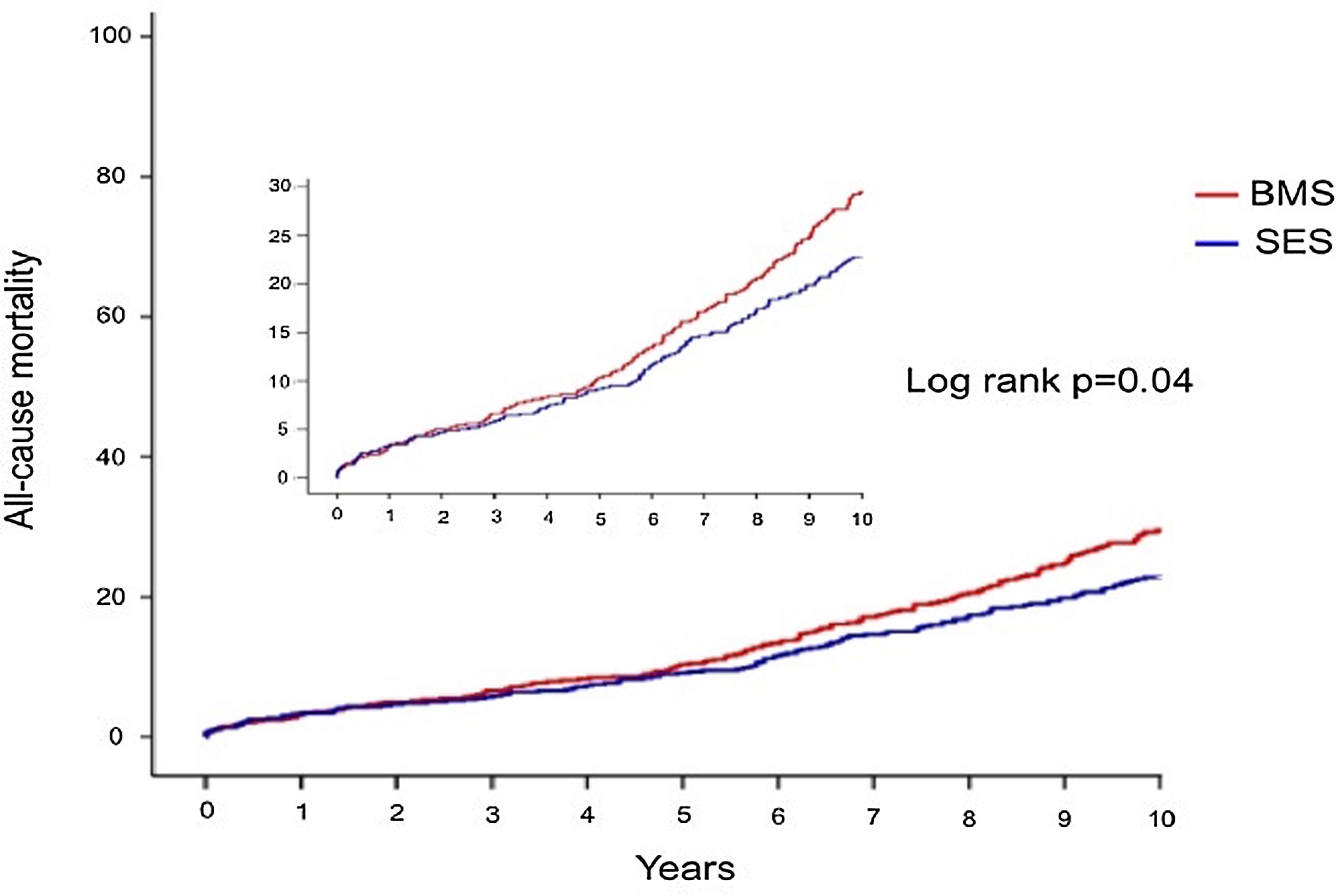
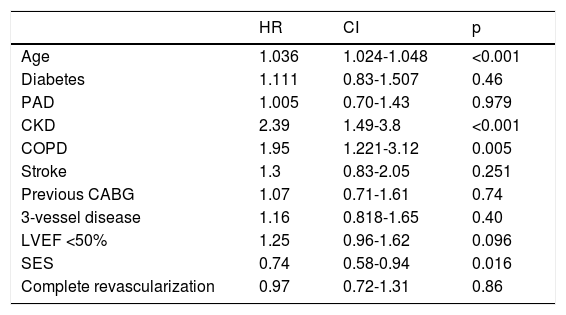
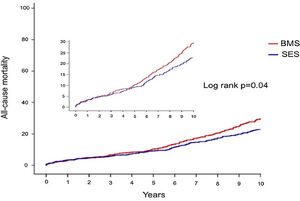
Crude all-cause mortality rates at the end of the 10-year follow-up were 25.4% in BMS-treated patients and 20.1% in the SES group (absolute difference -5.3% in favor of SES-treated patients) (Table 3). After adjustment for baseline differences, the use of first-generation SES was independently associated with a 26% reduction in the risk of all-cause death at 10 years (HR 0.74; 95% CI 0.58-0.94; p=0.015) (Table 5), which translates into a relative risk reduction of 19.8%, taking BMS as the reference group (Figure 2). Age, CKD and COPD persisted as independent predictors of all-cause death (Table 5).
Independent predictors of all-cause mortality at 10 years (multivariate analysis).
| HR | CI | p | |
|---|---|---|---|
| Age | 1.036 | 1.024-1.048 | <0.001 |
| Diabetes | 1.111 | 0.83-1.507 | 0.46 |
| PAD | 1.005 | 0.70-1.43 | 0.979 |
| CKD | 2.39 | 1.49-3.8 | <0.001 |
| COPD | 1.95 | 1.221-3.12 | 0.005 |
| Stroke | 1.3 | 0.83-2.05 | 0.251 |
| Previous CABG | 1.07 | 0.71-1.61 | 0.74 |
| 3-vessel disease | 1.16 | 0.818-1.65 | 0.40 |
| LVEF <50% | 1.25 | 0.96-1.62 | 0.096 |
| SES | 0.74 | 0.58-0.94 | 0.016 |
| Complete revascularization | 0.97 | 0.72-1.31 | 0.86 |
CABG: coronary artery bypass grafting; CI: confidence interval; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; LVEF: left ventricular ejection fraction; PAD: peripheral arterial disease; SES: sirolimus-eluting stent.
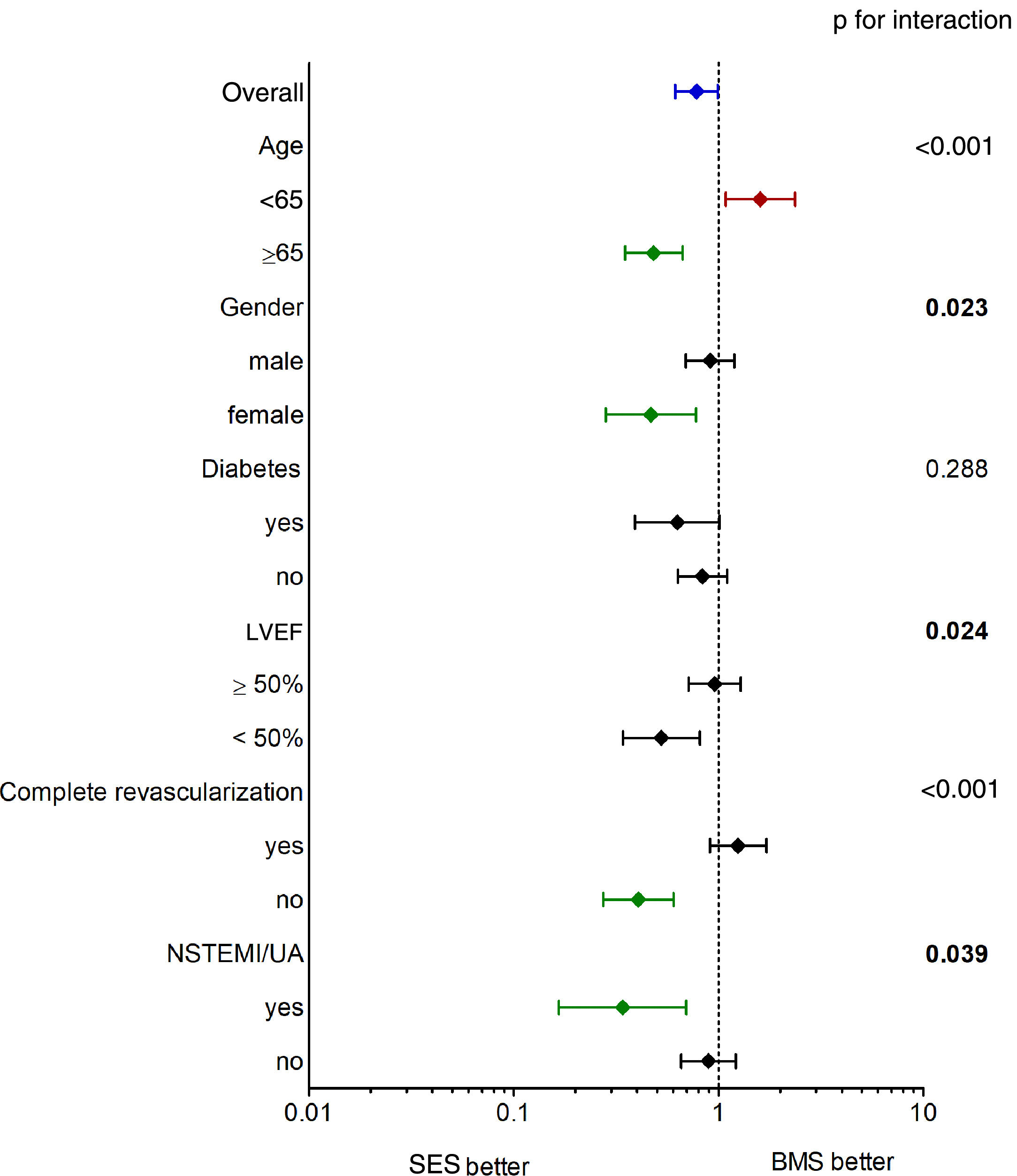
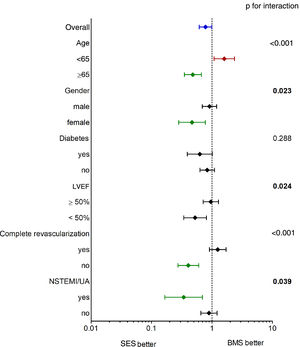
The main findings of our study were that (1) SES use was independently associated with better long-term survival; (2) the benefit of SES was more pronounced in the elderly, in female patients, in patients with LVEF <50% and in those with incomplete revascularization (Figure 3); and (3) CKD, COPD, depressed LVEF and age at the time of the procedure were the main determinants of poor prognosis after PCI.
Although no longer in use, first-generation SES have been implanted in millions of patients worldwide in different clinical scenarios. Knowledge of their impact on patient survival is therefore of paramount importance.
After the ‘ESC firestorm’, the safety and efficacy of first-generation DES were widely studied in observational registries, RCTs and meta-analyses. In general, the available evidence shows that patients treated with SES have better survival free of cardiovascular events, without a negative impact on all-cause and cardiovascular mortality.9,13,16 Although most studies have a relatively short follow-up, crude mortality rates in our cohort are within the range of prior studies in the literature.13,17,18 Five-year all-cause mortality was remarkably similar to that reported for both the SIRIUS randomized trial13 and the SCAAR registry,19 but was much lower than in the SCORPIUS trial.18 This may be due to the fact that in the latter, all patients were diabetic, which is known to correlate with adverse outcomes. As stated above, only one study has reported on the 10-year outcome of patients treated with first-generation SES.14 In the SIRTAX VERY LATE study (which originally compared SES with paclitaxel-eluting stents (PES) in 1012 patients,1 the cumulative incidence of all-cause death in SES-treated patients was 25%, comparable to the 20.1% reported in our similarly sized population. To our knowledge, no other study has ever reported on the very long-term outcome of first-generation SES as compared to BMS.
The reasons for the observed lower mortality associated with SES (Figure 3) and for it to become significant only over a relatively long time frame are not straightforward (Figure 4). Stent-related mortality can only be explained by (1) a device-related change in the rate of ischemic events; (2) coadjuvant medical therapy associated with that particular device (for example, the benefits of cardiovascular protective drugs); or (3) unmeasured comorbidity in the study population leading to bias. The fact that information was not collected on major clinical events other than mortality (including ACS and repeat revascularizations during follow-up) precludes any attempts to establish a reliable link between stent-related outcomes and total mortality in our study. Despite the known reduction in target lesion revascularization (TLR)8,13 associated with first-generation DES, a late catch-up phenomenon regarding late and very late ischemic events due to continuous neointimal growth, late-acquired malapposition, neoatherosclerosis and stent thrombosis2,5,6,20 has been described and raised concerns. However, this issue has recently been demystified by Yamaji et al.,1 who reported a significant annual reduction in risk of TLR and stent thrombosis reduction after five years in both first-generation SES and PES. This effect may have contributed to the late survival benefit. Moreover, in our population, SES-treated patients were slightly more likely to be medicated with statins, angiotensin-converting enzyme inhibitor inhibitors and beta-blockers at baseline (Table 1), although the differences were not statistically significant relative to the BMS group and we had no way of assessing therapy prescription and adherence over the entire follow-up period. Finally, a selection bias favoring SES patients cannot be definitively ruled out. Still, patients treated with SES had a higher prevalence of diabetes and multivessel disease, smaller vessels and longer lesions, all of which are surrogates of a greater disease burden and, if anything, would tend to worsen the clinical outcome of the SES group. Nevertheless, they experienced better long-term survival in comparison with those who received BMS. On the other hand, BMS were more often implanted in patients with ACS (excluding primary PCI in STEMI), which could in part contribute to the observed differences in mortality in the long run. However, ACS was not an independent predictor of mortality.
As in other non-cardiac and cardiac clinical settings, age, CKD and COPD21 were independent predictors of mortality in our cohort. These comorbidities and age reflect increasing patient frailty and overall decreased functional reserve to cope with any pathological insult. LVEF is a fundamental parameter in the clinical management of heart disease, and is one of the strongest prognostic determinants in heart disease, particularly in ischemic heart disease.22 These predictors are included and used in many predictive risk scores validated in several heart conditions.23–25
Subgroup analysis (Figure 3) revealed a greater and significant benefit of SES in older and female patients, which could be expected taking into account that these characteristics are classical predictors of BMS failure.26 Depressed LVEF and incomplete revascularization, as well as insulin-treated diabetes, also showed a potential survival benefit when treated with SES compared to BMS.
LimitationsOur study has some important limitations that should be considered when interpreting the results, some of which have already been addressed. The inherent limitations of a single-center study with an observational design make it prone to bias. Although the SES-treated patients were consecutive (except for those with exclusion criteria), there is no information on why BMS were still implanted in the remaining patients during that period. Some knowledge on the characteristics of this group, as well as additional information on their long-term outcome, could help clarify the true role of SES in the observed survival benefit.
Finally, long-term follow-up was not prespecified when data were collected at baseline and relevant information on adverse events and cardiac death were not reported, precluding the analysis of other major cardiovascular events.
ConclusionsTo date, this is the longest study ever showing a survival benefit of first-generation SMS versus BMS. Although they are no longer in clinical use, our findings are reassuring and build on the perceived efficacy and long-term safety of first-generation SES.
Conflicts of interestThe authors have no conflicts of interest to declare.