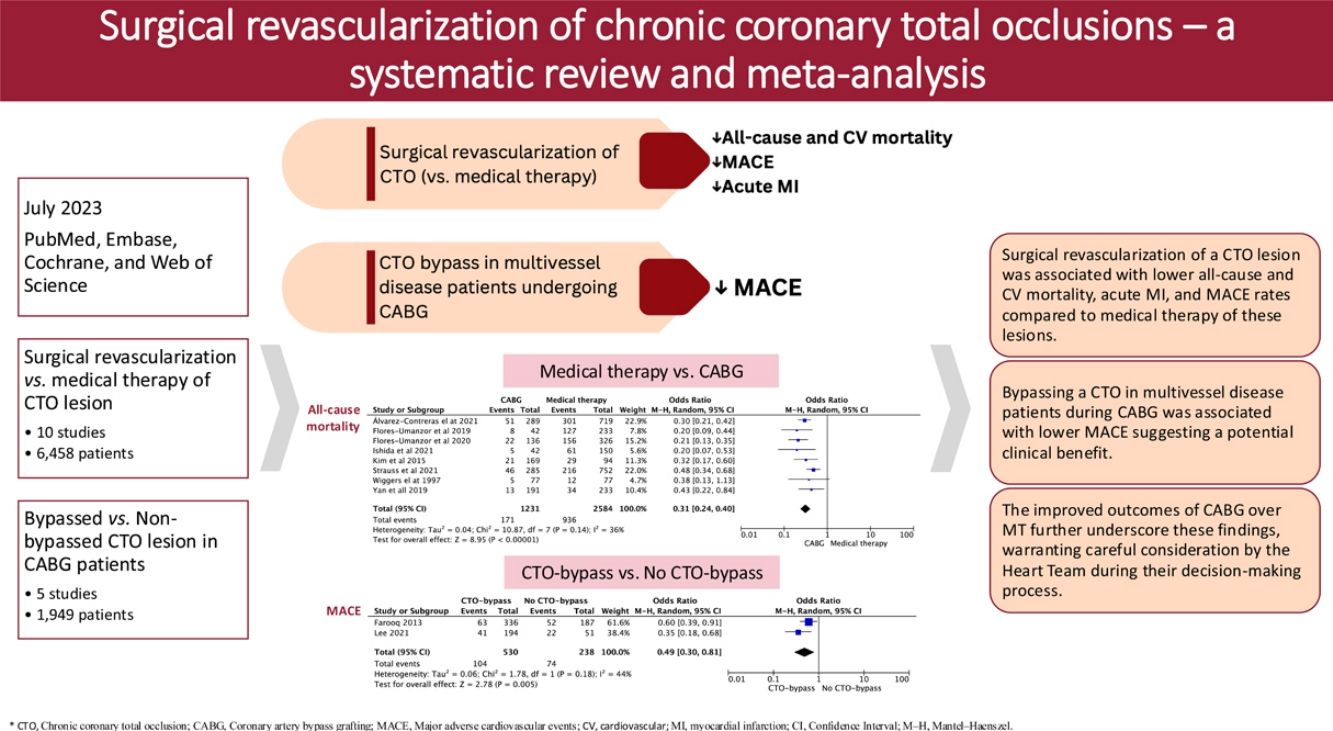
Chronic coronary total occlusion (CTO) optimal therapeutic management remains a topic of debate despite its association with adverse clinical outcomes. This study aimed to compare clinical outcomes of patients with CTOs treated with coronary artery bypass graft (CABG) versus medical therapy (MT), assessing the effect of CTO revascularization in patients with multivessel disease undergoing CABG.
MethodsIn July 2023, PubMed, Embase, Cochrane, and Web of Science databases were systematically searched for studies comparing CTOs treated with CABG versus MT. A sub-analysis of CABG patients, comparing complete surgical revascularization, including CTO bypass, to CABG without CTO bypass, was performed. A pooled odds ratio meta-analysis assessed four main outcomes: mortality, myocardial infarction (MI), stroke, and major adverse cardiovascular events (MACE). The primary outcome was all-cause mortality.
ResultsTen observational studies (6458 patients) comparing CABG-CTO with MT-CTO showed lower all-cause mortality in the CABG group (OR 0.31, 95% CI 0.24–0.40, p<0.001, I2=36%). Despite heterogeneity, CABG exhibited reduced CV mortality and MACE (OR 0.37, 95% CI 0.24–0.57, p<0.001, I2=59%; OR 0.37, 95% CI 0.15–0.92, p=0.03, I2=80%, respectively). The MI rate was lower in the CABG group (OR 0.41, 95% CI 0.30–0.56, p<0.001, I2=0%). Comparing bypassed to non-bypassed CTO groups (5 studies, 1949 patients), the bypassed-CTO group had considerably lower MACE (OR 0.49, 95% CI 0.30–0.81, p=0.005, I2=44%).
ConclusionThis study suggests a clinical benefit of bypassing a CTO in multivessel disease patients during CABG, with significantly lower MACE. The improved outcomes of CABG over MT further underscore these findings, warranting careful consideration by the Heart Team during their decision-making process.
A terapêutica ideal para as oclusões coronárias crónicas (CTO) continua a ser um tópico controverso, apesar de sua associação com eventos clínicos adversos. Este estudo tem como objetivo comparar os doentes submetidos à revascularização cirúrgica da CTO com aqueles tratados com terapêutica médica (TM), avaliando o impacto da revascularização das CTOs em pacientes com doença multivaso submetidos a cirurgia de revascularização miocárdica (CABG).
MétodosEm julho de 2023, estudos a comparar CTOs revascularizadas cirurgicamente com terapêutica médica foram sistematicamente pesquisados nas bases de dados PubMed, Embase, Cochrane e Web of Science. Foi realizada uma subanálise de doentes submetidos a CABG, comparando a revascularização completa, que inclui a revascularização da CTO, com CABG em que a CTO não foi revascularizada. Uma meta-análise agrupada de odds ratio avaliou quatro outcomes principais: mortalidade, enfarte agudo do miocárdio (EAM), acidente vascular cerebral e eventos cardiovasculares adversos major (MACE).
ResultadosDez estudos observacionais (6,458 doentes) que compararam CABG-CTO com TM-CTO mostraram redução da mortalidade global no grupo CABG (OR 0.31, 95% CI 0.24-0.40, p<0.001, I2=36%). Apesar da heterogeneidade, o grupo CABG-CTO apresentou redução da mortalidade cardiovascular e do MACE (OR 0.37, 95% CI 0.24-0.57, p<0.001, I2=59%; OR 0.37, 95% CI 0.15-0.92, p=0.03, I2=80%, respetivamente). A incidência de EAM foi inferior no grupo CABG (OR 0.41, 0.30-0.56, p<0.001, I2=0%). Comparando o grupo submetido a bypass da CTO com o não submetido (5 estudos, 1.949 doentes), o grupo com bypass na CTO apresentou redução significativa do MACE (OR 0.49, IC 95% 0.30-0.81, p=0.005, I2=44%).
ConclusãoEste estudo sugere um benefício clínico da revascularização da CTO em doentes com doença multivaso submetidos a CABG, com redução significativa do MACE. A melhoria dos resultados obtidos com a CABG em comparação com a TM reforça os achados supracitados, destacando a importância de uma avaliação cuidadosa pela Heart Team durante o processo de decisão.
Chronic coronary total occlusions (CTOs) are defined as angiographic evidence of complete occlusion of a coronary artery persisting for a minimum of three months.1,2 The right coronary artery is the most frequently affected (43–55%).3 Studies have reported an incidence of CTOs detected during coronary angiography ranging from 18% to 52%, yet their prevalence has shown a decline over recent decades.1,4 CTOs are associated with ischemic symptoms and adverse clinical outcomes, such as reduced left ventricular ejection fraction, elevated risks of cardiogenic shock, and increased mortality rates.4 Nevertheless, the management of CTOs remains a complex and contentious challenge.5
The three primary strategies for managing CTOs are medical therapy (MT), coronary artery bypass grafting (CABG), and percutaneous coronary intervention (PCI).5 Despite technological advances, a substantial proportion of patients with CTO continue to receive MT.6 Due to its technical complexity, safety concerns, and expected benefits, CTO-PCI remains a subject of ongoing debate.7 Although observational studies have linked CTO-PCI to improved clinical outcomes, randomized controlled trials (RCTs) have not corroborated these findings.8 The randomized EuroCTO trial demonstrated greater improvement in the angina frequency and quality of life in CTO patients undergoing PCI at 1 year as compared to MT, but major adverse cardiovascular events (MACE) were comparable between the 2 groups, which was supported by the DECISION-CTO trial.9,10
The predicted surgical mortality, the anatomical complexity of coronary artery disease, and the expected completeness of revascularization are important criteria for decision-making with respect to the type of revascularization.11 Since there is generally no difference in the techniques of revascularization whether the vessel is highly stenosis or completely occluded, the presence of CTO may not affect the complexity of CABG or postoperative outcomes.12 Strauss et al. showed that, compared to the MT group, CABG-CTO presented a more robust reduction in mortality at ten years of follow-up than PCI-CTO.13 Several studies have linked the improved cardiovascular outcomes of CABG over multivessel PCI to the completeness of revascularization it accomplished.2 However, there is a lack of evidence on the survival benefit of complete revascularization of CTOs in patients undergoing CABG.12
ObjectivesThis study aims to perform a comprehensive systematic review and meta-analysis, first comparing the clinical outcomes of patients with CTO treated with CABG versus MT, and then assessing the effect of CTO revascularization, comparing CABG with and without CTO revascularization. It will focus on critical endpoints including all-cause mortality, cardiovascular mortality, acute myocardial infarction (MI), stroke, and MACE.
MethodsStudy protocol and registrationThis study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard14 (Supplementary Material, Table 1). The study was also registered in the International Prospective Register of Systematic Reviews database (CRD42023433839).
Literature searchThe search strategy was conducted by ALS and GC. In July 2023, PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science databases were systematically searched, and the reference lists of eligible studies were scanned to capture additional articles by cross-referencing. The search strategy included “Coronary occlusion” or “chronic coronary total occlusion” or “chronic total occlusion of coronary artery” or “CTO”, and “coronary artery bypass grafting” or “surgical revascularization” or “CABG”, and “medical treatment” or “medical management”. The specific search strategy is presented in Supplementary Material, Table 2. Databases were searched without time restrictions, from their inception until July 2023. Literature searches were updated during peer review to include the most up-to-date literature. The search was restricted to involve human subjects only, and no language limits were imposed.
Eligibility criteriaWe identified studies investigating adult patients (≥18 years old) and reporting the comparison of surgical revascularization versus MT of the CTO lesion. Additionally, studies comparing bypassed to non-bypassed CTO lesions in patients undergoing CABG were also included. Reports where the comparison received another intervention (e.g., PCI) were excluded from the research.
Observational (cohort and case-control) and interventional (randomized controlled trials) were systematically searched in databases. Reviews, dissertations, theses, editorials, study protocols, clinical guidelines, commentaries, and letters were excluded. Studies without MT as a comparison group were banned.
Primary and secondary outcomesThe primary outcome was all-cause mortality. Secondary outcomes were cardiovascular mortality, acute myocardial infarction, stroke, MACE, and complete revascularization. The definition of MACE differed across studies, although the majority included the composite endpoint of all-cause or cardiovascular mortality, stroke, or myocardial infarction. Further details are provided in Table 2.
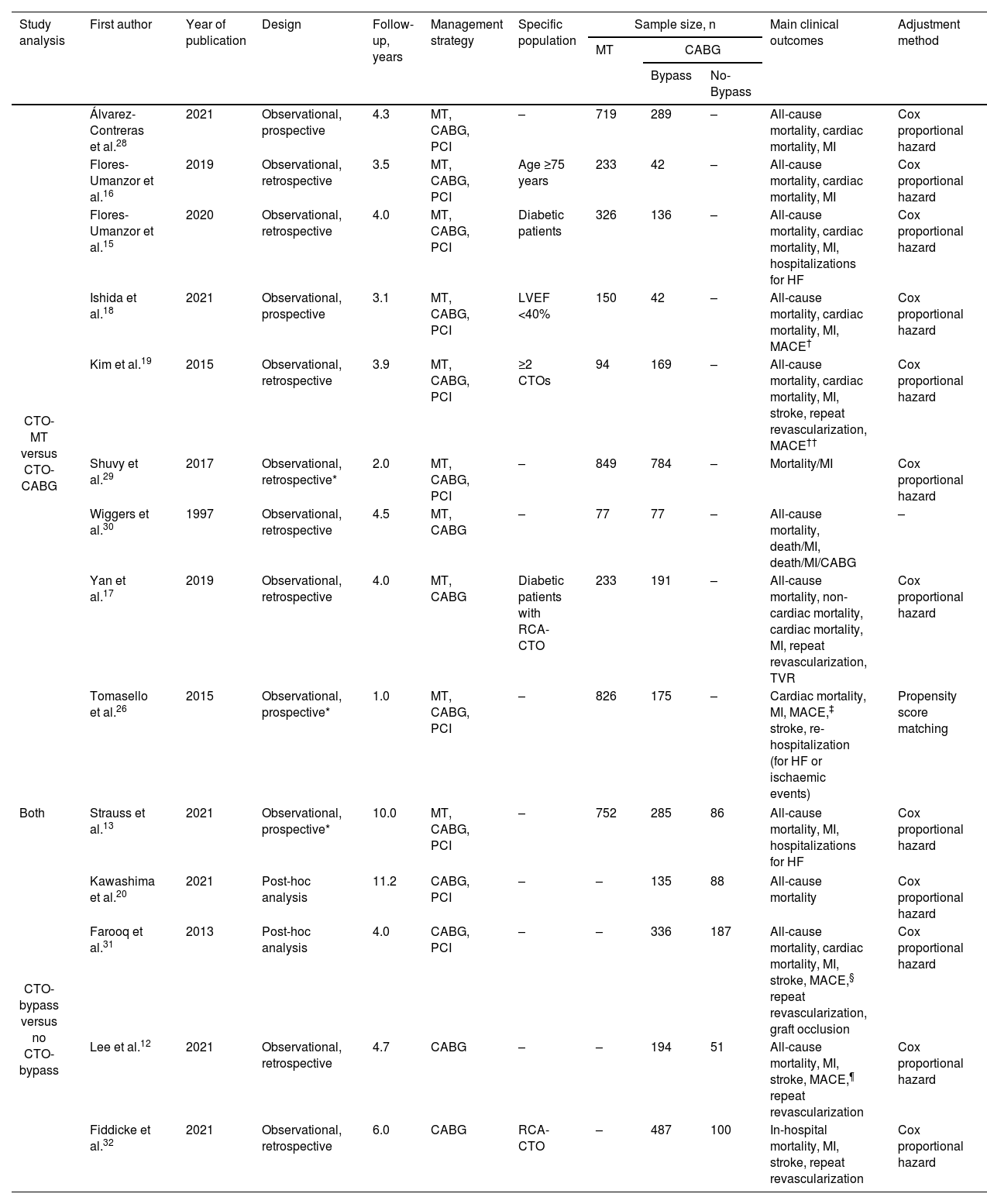
Study characteristics.
| Study analysis | First author | Year of publication | Design | Follow-up, years | Management strategy | Specific population | Sample size, n | Main clinical outcomes | Adjustment method | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MT | CABG | ||||||||||
| Bypass | No-Bypass | ||||||||||
| CTO-MT versus CTO-CABG | Álvarez-Contreras et al.28 | 2021 | Observational, prospective | 4.3 | MT, CABG, PCI | – | 719 | 289 | – | All-cause mortality, cardiac mortality, MI | Cox proportional hazard |
| Flores-Umanzor et al.16 | 2019 | Observational, retrospective | 3.5 | MT, CABG, PCI | Age ≥75 years | 233 | 42 | – | All-cause mortality, cardiac mortality, MI | Cox proportional hazard | |
| Flores-Umanzor et al.15 | 2020 | Observational, retrospective | 4.0 | MT, CABG, PCI | Diabetic patients | 326 | 136 | – | All-cause mortality, cardiac mortality, MI, hospitalizations for HF | Cox proportional hazard | |
| Ishida et al.18 | 2021 | Observational, prospective | 3.1 | MT, CABG, PCI | LVEF <40% | 150 | 42 | – | All-cause mortality, cardiac mortality, MI, MACE† | Cox proportional hazard | |
| Kim et al.19 | 2015 | Observational, retrospective | 3.9 | MT, CABG, PCI | ≥2 CTOs | 94 | 169 | – | All-cause mortality, cardiac mortality, MI, stroke, repeat revascularization, MACE†† | Cox proportional hazard | |
| Shuvy et al.29 | 2017 | Observational, retrospective* | 2.0 | MT, CABG, PCI | – | 849 | 784 | – | Mortality/MI | Cox proportional hazard | |
| Wiggers et al.30 | 1997 | Observational, retrospective | 4.5 | MT, CABG | – | 77 | 77 | – | All-cause mortality, death/MI, death/MI/CABG | – | |
| Yan et al.17 | 2019 | Observational, retrospective | 4.0 | MT, CABG | Diabetic patients with RCA-CTO | 233 | 191 | – | All-cause mortality, non-cardiac mortality, cardiac mortality, MI, repeat revascularization, TVR | Cox proportional hazard | |
| Tomasello et al.26 | 2015 | Observational, prospective* | 1.0 | MT, CABG, PCI | – | 826 | 175 | – | Cardiac mortality, MI, MACE,‡ stroke, re-hospitalization (for HF or ischaemic events) | Propensity score matching | |
| Both | Strauss et al.13 | 2021 | Observational, prospective* | 10.0 | MT, CABG, PCI | – | 752 | 285 | 86 | All-cause mortality, MI, hospitalizations for HF | Cox proportional hazard |
| CTO-bypass versus no CTO-bypass | Kawashima et al.20 | 2021 | Post-hoc analysis | 11.2 | CABG, PCI | – | – | 135 | 88 | All-cause mortality | Cox proportional hazard |
| Farooq et al.31 | 2013 | Post-hoc analysis | 4.0 | CABG, PCI | – | – | 336 | 187 | All-cause mortality, cardiac mortality, MI, stroke, MACE,§ repeat revascularization, graft occlusion | Cox proportional hazard | |
| Lee et al.12 | 2021 | Observational, retrospective | 4.7 | CABG | – | – | 194 | 51 | All-cause mortality, MI, stroke, MACE,¶ repeat revascularization | Cox proportional hazard | |
| Fiddicke et al.32 | 2021 | Observational, retrospective | 6.0 | CABG | RCA-CTO | – | 487 | 100 | In-hospital mortality, MI, stroke, repeat revascularization | Cox proportional hazard | |
CABG: coronary artery bypass grafting; CTO: chronic coronary total occlusion; HF: heart failure; LVEF: left ventricular ejection fraction; MACE: major adverse cardiovascular events; MI: myocardial infarction; MT: medical treatment; PCI: percutaneous coronary intervention; RCA: right coronary artery; TVR: target vessel revascularization.
Study selection and data extraction were performed in duplicate by two reviewers. Mendeley software was used to store, organize and manage references, and allow a transparent and reproducible systematic search. Both reviewers independently scanned study titles and abstracts against eligibility criteria. The full text of the included studies was assessed independently by two co-authors to comply with the inclusion criteria. Reviewers’ results were compared, and critical points were discussed when disagreements occurred until a consensus was reached. If necessary, other study authors were asked to resolve eligibility issues. The data extracted per study includes the author and year of publication, study design and population, baseline demographic and clinical characteristics, the intervention, and the outcomes mentioned above.
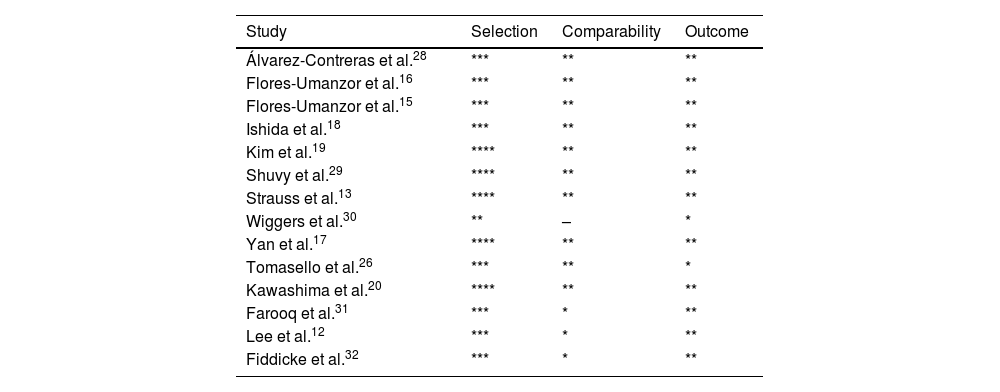
Risk of bias assessmentTwo co-authors performed the risk of bias assessment independently, using the Newcastle–Ottawa Scale for observational studies. The quality assessment for each study is presented in the Newcastle–Ottawa Scale (Table 1). Publication bias was assessed using funnel plots (Supplementary Material, Figures 1–9).
Newcastle–Ottawa Scale summary.
| Study | Selection | Comparability | Outcome |
|---|---|---|---|
| Álvarez-Contreras et al.28 | *** | ** | ** |
| Flores-Umanzor et al.16 | *** | ** | ** |
| Flores-Umanzor et al.15 | *** | ** | ** |
| Ishida et al.18 | *** | ** | ** |
| Kim et al.19 | **** | ** | ** |
| Shuvy et al.29 | **** | ** | ** |
| Strauss et al.13 | **** | ** | ** |
| Wiggers et al.30 | ** | – | * |
| Yan et al.17 | **** | ** | ** |
| Tomasello et al.26 | *** | ** | * |
| Kawashima et al.20 | **** | ** | ** |
| Farooq et al.31 | *** | * | ** |
| Lee et al.12 | *** | * | ** |
| Fiddicke et al.32 | *** | * | ** |
* Indicate the star rating according to the Newcastle-Ottawa Scale.
Statistical analysis was conducted using Review Manager 5.4 from the Cochrane Collaboration to perform meta-analyses for the defined endpoints. The measure of effect was the odds ratio, comparing CABG versus MT or bypassed-CTO versus non-bypassed CTO, with 95% confidence intervals estimated using the Mantel–Haenszel method under a random effects model. Given the clinical and methodological heterogeneity among the included studies, a random effects model was selected to account for between-study variability arising from differences in populations, follow-up durations, and study designs, ensuring a more conservative and generalizable effect size estimate.
The statistical significance of the overall effect was assessed using the Z-statistic and its corresponding p-value, with a significance threshold of 5%. Heterogeneity was visually represented through forest plots and statistically assessed using Cochran's Q test (significance level=0.05) and the I2 index, categorized as low (<25%), moderate (25–50%), or high (>50%). Despite the presence of high heterogeneity in some cases, meta-analyses were performed, with results interpreted in the context of this variability. The certainty of the evidence was evaluated using the GRADE approach (Supplementary Material, Table 3).
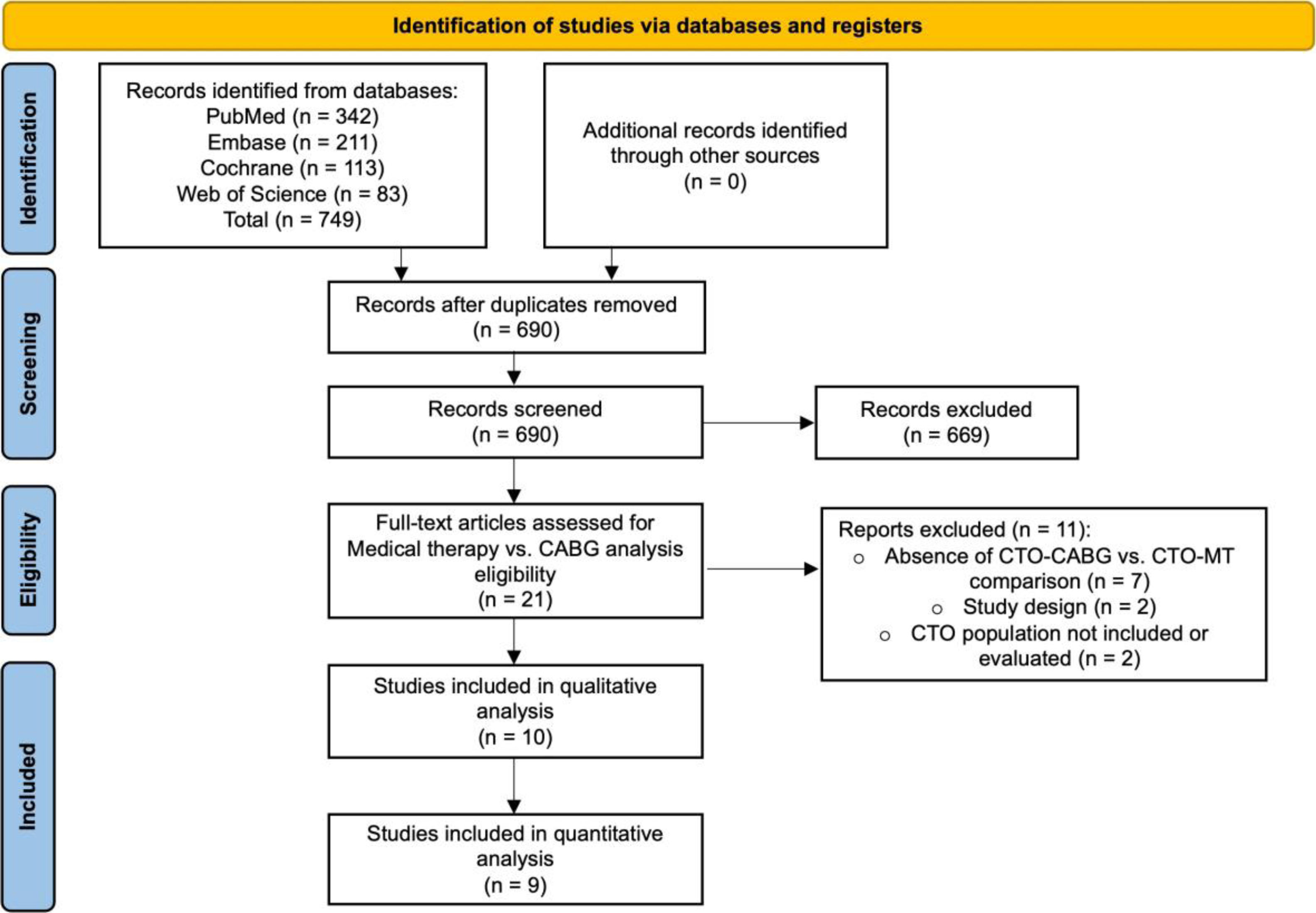
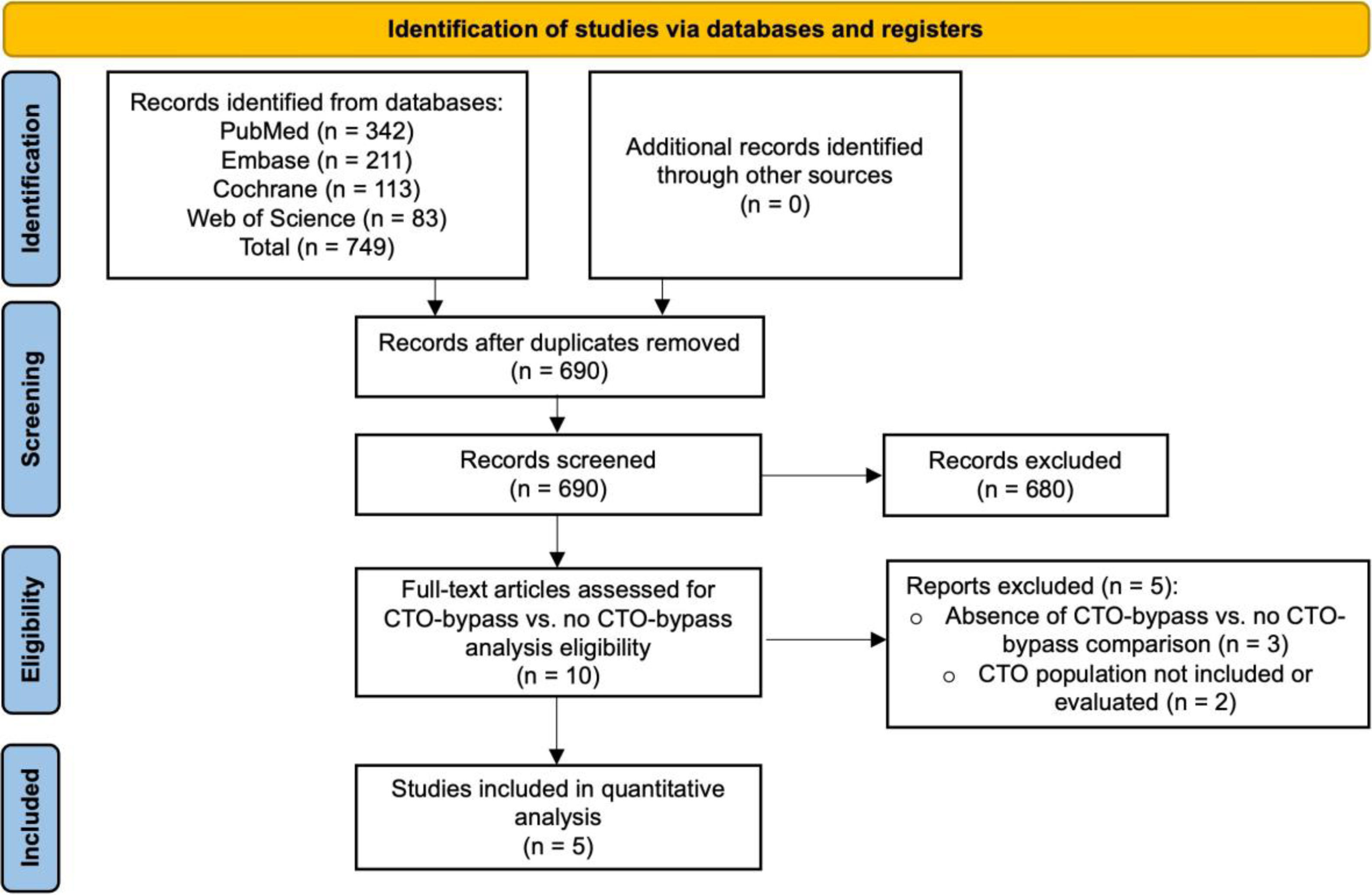
ResultsSearch resultsThe literature search identified 749 articles. After removing duplicates, 690 publications were screened. Based on titles, abstracts, study type, and study population assessments, 669 studies were excluded from the medical therapy versus CABG comparison (Figure 1), and 680 studies were excluded from the CTO-bypass versus no CTO-bypass analysis (Figure 2). For the medical therapy versus CABG comparison, 21 full-text articles were evaluated for eligibility (Figure 1). Of these, 11 were excluded due to the absence of a direct CTO-CABG versus CTO-MT comparison (n=7), inappropriate study design (n=2), or lack of relevant CTO population data (n=2). In the CTO-bypass versus no CTO-bypass analysis, 10 full-text articles were assessed for eligibility (Figure 2). Five articles were excluded due to the absence of a CTO-bypass versus no CTO-bypass comparison (n=3) or insufficient data on the CTO population (n=2).
Following this procedure, ten studies met the eligibility criteria for the CTO-CABG versus CTO-MT comparison and five for the CTO-bypass versus no CTO-bypass analysis. One study fulfilled the criteria for both comparisons and was included in both analyses. Additionally, one study was excluded from the quantitative analysis of medical therapy versus CABG comparison due to the absence of a comparable outcome.
The characteristics of the fourteen studies that met the eligibility criteria are summarized in Table 2, with one study included in both analyses. Ten studies conducted a comparative analysis of clinical outcomes between CABG and MT of CTO lesions, enrolling 6458 patients (34.1% underwent CABG, while 65.9% received MT). Additionally, five studies compared groups undergoing CABG with and without CTO bypass, involving a total of 1949 patients (73.7% with CTO bypass compared to 26.3% without). Demographic and baseline characteristics of included patients are represented in Table 3. Follow-up time ranged between 1 and 11.2 years, with a median of 4 (IQR 1.6) years.
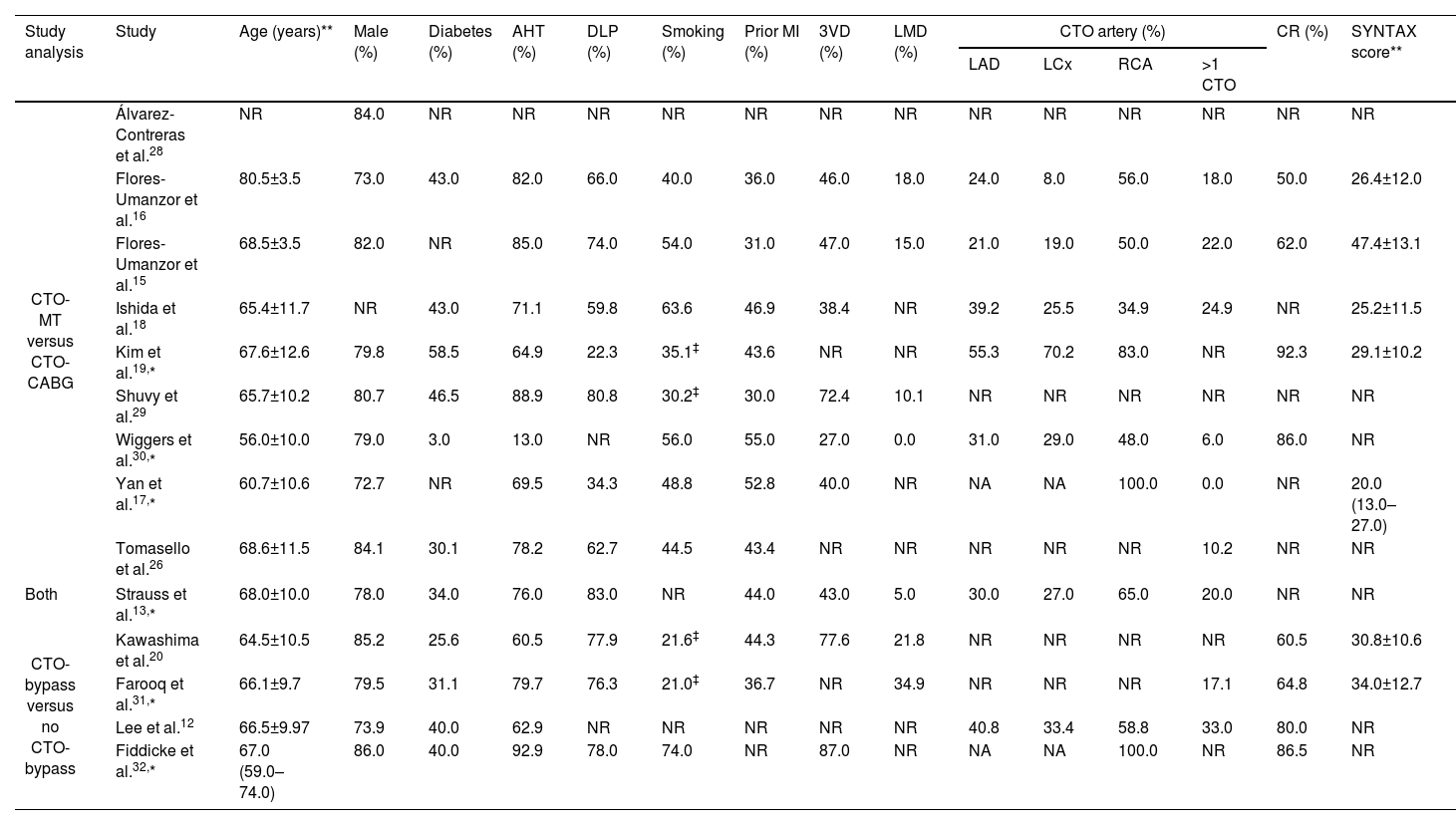
Demographic and baseline characteristics of included patients.
| Study analysis | Study | Age (years)** | Male (%) | Diabetes (%) | AHT (%) | DLP (%) | Smoking (%) | Prior MI (%) | 3VD (%) | LMD (%) | CTO artery (%) | CR (%) | SYNTAX score** | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAD | LCx | RCA | >1 CTO | |||||||||||||
| CTO-MT versus CTO-CABG | Álvarez-Contreras et al.28 | NR | 84.0 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Flores-Umanzor et al.16 | 80.5±3.5 | 73.0 | 43.0 | 82.0 | 66.0 | 40.0 | 36.0 | 46.0 | 18.0 | 24.0 | 8.0 | 56.0 | 18.0 | 50.0 | 26.4±12.0 | |
| Flores-Umanzor et al.15 | 68.5±3.5 | 82.0 | NR | 85.0 | 74.0 | 54.0 | 31.0 | 47.0 | 15.0 | 21.0 | 19.0 | 50.0 | 22.0 | 62.0 | 47.4±13.1 | |
| Ishida et al.18 | 65.4±11.7 | NR | 43.0 | 71.1 | 59.8 | 63.6 | 46.9 | 38.4 | NR | 39.2 | 25.5 | 34.9 | 24.9 | NR | 25.2±11.5 | |
| Kim et al.19,* | 67.6±12.6 | 79.8 | 58.5 | 64.9 | 22.3 | 35.1‡ | 43.6 | NR | NR | 55.3 | 70.2 | 83.0 | NR | 92.3 | 29.1±10.2 | |
| Shuvy et al.29 | 65.7±10.2 | 80.7 | 46.5 | 88.9 | 80.8 | 30.2‡ | 30.0 | 72.4 | 10.1 | NR | NR | NR | NR | NR | NR | |
| Wiggers et al.30,* | 56.0±10.0 | 79.0 | 3.0 | 13.0 | NR | 56.0 | 55.0 | 27.0 | 0.0 | 31.0 | 29.0 | 48.0 | 6.0 | 86.0 | NR | |
| Yan et al.17,* | 60.7±10.6 | 72.7 | NR | 69.5 | 34.3 | 48.8 | 52.8 | 40.0 | NR | NA | NA | 100.0 | 0.0 | NR | 20.0 (13.0–27.0) | |
| Tomasello et al.26 | 68.6±11.5 | 84.1 | 30.1 | 78.2 | 62.7 | 44.5 | 43.4 | NR | NR | NR | NR | NR | 10.2 | NR | NR | |
| Both | Strauss et al.13,* | 68.0±10.0 | 78.0 | 34.0 | 76.0 | 83.0 | NR | 44.0 | 43.0 | 5.0 | 30.0 | 27.0 | 65.0 | 20.0 | NR | NR |
| CTO-bypass versus no CTO-bypass | Kawashima et al.20 | 64.5±10.5 | 85.2 | 25.6 | 60.5 | 77.9 | 21.6‡ | 44.3 | 77.6 | 21.8 | NR | NR | NR | NR | 60.5 | 30.8±10.6 |
| Farooq et al.31,* | 66.1±9.7 | 79.5 | 31.1 | 79.7 | 76.3 | 21.0‡ | 36.7 | NR | 34.9 | NR | NR | NR | 17.1 | 64.8 | 34.0±12.7 | |
| Lee et al.12 | 66.5±9.97 | 73.9 | 40.0 | 62.9 | NR | NR | NR | NR | NR | 40.8 | 33.4 | 58.8 | 33.0 | 80.0 | NR | |
| Fiddicke et al.32,* | 67.0 (59.0–74.0) | 86.0 | 40.0 | 92.9 | 78.0 | 74.0 | NR | 87.0 | NR | NA | NA | 100.0 | NR | 86.5 | NR | |
AHT: arterial hypertension; CR: complete revascularization; CTO: chronic coronary total occlusion; DLP: dyslipidemia; LAD: left anterior descending; LCX: left circumflex; LMD: left main disease; MI: myocardial infarction; NA: not applicable; NR: not reported; RC: right coronary; 3VD: three-vessel disease.
Some studies narrow their scope to specific CTO populations. For instance, Flores-Umanzor et al. exclusively examined diabetic patients in one study,15 and older patients (≥75 years) in another.16 Yan et al. focused on diabetic patients with a CTO of the right coronary artery (RCA).17 Ishida's study included only patients with reduced ejection fraction (FEVE <40%), and Kim et al. specifically targeted patients with two or more CTOs.18,19
Study outcomes – medical therapy versus CABGFirst, we assessed the clinical outcomes of patients in which CTO was treated with CABG versus MT alone, focusing on all-cause mortality as primary endpoint, and cardiovascular mortality, acute MI, stroke, and MACE as secondary endpoints.
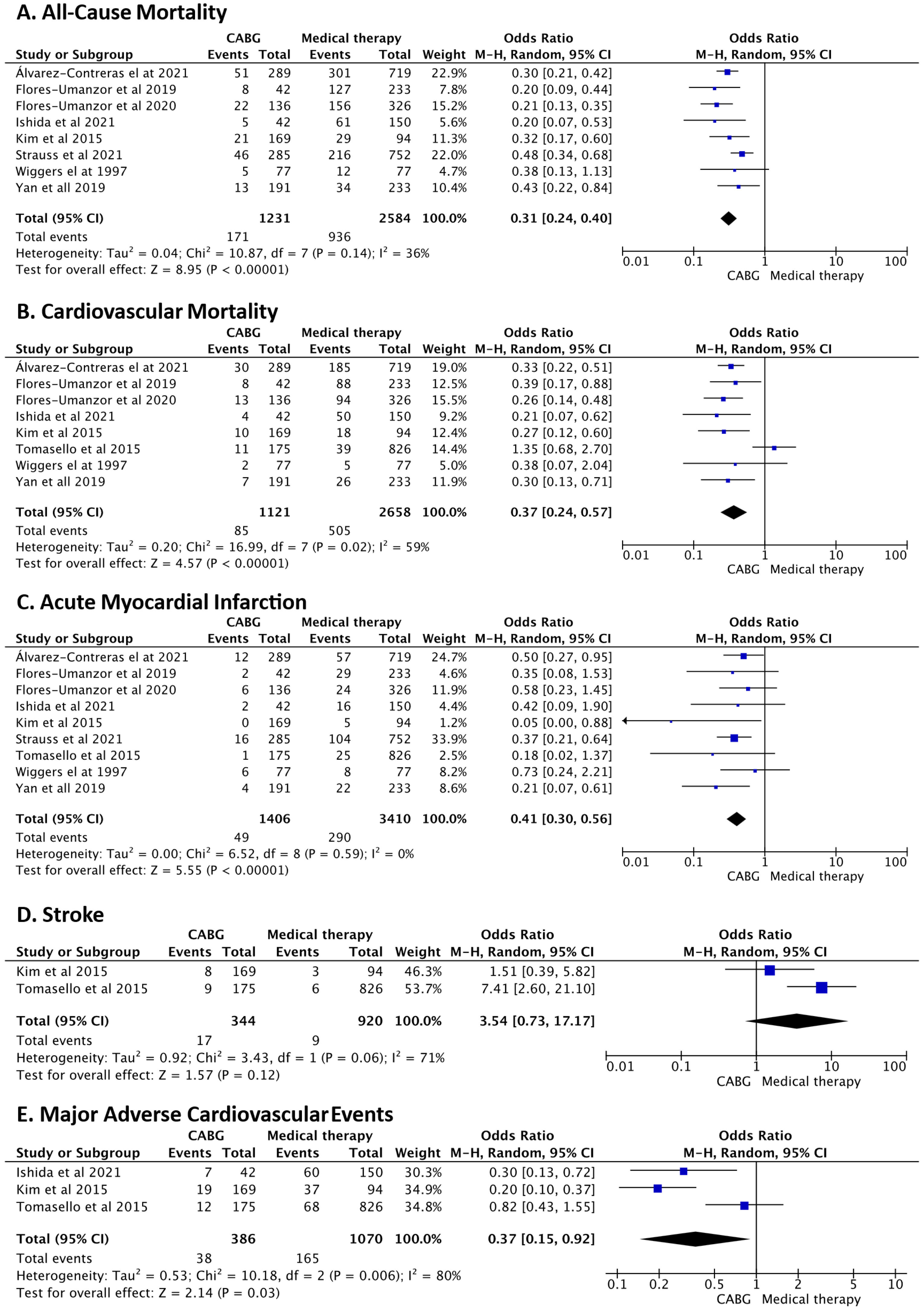
All-cause mortality rates between the above-mentioned groups were evaluated in eight studies. Of the 3815 patients included in the analysis, 1107 died during follow-up. Our study showed significantly lower all-cause mortality in the CABG group compared to the MT group, with a moderate heterogeneity in the magnitude of effect between studies (OR 0.31, 95% CI 0.24–0.40, p<0.001, I2=36%) (Figure 3).
Forest plots of (A) all-cause mortality, (B) cardiovascular mortality, (C) acute myocardial infarction, (D) stroke, and (E) major adverse cardiovascular events (MACE) comparing coronary artery bypass grafting (CABG) versus medical therapy. CI: confidence interval; M–H: Mantel–Haenszel.
This divergence persisted in cardiovascular mortality, where CABG patients showed fewer cardiac deaths compared to those under medical management, although with high heterogeneity between studies (OR 0.37, 95% CI: 0.24–0.57, p<0.001, I2=59%) (Figure 3). When examining acute MI, CABG showed a lower event rate when compared with MT, with no heterogeneity identified between the included studies (OR 0.41, 95% CI: 0.30–0.56, p<0.001, I2=0%) (Figure 3). The risk of stroke was assessed in two studies, with no statistically significant difference between the MT and the CABG groups (OR 3.54, 95% CI 0.73–17.17, p=0.12, I2=71%) (Figure 3). Regarding MACE, only three studies were included in the meta-analysis. Despite the high heterogeneity identified between studies, the analysis revealed lower event rates in the surgical revascularization group compared with the MT group (OR 0.37, 95% CI 0.15–0.92, p=0.03, I2=80%) (Figure 3). To ensure the robustness of our findings, a sensitivity analysis was conducted using a fixed effects model (Supplementary Material, Table S10).
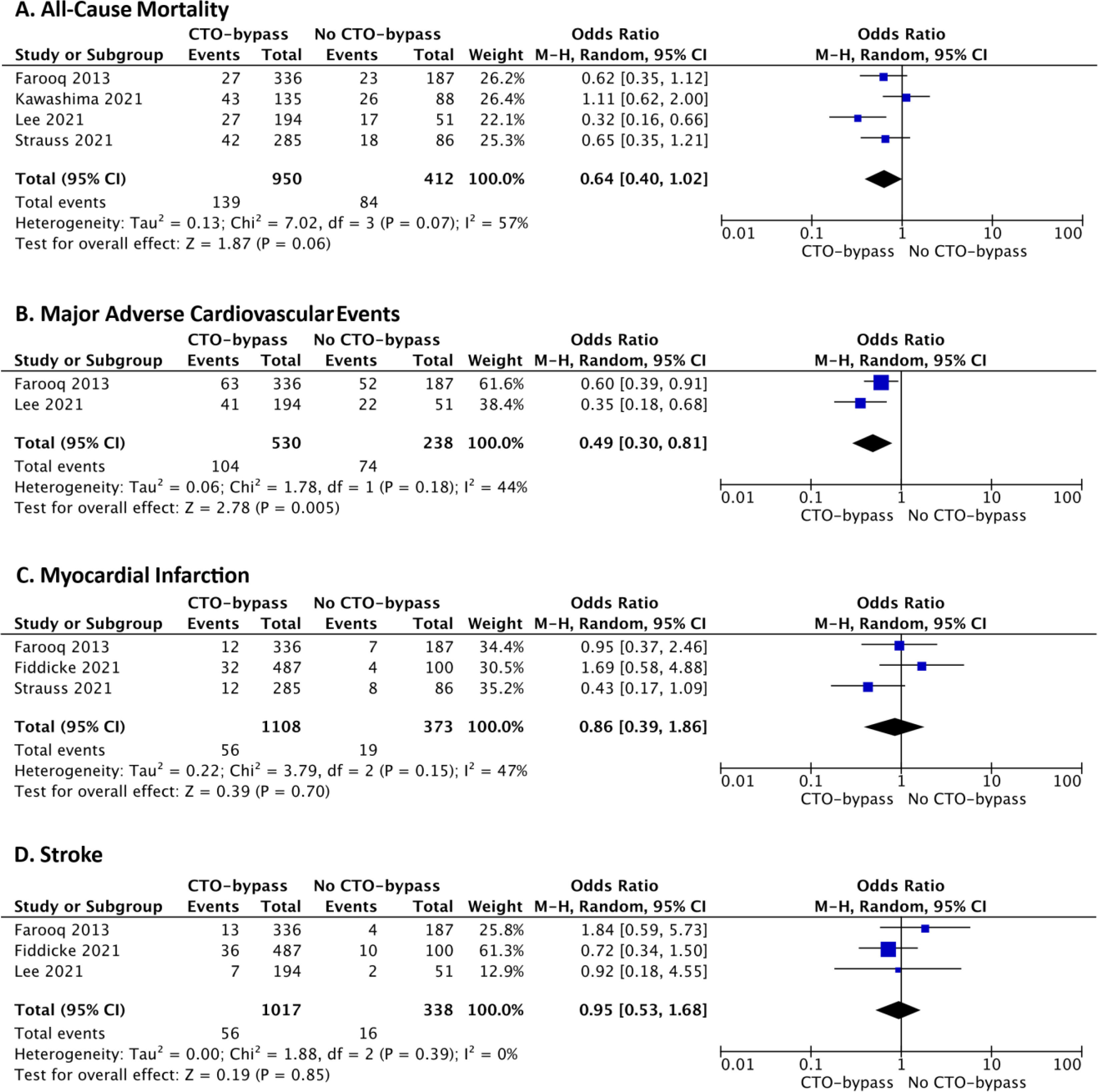
Study outcomes – CTO revascularization in CABG patientsAdditionally, we focused on studies involving patients with multivessel disease undergoing CABG, and compared clinical outcomes between those who received a bypass to CTO and those with a non-bypassed CTO. This assessment allowed us to evaluate the impact of complete revascularization. All-cause mortality, MACE, acute MI, and stroke were evaluated. Cardiovascular mortality was not assessed due to insufficient data in the individual studies.
In terms of all-cause mortality, our study demonstrated no statistically significant difference between the bypassed and non-bypassed CTO groups, although a trend was observed favoring CTO bypass (OR 0.64, 95% CI 0.40–1.02, p=0.06, I2=57%) (Figure 4). Conversely, there was a statistically significant difference in MACE between the two groups, demonstrating a lower event rate in the CTO-bypass group, with a moderate heterogeneity identified between the two included studies (OR 0.49, 95% CI 0.30–0.81, p=0.005, I2=44%) (Figure 4). Regarding acute MI and stroke, no differences were found between the bypass-CTO and no bypass-CTO groups (acute MI: OR 0.86, 95% CI 0.39–1.86, p=0.70, I2=47%, Figure 4; stroke OR 0.95, 95% CI 0.53–1.68, p=0.85, I2=0%, Figure 4).
Forest plots of (A) all-cause mortality, (B) major adverse cardiovascular events (MACE), (C) acute myocardial infarction, and (D) stroke comparing CTO-bypass versus no CTO-bypass in patients undergoing coronary artery bypass grafting (CABG). CI: confidence interval; M–H: Mantel–Haenszel.
Our systematic review and meta-analysis suggest that surgical revascularization of a CTO lesion is associated with lower all-cause mortality, cardiovascular mortality, acute MI, and MACE rates when compared to medical management of these lesions. The risk of stroke did not differ significantly between the two treatment groups. Additionally, patients who underwent CABG and received a bypass for the CTO exhibited notably reduced rates of MACE compared to those whose CTO was not revascularized, which might suggest a potential advantage in bypassing the CTO for patients undergoing CABG. There was a tendency towards a reduction in all-cause mortality although it did not reach statistical significance. This represents the first meta-analysis specifically assessing the prognosis of CTO revascularization in patients undergoing CABG.
The improved outcomes with CABG have been suggested previously in several studies and may be attributed to the completeness of revascularization.2 As previously described, complete CTO revascularization is more likely accomplished with CABG than with PCI.2 In the SYNTAX trials, the presence of a CTO made incomplete revascularization more than twice as likely with PCI, leading to higher event rates in the PCI group compared with CABG.2 Also, in the Kawashima et al. study, CTO revascularization success was more frequent in CABG (60.5%) than PCI (43.5%).20 In fact, Lee et al. concluded that incomplete revascularization of CTOs was an independent risk factor for 30-day and overall mortality.12 Successful revascularization is associated with better clinical outcomes, which may be due to the preservation or recovery of left ventricular systolic function (LVSF), decreased incidence of ventricular arrhythmias, and improved exercise capacity.6,21,22 These are aligned with our findings, suggesting that bypassing a CTO in patients undergoing CABG due to multivessel disease may be associated with improved clinical outcomes compared to those in which the CTO is not revascularized. This might carry significant practical implications as a substantial proportion of individuals who undergo CABG do not receive a bypass for the CTO lesion. For instance, findings from the Canadian Multicenter Chronic Total Occlusion Registry revealed that roughly three-quarters of CABG patients did not receive CTO grafting.13
The improvement in systolic function with surgical revascularization may be related to myocardial viability (MV).23 Despite data on the benefit of MV in patients with ischemic cardiomyopathy undergoing CABG being controversial, with recent studies reporting no association between MV and long-term benefit, it is consistently described that MV is associated with an improvement in LVSF, subsequently improving symptoms and quality of life.23 In fact, current guidelines on myocardial revascularization recommend assessing viability in the presence of regional wall motion abnormalities in the territory of the CTO, additionally to the evaluation of symptoms and ischemia burden, to decide whether to perform revascularization.11
To the best of our knowledge, no randomized controlled trials were performed to support the clinical benefit of surgical revascularization over MT of a CTO lesion. Although not directly related to the CTO lesions, the surgical treatment for ischemic heart failure (STICH) trial and its extension study (STICHES) demonstrated that after a median follow-up of 10 years, patients with ischemic cardiomyopathy who underwent CABG had better outcomes than those who received MT alone, which may be aligned with our findings.23–25
In a prior meta-analysis that compared MT to CTO revascularization (PCI or CABG), a subgroup analysis revealed that CABG was associated with reduced mortality and fewer MACE but not a reduced risk of MI or cardiovascular mortality.5 This contrasts with our study, in which the reduced events in the CABG group compared to the MT group remained consistent across all the above-mentioned outcomes, irrespective of the specific population enrolled. The heterogeneity in cardiac mortality and MACE identified in our study could be a consequence of the considerably shorter follow-up period of the Tomasello et al. study compared to the remaining investigations, which spanned only one year.26
As it was presented previously in Table 2, several included studies integrated CTO patients with specific criteria. This aspect may be a limitation of our meta-analysis, as it may introduce heterogeneity. However, when we examine the outcomes of these studies individually, we find consistent findings that agree with our overall analysis, reinforcing the clinically significant benefits of CABG over MT of a CTO lesion. For instance, Yan et al. demonstrated that even in a subpopulation of diabetic patients with RCA-CTO, CTO-CABG was superior to CTO-MT in all-cause and cardiovascular mortality.17 Also, in a study conducted by Flores-Umanzor et al. involving 538 CTO patients with diabetes, the analysis comparing optimal medical management with revascularization (either CABG or PCI) revealed that individuals who underwent revascularization experienced superior outcomes in terms of reduced mortality and decreased heart failure hospitalizations, and that the survival benefit was particularly pronounced and statistically significant among patients who underwent surgical revascularization.15,27
Despite the positivity of our findings suggesting a clinical benefit of bypassing a CTO lesion, additional validation is warranted through future randomized controlled trials. In addition, our study only evaluated hard endpoints, but it is worth noting that intermediate endpoints like hospitalizations for heart failure or ischemic events, ventricular arrhythmias, angina frequency, exercise capacity, and improvement in quality of life may also hold significance for the Heart Team's decision-making process.
Study limitationsThis meta-analysis has several limitations. First, the included studies have different study populations (including baseline and demographic clinical characteristics), entry criteria, clinical outcomes, and follow-up times. Despite a multivariate analysis with adjustments for baseline differences being performed in most studies, it may not fully compensate for the differences that influence the outcomes. A key limitation of this meta-analysis is the heterogeneity of the included studies and the broad categorization of CTOs. Factors such as vessel size, myocardial mass at risk, myocardial viability, and collateral circulation were not consistently reported, despite their significant impact on outcomes. For example, an occluded but well-collateralized proximal LAD differs substantially from a non-dominant posterior descending artery without viable myocardium.
Another important limitation of our study is the lack of consistent data on the completeness of revascularization (CR) in studies comparing MT versus CABG. Although some studies included information on CR during CABG, many lacked such data, and those that reported it demonstrated substantial variability in CR rates, potentially influencing the outcomes.
Additionally, the lack of detailed information on why occluded vessels were not bypassed in the non-bypassed CTO group introduces bias. Decisions not to bypass are often due to diffuse disease, small vessel size, or poor myocardial viability – markers of higher cardiovascular risk that likely influenced outcomes independently of revascularization strategy. These findings should therefore be interpreted cautiously, as they may reflect the inherent risk profiles of these patients.
On the other hand, our meta-analysis only included observational studies, which, as noted in the literature, are subject to inherent limitations that statistical adjustments cannot fully correct. This is consistent with findings from Simsek et al., which demonstrated better outcomes in 19 observational studies comparing CTO-PCI versus no PCI, but not in the 6 RCTs included.8 This is an example of how observational designs may overestimate the benefits of revascularization. Despite our findings providing valuable insights into real-world outcomes, they should be interpreted cautiously, as only prospective RCTs can definitively establish causality. Thus, due to the non-randomized nature of our study, selection bias cannot be completely ruled out, which limits the power and generalizability of our analysis.
Furthermore, the selection of the treatment group in the included studies was likely influenced by patients’ characteristics and Heart Team preferences, which means that the MT group might have a poorer baseline prognosis.
Lastly, given that there was no difference in all-cause mortality between CABG with and without CTO revascularization, the secondary outcomes are exploratory and should be interpreted with caution.
ConclusionsOur study suggests a potential clinical benefit of bypassing a CTO lesion in patients with multivessel disease undergoing CABG, with a significantly lower MACE. The improved clinical outcomes of CABG over MT of CTO lesions identified in this research for all-cause and cardiovascular mortality, MACE, and acute MI further underscore the potential advantages of revascularizing a CTO during CABG rather than leaving it untreated in patients undergoing this procedure, warranting careful consideration by the Heart Team during their decision-making.
Conflict of interestThe authors have no conflicts of interest to declare.