Since 2011, the European guidelines have included a specific low-density lipoprotein cholesterol (LDL-C) target, <70 mg/dl, for very high cardiovascular risk (CVR) patients. However, registries have shown unsatisfactory results in obtaining this level of adequate lipid control.
ObjectivesTo assess temporal trends in the use of lipid-lowering therapy (LLT) and attainment of adequate control in very high CVR patients since 2011.
MethodsWe performed a retrospective observational study including very high CVR patients admitted in two periods: the first two years since the 2011 guidelines (2011/2012) and five years later (2016/2017). Lipid values, LLT, clinical variables and adequate lipid control rates were analyzed.
ResultsA total of 1314 patients were reviewed (2011/2012: 638; 2016/2017: 676). Overall, 443 patients (33.7%) were not under LLT and only a slight improvement in drug prescription was observed from 2011/2012 to 2016/2017. In LLT users, the proportion of high-intensity LLT increased significantly in the later years (6.4% vs. 24.0%; p<0.001), but this was not associated with adequate lipid control. Overall, mean LDL-C was 95.4±37.2 mg/dl and adequate control was achieved in 320 patients (24.4%), without significant differences between 2011/2012 and 2016/2017 (p=0.282). Independent predictors of adequate control were male gender, older age, diabetes, chronic kidney disease, prior acute coronary syndrome, prior stroke and LLT, while stable coronary artery disease was associated with higher risk of failure.
ConclusionEven after the introduction of specific LDL-C targets, these are still not reached in most patients. Over a five-year period, LLT prescription only improved slightly, while adequate lipid control rates remained unchanged.
Desde 2011, as guidelines europeias introduziram um alvo específico de colesterol de lipoproteínas de baixa densidade (C-LDL) para pacientes de risco cardiovascular muito alto (RCVMA): C-LDL <70 mg/dL. No entanto, os registos demonstraram resultados insatisfatórios na obtenção desse controlo lipídico adequado (CLAd).
ObjetivosAvaliar tendências temporais no uso de terapêutica hipolipemiante (TH) e no alcance de CLAd em pacientes de RCVMA desde 2011.
MétodosEstudo observacional retrospetivo, incluindo pacientes de RCVMA admitidos em dois períodos: dois primeiros anos desde as guidelines de 2011 (2011/2012) e cinco anos depois (2016/2017). Os valores lipídicos, TH, variáveis clínicas e taxas de CLAd foram analisados.
ResultadosForam revistos 1314 pacientes (2011/2012: 638; 2016/2017: 676). No geral, 443 pacientes (33,7%) não estavam sob TH e foi observada apenas uma ligeira melhoria na prescrição destes fármacos de 2011/2012 a 2016/2017. Em pacientes sob TH, a proporção destes com terapêutica de alta intensidade aumentou significativamente nos últimos anos (6,4% versus 24,0%; p<0,001), mas não foi associada ao CLAd. No geral, o C-LDL médio foi de 95,4±37,2 mg/dL e o CLAd foi alcançado em 320 pacientes (24,4%), sem diferenças significativas entre 2011/2012 e 2016/2017 (p=0,282). Os preditores independentes de CLAd foram: género masculino, idade avançada, diabetes, doença renal crónica, síndrome coronária aguda prévia, acidente vascular cerebral prévio e TH, enquanto a doença coronária estável foi associada a maior risco de falhar esse objetivo.
ConclusãoMesmo após a introdução de metas específicas de C-LDL, estas ainda não são atingidas na maioria dos pacientes. Durante um período de cinco anos, a prescrição de TH apenas melhorou ligeiramente, enquanto as taxas de obtenção de CLAd permanecerem inalteradas.
Cardiovascular disease (CVD), of which atherosclerotic disease is the main component, is the leading cause of mortality worldwide, accounting for 31% of all global deaths1 and 45% of deaths in Europe.2 CVD prevention is an effective and fundamental tool to reduce morbidity and mortality associated with these diseases,3,4 and includes, among others, optimal management of dyslipidemias. It has been shown that lowering low-density lipoprotein cholesterol (LDL-C) levels leads to proportional reductions in major cardiovascular events and in both cardiovascular and all-cause mortality.5,6 Furthermore, the protective effect of LDL-C lowering is independent of the drug used, depending only on its absolute reduction.6–10
Since 2011, the European Society of Cardiology (ESC) guidelines for the management of dyslipidemias have included specific LDL-C targets for each level of cardiovascular risk.11 For patients at very high cardiovascular risk (CVR), the goal defined was LDL-C <70 mg/dl or a ≥50% reduction from baseline LDL-C. The achievement of this goal is one of the essential secondary prevention measures and requires widespread use of statins or other lipid-lowering therapy (LLT).4,12 However, population studies performed since then in different European countries have shown disappointing results, with less than a third of patients being adequately controlled.13–16 Similar results were found in Portuguese studies (the Portuguese arm of DYSIS17 and DISGEN-LIPID18). Furthermore, despite the wide use of statins, the proportion of those on high-intensity LLT is still far from desirable.13,15,17,19
ObjectivesThe objective of this study was to assess the lipid control of very high CVR patients in the first two years (2011/2012) after the introduction of specific LDL-C targets in the 2011 ESC guidelines11 and to compare these results with those obtained five years later (2016/2017), concerning LLT and attainment of lipid goals.
MethodsPatient populationThis retrospective observational study was performed in the Cardiology Department of Centro Hospitalar Universitário de São João, Porto.
Patients aged ≥18 years were recruited if they fulfilled the criteria for very high CVR, defined by the 2011 ESC guidelines11 as at least one of the following: documented CVD; type 2 diabetes; type 1 diabetes with target organ damage; moderate to severe CKD (glomerular filtration rate <60 ml/min/1.73 m2); or calculated 10-year Systematic COronary Risk Evaluation (SCORE) ≥10%.
Recruitment included all consecutive patients at very high CVR admitted to this department in two time periods: from January 2011 to December 2012 (2011/2012) and from January 2016 to December 2017 (2016/2017). Patients who did not have a complete lipid profile collected within 24 hours of admission were excluded from the analysis.
Data collectionA retrospective assessment was performed by consulting patients’ clinical records. The data collected included gender, age, cardiovascular risk factors, documented CVD, relevant comorbidities, fasting lipid profile and LLT. Lipid profile was collected within 24 hours of admission and included total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). Treatment with LLT prior to admission included statins, a combination of statins and ezetimibe, or neither. On this basis, patients were classified as non-users (neither statin nor ezetimibe) or high-intensity LLT (atorvastatin 40-80 mg, rosuvastatin 20-40 mg or any moderate-intensity statin plus ezetimibe), medium-intensity LLT (atorvastatin 10-20 mg, rosuvastatin 5-10 mg, simvastatin 20-40 mg, pravastatin 40-80 mg, lovastatin 40 mg, fluvastatin 80 mg or pitavastatin 2-4 mg) or low-intensity LLT (simvastatin 10 mg, pravastatin 10-20 mg, lovastatin 20 mg, fluvastatin 20-40 mg or pitavastatin 1 mg) users.
In the 2011 ESC guidelines,11 a goal of <70 mg/dl LDL-C or a ≥50% reduction from baseline LDL-C was set for patients at very high CVR. However, in some of our study population it was not possible to obtain baseline values with which to calculate a percentage value of LDL-C reduction. Consequently, only the goal of absolute LDL-C value was analyzed. Therefore, patients were classified into two groups: those with LDL-C <70 mg/dl, i.e. with adequate lipid control, and those with LDL-C ≥70 mg/dl, i.e. with inadequate lipid control.
Statistical analysisStatistical analysis was performed using IBM SPSS version 25.0. A descriptive analysis was performed to characterize the sample profile. Continuous variables are expressed as means and standard deviations and categorical variables are shown as percentages. Comparison of means was performed with the Student's t test, while the chi-square or Fisher's exact test were used for comparison of percentages. Kolmogorov-Smirnov and Shapiro-Wilk tests were used to assess normality of distribution. Binary logistic regression was used to identify associations with adequate lipid control. Statistical significance was defined as a p-value <0.05 (significance level of 95%).
ResultsThe baseline characteristics of the study population are shown in Table 1. A total of 1314 patients were included: 638 from 2011/2012 (48.6%) and 676 from 2016/2017 (51.4%). Of the total population, 929 (70.7%) were male, with a mean age of 67.6±11.7 years. Patients from the 2016/2017 group were older (68.4±11.7 vs. 66.7±11.7 years; p=0.009). Most patients were hypertensive (76.7%) and diabetic (56.2%), with similar proportions between groups. CKD was present in 239 patients of the total population (18.2%), with significantly higher rates in the later period. Overall, the majority had prior documented CVD (n=898; 68.3%), including 572 with prior acute coronary syndrome (ACS), 128 with stable coronary artery disease (SCAD) but no prior ACS, 171 patients with prior stroke and 195 with peripheral arterial disease (PAD). In the 2016/2017 group there were more patients with SCAD, stroke and CKD, but fewer with prior ACS.
Baseline characteristics of the study population.
| Characteristic | Overall (n=1314) | 2011/2012 (n=638) | 2016/2017 (n=676) | p |
|---|---|---|---|---|
| Male gender | 929 (70.7%) | 455 (71.3%) | 474 (70.1%) | 0.633 |
| Age, years | 67.6±11.7 | 66.7±11.7 | 68.4±11.7 | 0.009 |
| Diabetes | 738 (56.2%) | 362 (56.7%) | 376 (55.6%) | 0.683 |
| Hypertension | 1008 (76.7%) | 487 (76.3%) | 521 (77.1%) | 0.752 |
| CKD | 239 (18.2%) | 96 (15.0%) | 143 (21.2%) | 0.004 |
| Documented CVD | 898 (68.3%) | 435 (68.2%) | 463 (68.5%) | 0.904 |
| SCAD | 128 (9.7%) | 37 (5.8%) | 91 (13.5%) | <0.001 |
| Prior ACS | 572 (43.5%) | 319 (50.0%) | 253 (37.4%) | <0.001 |
| Prior stroke | 171 (13.0%) | 69 (10.8%) | 102 (15.1%) | 0.021 |
| PAD | 195 (14.8%) | 85 (13.3%) | 110 (16.3%) | 0.133 |
ACS: acute coronary syndrome; CKD: chronic kidney disease; CVD: cardiovascular disease; PAD: peripheral arterial disease; SCAD: stable coronary artery disease.
Data presented as mean ± standard deviation or n (%).
The mean serum lipid values for the overall population (Table 2) were: TC: 165.1±44.5 mg/dl; HDL-C: 40.5±10.5 mg/dl; LDL-C: 95.4±37.1 mg/dl; TG: 146.4±90.1 mg/dl. There were no significant differences in these values between the groups.
Serum lipid values.
| Characteristic | Overall (n=1314) | 2011/2012 (n=638) | 2016/2017 (n=676) | p |
|---|---|---|---|---|
| TC | 165.1±44.5 | 166.4±43.4 | 163.9±45.5 | 0.306 |
| HDL-C | 40.5±10.5 | 40.7±10.2 | 40.3±10.7 | 0.444 |
| LDL-C | 95.4±37.1 | 96.7±36.7 | 94.3±37.5 | 0.246 |
| TG | 146.4±90.1 | 144.9±81.5 | 147.8±97.6 | 0.559 |
HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TC: total cholesterol; TG: triglycerides.
Data presented as mean ± standard deviation.
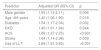
A significant proportion of patients were not receiving LLT (33.7%) (Table 3), which led to significantly higher TC (182.8±45.7 vs. 156.1±41.1; p<0.001), TG (154.9±103.1 vs. 142.1±82.5; p=0.023) and LDL-C (111.3±39.7 vs. 87.4±32.9; p<0.001), but similar HDL-C (p=0.370). The use of these drugs increased from 2011/2012 to 2016/2017 (63.5% vs. 68.9%; p=0.037).
Lipid-lowering therapy use and intensity.
| Characteristic | Overall (n=1314) | 2011/2012 (n=638) | 2016/2017 (n=676) | p |
|---|---|---|---|---|
| Use of LLT | 871 (66.3%) | 405 (63.5%) | 466 (68.9%) | 0.037 |
| High-intensity LLT | 138 (15.8%) | 26 (6.4%) | 112 (24.0%) | <0.001 |
| Medium-intensity LLT | 676 (77.6%) | 344 (84.9%) | 332 (71.2%) | <0.001 |
| Low-intensity LLT | 57 (6.5%) | 35 (8.6%) | 22 (4.7%) | 0.020 |
LLT: lipid-lowering therapy.
Data presented as n (%).
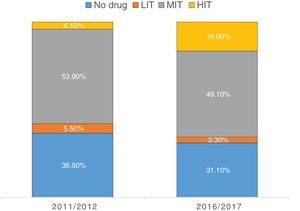
Regarding LLT intensity, most patients received medium-intensity LLT (77.6%) and only 15.8% received high-intensity LLT (Figure 1). However, the use of high-intensity LLT increased significantly in the later years (6.4% vs. 24.0%; p<0.001). Overall, atorvastatin (33.5%), simvastatin (30.3%) and rosuvastatin (20.7%) were the most frequently prescribed drugs (Figure 2). Ezetimibe was only prescribed in a small minority of patients (1.8%) in both time periods (p=0.496).
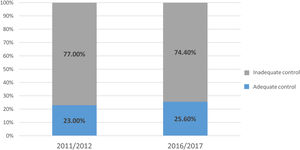
Overall, 320 patients had adequate lipid control (24.4%), while the other 994 (75.6%) did not. As seen in Figure 3, the proportion of patients achieving adequate lipid control did not differ between the two time periods (2011/2012: 23.0% vs. 2016/2017: 25.6%; p=0.282).
Clinical variables associated with adequate lipid control (Table 4) were male gender (26.3% vs. 19.7%; p=0.012), age >65 years (28.0% vs. 19.4%; p<0.001), diabetes (26.6% vs. 21.5%; p=0.035), CKD (33.5% vs. 22.3%; p<0.001), prior ACS (32.0% vs. 18.5%; p<0.001), prior stroke (33.3% vs. 23.0%; p=0.003) and PAD (31.3% vs. 23.1%; p=0.015). On the other hand, patients with SCAD were less likely to achieve the target (7.0% vs. 26.2%; p<0.001). Regarding drug therapy, the use of LLT was associated with significantly higher rates of adequate lipid control (30.9% vs. 11.5%; p<0.001). However, among users of these drugs, high-intensity LLT was not associated with attainment of the goal (30.4% vs. 31.0%; p=0.901).
Characteristics associated with adequate or inadequate lipid control.
| Characteristic | Adequate control (n=320) | Inadequate control (n=994) | p |
|---|---|---|---|
| Period | |||
| 2011/2012 | 23% (147) | 77% (491) | 0.282 |
| 2016/2017 | 25.6% (173) | 74.4% (503) | |
| Gender | |||
| Male | 26.3% (244) | 73.7% (685) | 0.012 |
| Female | 19.7% (76) | 80.3% (309) | |
| Age | |||
| Mean, years | 69.5±11.1 | 67.0±11.9 | 0.001 |
| Age >65 years | 28% (212) | 72% (546) | <0.001 |
| Age ≤65 years | 19.4% (108) | 80.6% (448) | |
| Hypertension | 25.1% (253) | 74.9% (755) | 0.253 |
| No hypertension | 21.9% (67) | 87.1% (239) | |
| Diabetes | 26.6% (196) | 73.4% (542) | 0.035 |
| No diabetes | 21.5% (124) | 78.5% (452) | |
| CKD | 33.5% (80) | 66.5% (159) | <0.001 |
| No CKD | 22.3% (240) | 77.7% (835) | |
| Prior ACS | 32.0% (183) | 68.0% (389) | <0.001 |
| No prior ACS | 18.5% (137) | 81.5% (605) | |
| SCAD | 7.0% (9) | 93.0% (119) | <0.001 |
| No SCAD | 26.2% (311) | 73.8% (875) | |
| Prior stroke | 33.3% (57) | 66.7% (114) | 0.003 |
| No prior stroke | 23.0% (263) | 77.0% (880) | |
| PAD | 31.3% (61) | 68.7% (134) | 0.015 |
| No PAD | 23.1% (259) | 76.9% (860) | |
| Use of LLT | 30.9% (269) | 69.1% (602) | <0.001 |
| No LLT | 11.5% (51) | 88.5% (392) | |
| High-intensity LLT | 30.4% (42) | 69.6% (96) | 0.901 |
| No high-intensity LLT | 31.0% (227) | 69.0% (506) | |
ACS: acute coronary syndrome; CKD: chronic kidney disease; LLT: lipid-lowering therapy; PAD: peripheral arterial disease; SCAD: stable coronary artery disease.
Data presented as mean ± standard deviation or n (%).
On multivariate analysis, male gender (odds ratio [OR] 1.55; p=0.006), age >65 years (OR 1.40; p=0.018), diabetes (OR 1.54; p=0.002), CKD (OR 1.40; p=0.044), prior ACS (OR 1.69; p<0.001), stroke (OR 1.64; p=0.009) and use of LLT (OR 2.69; p<0.001) were all independent predictors of adequate lipid control (Table 5), but not PAD.
Multivariate analysis of predictors of adequate lipid control.
| Predictor | Adjusted OR (95% CI) | p |
|---|---|---|
| Male gender | 1.55 (1.13-2.11) | 0.006 |
| Age >65 years | 1.40 (1.06-1.90) | 0.018 |
| Diabetes | 1.54 (1.17-2.04) | 0.002 |
| CKD | 1.40 (1.01-1.94) | 0.044 |
| ACS | 1.69 (1.27-2.25) | <0.001 |
| Stroke | 1.64 (1.14-2.38) | 0.009 |
| Use of LLT | 2.69 (1.91-3.80) | <0.001 |
ACS: acute coronary syndrome; CI: confidence interval; CKD: chronic kidney disease; LLT: lipid-lowering therapy; OR: odds ratio.
Lipid control in patients at very high CVR is still clearly insufficient. Mean LDL-C in this overall population was >90 mg/dl, far from the goals established in the previous 2011 and 2016 ESC guidelines.11,20,21 Considering that most of these patients had established CVD, this is a reason if anything for greater concern, given that LDL-C lowering produces significant reductions in cardiovascular events.5,6
Furthermore, about one third of the population in this study were not receiving any LLT, which obviously was linked to significantly worse lipid control. Compared to other studies in patients at very high CVR in which these drugs were prescribed in 86-99% of patients,13,15,16,19 the proportion receiving this therapy in our population was considerably lower, even in the later years (68.9%).
One of the goals of this study was to assess the temporal evolution of LLT prescription and lipid control over a five-year period. There was a slight increase in drug prescription from 2011/2012 to 2016/2017 (8.5%; p=0.037), but this was still far from desirable. There was a positive trend in the use of high-intensity LLT, which almost quadrupled in the later years, but this did not result in better lipid control. Furthermore, despite the increased use of high-intensity LLT in the more recent period, it was still lower (24.0%) than observed in other studies, in which high-intensity LLT was prescribed in 33-58% of patients receiving LLT.13,15,19,22
Additionally, the prescription rate of dual therapy with a statin and ezetimibe in this study was only marginal, even in the more recent period, and considerably lower than in other studies,13,15,19 which may be related to these unsatisfactory results.
Of the overall population, only 24.4% achieved adequate lipid control and there was no significant improvement between the two periods. Similar studies in other European countries have also shown poor lipid control in patients at very high CVR, in the range of 20-32%,13,15,16,19 while in another Portuguese study (DISGEN-LIPID18) rates of adequate lipid control were even lower (10-15%).
Male gender, older age, diabetes, CKD, prior ACS and stroke were all independent predictors of adequate lipid control, but the most powerful predictor was the use of LLT, which almost tripled the chances of achieving the goal. High-intensity LLT was not associated with an increased likelihood of reaching the LDL-C target. Unlike the other forms of CVD, SCAD was associated with higher risk of failure to achieve adequate lipid control.
Gender was not a differentiating factor when prescribing LLT (p=0.317) or high-intensity LLT (p=0.788), making it more difficult to understand the differences between men and women. By contrast, older age was associated with greater prescription of LLT (71.8% vs. 58.8%; p<0.001) and high-intensity LLT (19.0% vs. 14.0%; p=0.05), which could explain the higher rates of adequate lipid control in this group. With regard to diabetes, CKD, prior ACS and stroke, these predictors could probably be explained by the greater attention given by physicians to these patients, considering them more severe or progressive conditions than other forms of very high CVR (PAD, SCAD or SCORE ≥10%), since LLT and high-intensity LLT prescription were not a differentiating factor in most of them (with the exception of prior ACS).
Updated ESC guidelines on dyslipidemias were published in 2019,12 with even more ambitious lipid targets for patients at very high CVR: LDL-C reduction of ≥50% from baseline and an LDL-C goal of <55 mg/dl (<40 mg/dl in some cases). However, what was shown in this and other similar studies, as well as in European registries,13,15,16,19 is that even the previous and less ambitious targets in the 2011 and 2016 ESC guidelines11,20,21 were only achieved in a minority of patients.
It has been reported that physicians have a poor proactive attitude in response to unachieved lipid goals and only a minority make therapeutic changes to attain better lipid control.19,23 Additionally, patients’ adherence to LLT is low, with significant discontinuation rates,24–28 which results in higher mortality.29 Therapeutic inertia by physicians and poor drug adherence by patients may thus be important factors in these disappointing results. These two issues should be an essential part of any strategy to ensure improved lipid control. The more widespread use of ezetimibe in recent years13,15,19 and the appearance of new and more potent lipid-lowering drugs such as PCSK9 inhibitors7–10 are also essential to reach lipid targets in a much more significant number of patients at very high CVR.
The results of the above-mentioned registries in Portugal and other European countries, together with this study, show the urgent need for improved lipid control, including greater use of high-intensity LLT, combination therapy with ezetimibe, the new PCSK9 inhibitors and, last but not least, the pivotal role of nutrition, dietary factors and lifestyle modifications to improve plasma lipid profile, in the prevention of CVD.12
It is also essential to combine these measures with a more proactive attitude on the part of physicians and improved patient adherence to drug therapy.
ConclusionsThis observational study shows that lipid control in very high CVR patients is clearly insufficient, as only about one in four patients achieved adequate control. Moreover, this rate showed no improvement over a five-year period. Male gender, older age, diabetes, CKD, prior ACS and stroke and the use of LLT were independently associated with achievement of adequate lipid control, whereas SCAD was associated with failure to reach the target.
In addition, the use of LLT is still not widely implemented in a population with significant rates of established CVD. There were minor improvements in drug prescription in LLT and high-intensity therapy in particular in recent years, but it remained clearly unsatisfactory and did not result in better lipid control.
Overall, these disappointing results reveal a significant gap between recommendations in the guidelines and real-world results, and should prompt serious rethinking of treatment strategies for these patients, especially now that even more ambitious lipid targets have emerged from the recent 2019 ESC guidelines.
Conflicts of interestThe authors have no conflicts of interest to declare.