Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in Portugal. Hypercholesterolemia has a causal role in atherosclerotic CVD. Guidelines recommend that cardiovascular (CV) risk reduction should be individualized and treatment goals identified. Low-density lipoprotein cholesterol (LDL-C) is the primary treatment target.
MethodsDISGEN-LIPID was a cross-sectional observational study conducted in 24 centers in Portugal in dyslipidemic patients aged ≥40 years, on lipid-lowering therapy (LLT) for at least three months and with an available lipid profile in the previous six months.
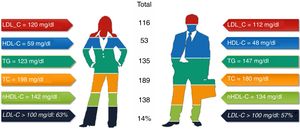
ResultsA total of 368 patients were analyzed: 48.9% men and 51.1% women (93.9% postmenopausal), of whom 73% had a SCORE of high or very high CV risk. One quarter had a family history of premature CVD; 31% had diabetes; 26% coronary heart disease; 9.5% cerebrovascular disease; and 4.1% peripheral arterial disease. Mean baseline lipid values were total cholesterol (TC) 189 mg/dl, LDL-C 116 mg/dl, high-density lipoprotein cholesterol (HDL-C) 53.5 mg/dl, and triglycerides (TG) 135 mg/dl. Women had higher TC (p<0.001), LDL-C (non-significant) and HDL-C (p<0.001), and lower TG (p=0.002); 57% of men and 63% of women had LDL-C>100 mg/dl (p=0.28), and 58% of men and 47% of women had LDL-C>70 mg/dl (p=0.933).
ConclusionThese observational data show that, despite their high-risk profile, more than half of patients under LLT, both men and women, did not achieve the recommended target levels for LDL-C, and a large proportion also had abnormal HDL-C and/or TG. This is a renewed opportunity to improve clinical practice in CV prevention.
A doença cardiovascular (DCV) é a principal causa de morbimortalidade em Portugal. Hipercolesterolemia é um reconhecido fator causal na DCV aterosclerótica. As recomendações aconselham a individualização da redução do risco cardiovascular (CV) e da identificação dos objetivos terapêuticos. O LDL-C é o principal alvo do tratamento.
MétodosO DISGEN-LIPID foi um estudo transversal, observacional, com 24 centros em Portugal, que incluiu doentes ≥40 anos e dislipidemia, com tratamento antidislipidémico havia pelo menos três meses e perfil lipídico nos últimos seis meses.
ResultadosForam analisados 368 pacientes: 48,9% homens e 51,1% mulheres (93,9% na pós-menopausa); 73% dos doentes tinham um risco CV alto ou muito alto. Um quarto tinha história familiar de DCV prematura; 31% DMT2, 26% DC; 9,5% doença vascular cerebral; e 4,1% DAP. O perfil lipídico basal médio era CT de 189 mg/dl, LDL-C de 116 mg/dl, HDL-C de 53,5 mg/dl e TG de 135 mg/dl. As mulheres apresentavam um CT (p<0,001), LDL-C (não significativo), HDL-C (p<0,001) mais elevado do que os homens e níveis mais baixos de TG (p=0,002); 57% dos homens e 63% das mulheres tinham um LDL-C>100 mg/dl (p=0,28) e 58% dos homens e 47% das mulheres apresentavam um valor de LDL-C>70 mg/dl (p=0,933).
ConclusãoOs dados mostram que mais de metade dos doentes, homens e mulheres, não alcançou o alvo de LDL-C e um grande número tinha valores indesejáveis de HDL-C e/ou TG. Esta é uma oportunidade para melhorar a prática clínica em prevenção CV.
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in Portugal.1,2 In 2014, despite a downward trend in recent years, most deaths were due to circulatory system diseases (30.7%), with a marked increase of 2.4% compared to 2013. There were 11 751 deaths from stroke in 2013 (mortality 54.6 per 100 000 population) and coronary heart disease (CHD) was responsible for 6526 deaths (standardized mortality per 100 000 population of 32.9). By comparison, there were 4292 deaths from myocardial infarction (MI) (standardized mortality rate of 22.2).2
Hypercholesterolemia is a major contributor to atherosclerosis and CVD.3–6 Increased levels of cholesterol-rich apolipoprotein B (apoB)-containing lipoproteins – especially low-density lipoprotein cholesterol (LDL-C) – are causatively related to atherosclerotic CVD (ASCVD). The role of triglyceride (TG)-rich lipoproteins is under ongoing analysis. Mendelian randomization studies have identified remnant lipoproteins as proatherogenic.7 In contrast, although it is included in the Systematic Coronary Risk Evaluation (SCORE) risk estimation tool, the protective role of high-density lipoprotein (HDL-C) is currently the subject of intense debate.8 Epidemiological studies suggest that low HDL-C, in both genders and in all age groups (but especially in the elderly), can be taken as an additional marker of increased cardiovascular (CV) risk.3,4 However, Mendelian randomization studies have consistently failed to demonstrate a protective role of HDL-C in ASCVD.9
The prevalence of dyslipidemia in Portugal has been thoroughly studied in recent decades.10–13 In 2010, the prevalence of hypercholesterolemia was estimated at 55.5% of the population aged >18 years (56.7% male and 54.5% female),14 with growing social (in 2010, 1689 deaths were attributed to hypercholesterolemia, 1.6% of all deaths15) and economic costs: the estimated direct cost attributable to hypercholesterolemia was 320 million euros at 2013 prices, and indirect costs (generated by disability) amounted to 198 million euros. The overall cost of disease was estimated at 518 million euros (around 0.3% of Portugal's gross domestic product).15
According to the European guidelines,3,4 adequate screening, diagnosis, monitoring and treatment are all essential to the management of dyslipidemias and CVD prevention. Multiple randomized clinical trials (RCTs) provide unequivocal evidence that reducing TC or LDL-C, and attaining recommended target levels, at least in high and moderate CV risk patients, is associated with reductions in CV events and mortality. Every 38.6 mg/dl reduction in LDL-C is linked with a 20-25% reduction in CVD mortality and non-fatal MI, a 23% reduction in major coronary events, a 17% reduction in stroke and a 10% proportional reduction in all-cause mortality,16,17 in all subgroups and in both genders.17,18
Residual (persistent) dyslipidemia in patients treated with statins (with or without other lipid-lowering therapy [LLT]) – with prognostic implications for subsequent CV outcomes19 – has also been a cause for concern in several Portuguese cross-sectional studies.19,20 In 16 856 individuals (mean age 58.1±15.1 years; 61.6% women) in the VALSIM study, 54.1% of the population aged ≥40 years met criteria for LLT and 44.7% were medicated with statins, but only 16.0% had TC≤175 mg/dl.19 In the Portuguese results of the DYSlipidemia International Study (DYSIS), analyzing 916 patients (mean age 64.1±9.9 years; 47.1% women, 66.7% with high CV risk), 62.9% and 68% of subjects had not attained LDL-C and total cholesterol (TC) target levels, respectively.21 Moreover, 22% of the patients presented low HDL-C and 39% had high TG levels.
We aimed to present the prevalence of lipid abnormalities and the achievement of lipid targets in men and women with dyslipidemia treated with statins and other LLT in the DISparidade de GÉNero na abordagem dos LÍPIDos (DISGEN-LIPID) study, assessing individual risk and clinical CV conditions.
MethodsStudy design, procedures and populationThe DISGEN-LIPID study was a cross-sectional observational study conducted in Portugal between November 2014 and November 2015 that aimed to compare lipid management between men and women with dyslipidemia relative to baseline TC (primary objective). The secondary objectives were to assess lipid management in both sexes relative to baseline LDL-C and to describe current clinical practice and therapeutic decisions – based on clinical history – in patients who do not reach the therapeutic targets for TC and LDL-C. Finally, we compared lipid profile at baseline in men and women with dyslipidemia treated with statins, with a focus on individual risk according to SCORE and the targets recommended by the guidelines3 (Figure 1).
Patients with dyslipidemia eligible for the study were those ≥40 years old, considered clinically stable (no hospitalization in the previous three months), on LLT therapy for at least three months, and with an available lipid profile in the previous six months (including TC), and who were available to be re-observed 3-6 months after inclusion. To avoid selection bias, physicians were encouraged to enroll all consecutive patients who fulfilled the inclusion criteria. Patients enrolled in RCTs were not eligible. Other exclusion criteria were life-threatening chronic disease or severe renal or hepatic dysfunction, and unavailability of the patient's medical history.
DISGEN-LIPID was strictly observational and all procedures were performed according to usual clinical practice. The study was conducted in accordance with good epidemiological and clinical practice, and was approved by the Portuguese authorities. All subjects provided written informed consent.
Data were collected on sociodemographic characteristics (gender and educational level), physical examination (height, weight, waist circumference, blood pressure [BP], and heart rate), CV risk factors, comorbidities and family history (hypertension, diabetes, CHD, cerebrovascular disease, heart failure, peripheral arterial disease [PAD], chronic kidney disease [CKD], family history of premature CVD in a first-degree relative), lifestyle (smoking, exercise level, alcohol consumption), medication (LLT and other concomitant drugs, medical indication and dosage for statins, other LLT, ongoing non-specific antihypertensive, antidiabetic, and antiplatelet and anticoagulant drugs), and most recent laboratory results including TC and LDL-C, HDL-C and non-HDL cholesterol (nHDL-C), TG, apoB, fasting blood glucose and HbA1C, serum creatinine and hemoglobin.
ObjectivesThe primary objective was to analyze documented real-life lipid concentrations, describing baseline patient characteristics and individual risk profiles according to the lipid targets established in the 2016 guidelines for CVD prevention3 and for the management of dyslipidemias,4 and the secondary objective was to document LLT usage patterns.
Statistical analysisThe sample size was defined considering that DISGEN-LIPID was an exploratory study. It was assumed that in the DYSIS registries21,22 65-70% of those included did not achieve the TC therapeutic targets. It was calculated that 1000 patients should be included, half of them women.
Continuous variables were expressed as mean ± standard deviation and median. Categorical variables were expressed as absolute values and relative frequencies. Mean TC and LDL-C levels were compared with the t test for independent samples, and therapies were compared by gender and lipid profile with the chi-square test. A significance level of 0.05 was assumed. All analyses were performed using SAS/STAT® software. Data management procedures were designed to ensure the integrity of all data, which were anonymized to protect confidentiality.
ResultsDISGEN-LIPID was conducted in 24 centers. Enrollment was ended early due to slow recruitment. Of the 392 patients enrolled, 24 did not meet the inclusion criteria. Of the 368 patients analyzed (Table 1), 180 (48.9%) were men and 188 (51.1%) women, of whom most were postmenopausal (n=169; 93.9%).
Baseline characteristics of the study population.
| Parameter | Total (n=392) | Men (n=180; 48.9%) | Women (n=188; 51.1%) | p |
|---|---|---|---|---|
| Educational level | ||||
| Illiterate | 13 (3.3%) | |||
| Primary school | 165 (42.2%) | |||
| High school | 148 (37.9) | |||
| College | 65 (16.6%) | |||
| Anthropometric data | ||||
| Height, cm (SD) | 163.2±8.3 | 169.2±6.2 | 157.6±5.7 | <0.001 |
| Weight, kg (SD) | 74.5±12.7 | 80.4±11.1 | 69.0±11.6 | <0.001 |
| BMI, kg/m2 (SD) | 27.9±3.8 | 28.1±3.4 | 27.7±4.1 | 0.018 |
| Waist circumference, cm (SD) | 97.2±12.0 | 101.6±11.1 | 93.1±11.4 | <0.001 |
| Smoking | ||||
| Never-smoker | 238 (64.9%) | 75 (41.9%) | 163 (86.7%) | <0.001 |
| Former smokera | 112 (30.5%) | 92 (51.4%) | 20 (10.6%) | |
| Current smoker | 17 (4.6%) | 12 (6.7%) | 5 (2.7%) | |
| Alcohol consumptionb | ||||
| 0-5 | 167 (58.8%) | 55 (36.4%) | 112 (84.2%) | <0.001c |
| 5-10 | 65 (22.9%) | 48 (31.8%) | 17 (12.8%) | |
| 10-15 | 32 (11.3%) | 28 (18.5%) | 4 (3.0%) | |
| 15-30 | 11 (3.9%) | 11 (7.3%) | 0 (0%) | |
| 30-45 | 7 (2.5%) | 7 (4.6%) | 0 (0%) | |
| >45 | 2 (0.7%) | 2 (1.3%) | 0 (0%) | |
| Hemodynamic variables | ||||
| Systolic BP, mmHg (SD) | 133.8±16.2 | 133.6±16.0 | 134.0±16.4 | 0.627 |
| Diastolic BP, mmHg (SD) | 75.7±9.7 | 76.2±10.8 | 75.3±8.5 | 0.001 |
| Heart rate, bpm (SD) | 68.6±10.1 | 67.3±10.8 | 70.0±9.1 | 0.066 |
| Laboratory variables | ||||
| Hgb, g/100 ml (SD) | 13.9±1.5 | 14.6±1.5 | 13.3±1.1 | 0.003 |
| Creatinine, mg/dl (SD) | 1.0±0.5 | 1.1±0.6 | 0.9±0.2 | 0.033 |
| FPG, mg/dl (SD) | 107.9±31.3 | 111.6±30.7 | 104.3±31.7 | 0.405 |
| HbA1c, % (SD) | 6.7±1.5 | 6.6±1.1 | 6.8±1.9 | 0.090 |
| Family history of premature CVD (%) | 90 (24.9%) | 37 (20.9%) | 53 (28.6) | 0.088 |
| Clinical history | ||||
| Hypertension | 297 (81.4%) | 147 (82.6%) | 150 (80.2%) | 0.56 |
| Diabetes | 114 (31.0%) | 66 (37.8%) | 46 (24.5%) | 0.006 |
| Coronary heart disease | 96 (26.1%) | 66 (36.7%) | 30 (16.0%) | <0.001 |
| Stroke/TIA | 35 (9.5%) | 19 (10.6%) | 16 (8.5%) | 0.50 |
| Atrial fibrillation | 41 (11.2%) | 26 (14.5%) | 15 (8.0%) | 0.047 |
| Heart failure | 29 (8.0%) | 19 (10.7%) | 10 (5.4%) | 0.062 |
| PADd | 16 (4.4%) | 12 (6.7%) | 4 (2.1%) | 0.034 |
| ACR≥30 mg/g | 14 (3.8%) | 8 (4.5%) | 6 (3.2%) | 0.52 |
| GFR<60 ml/min/1.73 m2 | 15 (4.1%) | 11 (6.1%) | 4 (2.1%) | 0.052 |
ACR: albumin/creatinine ratio; BMI: body mass index (kg/m2); BP: blood pressure; CVD: cardiovascular disease; FPG: fasting plasma glucose; GFR: glomerular filtration rate; HbA1c: glycated hemoglobin; Hgb: hemoglobin; PAD: peripheral arterial disease; SD: standard deviation; TIA: transient ischemic attack.
One quarter (n=90; 24.9%) of the patients had a family history of premature CVD in a first-degree relative, 17 (4.6%) were smokers, 140 (38.0%) presented sedentary behavior, and 181 (49.2%) regularly consumed alcohol (9.1±8.0 units per week; median 7.0). Mean body mass index (BMI) was 27.9±3.8 kg/m2 (over 75% had BMI>25 kg/m2), 114 (31%) patients had diabetes (fasting blood glucose ≥126 mg/dl, HgA1c<6.5% or on treatment with oral antidiabetic drugs), 96 (26.1%) had CHD, 41 (11.2%) had atrial fibrillation (AF), 29 (8%) had heart failure in New York Heart Association functional class II-IV, 35 (9.5%) had had stroke/transient ischemic attack, and 16 (4.1%) had PAD. Current antihypertensive drug treatment or a previous diagnosis of hypertension (BP≥140/90 mmHg) was reported by 297 (81.4%) patients; mean systolic and diastolic BP were 138.8±16.2 mmHg and 75.7±9.7 mmHg, respectively, and mean heart rate was 68.6±10.1 bpm. CKD23 presented as albuminuria (albumin/creatinine ratio ≥30 mg/g) in 14 (3.8%) of the patients and as glomerular filtration rate (GFR) <60 ml/min/1.73 m2 in 15 (4.1%). On the basis of these results, 285 (72.7%) of the patients were at high or very high CV risk. Laboratory variables are presented in Table 1.
The 368 patients were on LLT for 53±50.9 months (median: 36 months). The most frequently prescribed statins were simvastatin (34.5%), atorvastatin (19.9%), rosuvastatin (17.8%) and pitavastatin (13.1%), and less often pravastatin (8.0%), fluvastatin (1.9%), and lovastatin (0.5%). Fibrate (11.1%) followed by ezetimibe (8.4%) were the most frequently used non-statin LLT (Table 2). Concomitant to LLT, 76.8% patients took antihypertensive medication, 30.1% oral antidiabetic drugs, 47.0% antiplatelets and 12.0% oral anticoagulants. Baseline lipid values are presented in Table 3.
Lipid-lowering therapy with statins and other drugs, with dosage patterns.
| LLT, mg/day | n (%) | Mean ± SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| Atorvastatin | 73 (19.9) | 21.4±11.4 | 20.0 | 5.0 | 40.0 |
| Fluvastatin | 7 (1.9) | 73.3±16.3 | 80.0 | 40.0 | 80.0 |
| Lovastatin | 2 (0.5) | 20 | 20.0 | 20.0 | 20.0 |
| Pitavastatin | 48 (13.1) | 2.2±0.9 | 2.0 | 1.0 | 4.0 |
| Pravastatin | 28 (8.0) | 32.1±14.0 | 40.0 | 10.0 | 80.0 |
| Rosuvastatin | 65 (17.8) | 11.2±5.5 | 10.0 | 5.0 | 20.0 |
| Simvastatin | 127 (34.5) | 21.5±8.6 | 20.0 | 10.0 | 80.0 |
| Ezetimibe | 31 (8.4) | 9.8±0.9 | 10.0 | 5.0 | 10.0 |
| Fibrate | 43 (11.1) | 204.1±111.1 | 160.0 | 145.0 | 600.0 |
SD: standard deviation.
Baseline lipid values in the overall population and in men and women on lipid-lowering therapy for at least three months.
| Variable, mg/dl (mean ± SD) | Total (n=368) | Men (n=180) | Women (n=188) | p |
|---|---|---|---|---|
| TC | 189.2±42.4 (n=367) | 180.2±41.6 (n=180) | 197.9±41.5 (n=187) | <0.001 |
| LDL-C | 116.0±37.9 (n=248) | 111.7±37.8 (n=120) | 120.0±37.8 (n=128) | 0.087 |
| HDL-C | 53.5±14.5 (n=346) | 47.7±12.7 (n=169) | 59.0±14.0 (n=177) | <0.001 |
| TG | 134.7±72.4 (n=351) | 146.8±80.5 (n=170) | 123.4±61.9 (n=181) | 0.002 |
| ApoB | 104.5±30.9 (n=42) | 101.6±32.2 (n=17) | 106.6±30.5 (n=25) | 0.61 |
| nHDL-C | 138.0±47.1 (n=86) | 134.5±43.8 (n=46) | 142.0±51.0 (n=40) | 0.46 |
ApoB: apolipoprotein A; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; nHDL-C: non-high-density lipoprotein cholesterol; SD: standard deviation; TC: total cholesterol; TG: triglycerides.
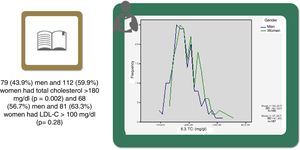
Statistically significant differences were found in distribution by gender in the baseline prevalence of diabetes (p=0.006), CHD (p<0.001), PAD (p=0.034), AF, and, although borderline (p=0.05), GFR, with higher rates in men than in women (Table 1). Among baseline lipid values (Table 3), TC (p<0.001), LDL-C (p=NS), HDL-C (p<0.001), HDL-C (p=NS), and apoB (p=NS) were higher and TG (p=0.002) were lower in women (Figure 2). Appendix B presents baseline lipid profiles in patients with various medical conditions by gender. Figure 3 illustrates the distribution of frequencies of TC. There were a total of 116 men and 79 women with vascular disease (diabetes, CHD or stroke). Of these, 67 (57.8%) men and 37 (46.8%) women had LDL-C >70 mg/dl (p=0.933) and 72 (62.1%) men and 60 (75.9%) women had TC >160 mg/dl (p=0.04). For most types of LLT, there were no differences in the percentage of men and women (Table 4), but there were significantly more women than men taking pitavastatin (p=0.013).
Lipid-lowering therapy with statins and ezetimibe by gender.
| Men | Women | p (men vs. women) | Total | |
|---|---|---|---|---|
| Atorvastatin | 40 (22.3%) | 33 (17.6%) | 0.295 | 73 (19.9%) |
| Fluvastatin | 5 (2.8%) | 2 (1.1%) | 0.274 | 7 (1.9%) |
| Lovastatin | 1 (0.6%) | 1 (0.5%) | 1.00 | 2 (0.5%) |
| Pitavastatin | 15 (8.4%) | 33 (17.6%) | 0.013 | 48 (13.1%) |
| Pravastatin | 10 (5.9%) | 18 (10.1%) | 0.171 | 28 (8.0%) |
| Rosuvastatin | 37 (20.7%) | 28 (15.0%) | 0.172 | 65 (17.8%) |
| Simvastatin | 61 (33.9%) | 66 (35.1%) | 0.827 | 127 (34.5%) |
| Ezetimibe | 19 (10.6%) | 12 (6.4%) | 0.105 | 31 (8.4%) |
DISGEN-LIPID provides contemporary insights into lipid profiles and residual dyslipidemia, and also a picture of patterns of LLT use in outpatients after at least three months on LLT. Despite their high-risk profile – 73% of the patients were at high or very high CV risk – more than half had LDL-C>100 mg/dl. Moreover, a large proportion also had abnormal nHDL-C, HDL-C and/or TG. This is therefore a renewed opportunity to try to understand the reasons for the persistently suboptimal rate of adherence to the European guidelines and to the standards of good clinical practice laid down by the Portuguese Directorate-General of Health (DGS),24,25 as well as a chance to improve clinical practice in secondary and primary prevention of ASCVD.
The evidence from RCTs with LLT, particularly statins, established that reductions not only in CV events, but also in CV and total mortality, are proportional to the extent of LDL-C lowering.6,16 Hence, providing that intensive LDL-C lowering is safe,17 it is imperative to ensure effective control of LDL-C and consequent reduction of residual risk.
Nevertheless, it is vital to call attention to the importance of moving from population-based risk models to established methodologies that include individual treatment scores (and individual numbers needed to treat) and to discuss the benefits and harms of starting a statin with the individual patient.26 On-treatment monitoring and targets are an important aspect of clinical practice that can facilitate communication between doctors and patients and helps improve patient compliance. American and European medical associations both stress the merits of this strategy in order to ensure that the intensity of therapy to lower TC and LDL-C is appropriate to the absolute risk for an ASCVD event.4,6,27 The favorable changes in lipid parameters after three months’ follow-up in our study – although incomplete and limited – exemplify the value of this approach.
Among the barriers to translating guideline-recommended targets into real-world clinical practice are suboptimal dose selection, failure to titrate therapy, patient non-adherence and limited efficacy. Statins are the cornerstone of LLT. Several different types of statins with different pharmacological structures are now available, as are other types of LLT.28 The clinical benefit is independent of the type of statin but the degree of LDL-C reduction is dose-dependent – although there is often wide interindividual variability in statin response – and varies between different statins.4,29 The range of LLT and the pattern of dosages in DISGEN-LIPID (Table 2) were diverse, with most patients receiving simvastatin (mean dose 21.5±8.6 mg/day), atorvastatin (mean dose 21.4±11.4 mg/day) and rosuvastatin (mean dose 11.2±5.5 mg/day). It is time to recognize the importance of up-titration of statin dose or, in some cases, of initiating combination therapy with ezetimibe.
In clinical practice, only a minority of patients who do not achieve the targets report drug intolerance,17,29 but, regrettably, data on adverse effects were not recorded in the DISGEN-LIPID study protocol. However, taking into account the improvements in lipid profile trend during follow-up – although this was short, with few patients, and not adjusted for differences in baseline characteristics to be conclusive (Table 4) – it is likely that at least some investigators reacted by increasing LLT dose during follow-up.
More than three-quarters of dyslipidemic patients were at high or very high risk. Less than half of the patients (37% of women and 43% of men) achieved the LDL-C treatment goal of <100 mg/dl and only a minority (10-15%) reached LDL-C <70 mg/dl (see Appendix B).
The data obtained in VALSIM and the Portuguese arm of DYSIS20,21 have been mentioned above. Paradoxically, in the context of ASCVD in Portugal there do not appear to have been changes in the real-world approach to CV risk and dyslipidemias. Portugal is not an exception in this. Although the more recent European Action on Secondary and Primary Prevention by Intervention to Reduce Events (EUROASPIRE IV) survey showed increased prescription of LLT,30 most patients (80.5%) did not achieve LDL-C≤70 mg/dl, even with 85.7% of them on statin medication. These findings reveal the need for better lipid control in the management of residual risk, proceeding to more aggressive LLT, including more potent drugs, higher doses or combined treatment.
There are differences in ASCVD risk between men and women.31 The lifetime risk for CHD after 40 years of age is 49% for men and 32% for women, but the incidence of coronary events rises with age, with women lagging 10 years behind men. A recent meta-analysis of statin prevention trials with gender-specific outcomes, adjusted for CV risk, demonstrated a similar benefit in CV events and mortality.32 However, women are generally less likely to be under LLT or to achieve recommended LDL-C goals, a gender disparity that persists.31,33–35
DISGEN-LIPID did not confirm gender disparities in dyslipidemia management. Nevertheless, at baseline, women had significantly higher TC and HDL-C (p<0.001 for both), non-significantly higher LDL-C, nHDL-C, and apoB, and significantly lower TG (p=0.002). It should also be noted that nearly 94% of the women were post-menopausal, which potentially results in increased TC, changes in LDL composition and increased LDL-C, and slight changes in HDL-C levels.36 In DISGEN-LIPID, there were no differences between genders in LLT generally and statin use in particular, except for pitavastatin. Although the most likely reason for this is chance, it is interesting to speculate whether it may be due to pitavastatin's more favorable metabolic profile, since incident diabetes is more prevalent in women.37,38 However, in DISGEN-LIPID, at baseline, BMI and weight were significantly lower in women, as were blood glucose and HbA1c (not significant), and the prevalence of diabetes was significantly higher in men.
Despite the relatively small number of patients and the study's limitations, this is the first cross-sectional study of real-world practice to examine this topic in Portugal, and will certainly give food for thought in the near future. Meanwhile, it is a call to follow the most recent guidelines,3,4 which clearly indicate how ASCDV prevention and dyslipidemias should be managed in both men and women.
LimitationsOur study has several limitations. DISGEN-LIPID did not set out to assess long-term outcomes; ASCVD risk was estimated based on current or retrospective data. Lipid parameters were taken from medical records without blood sample collection or central core laboratory analysis, which would have provided a better picture of real-world medical practice. The lack of certain baseline lipid parameters in a number of patients is a significant limitation of the study, which was after all an observational descriptive study. As defined by the Working Group on Relative Effectiveness set up by the European Commission, real-life trials are a way to analyze medical data collected under real-life conditions.39 They are conducted in usual care settings, and thus provide insights into the real-life effectiveness of medical condition and/or interventions; there will therefore naturally be differences in the data collected (e.g. lipid parameters). The characteristics of DISGEN-LIPID are useful tools for analyzing how lipid profile is being assessed and followed in clinical practice. In this context, the “Standardization of laboratory lipid profile assessment”, a consensus endorsed by the Cardiovascular Risk and Prevention Group of the Portuguese Internal Medicine Society, the Portuguese Atherosclerosis Society, the Portuguese Society of Cardiology, the Portuguese Society of Laboratory Medicine, and the Portuguese Association of Clinical Chemistry, emphasizes the importance of accurate laboratory assessment of patients’ lipid profile, and stresses that LDL-C should be the primary therapeutic target.40,41 It should be noted that, in Portugal, in most cases LDL-C is calculated by the Friedewald formula (based on fasting plasma TC, TG, and HDL-C values), although we are aware of its limitations, including the potential for inaccuracies in measurements of these parameters, the challenging nature of determining HDL-C and TG, particularly the former, and the assumptions that all plasma TG are carried in VLDL and that the TG/cholesterol ratio of VLDL is invariable. Neither of these assumptions is necessarily true. Interestingly, our consensus has been supported by a recent Special Report from the European Atherosclerosis Society.42
We did not confirm the accuracy of the data transcribed onto the case report forms. Our study did not collect details of patient lifestyle, genetic factors (only family history was assessed), side effects of drugs or treatment adherence. We attempted to minimize bias by asking physicians to enroll consecutive eligible patients, but the nonrandom selection process and the requirement for consent limit the generalizability of the findings. The physicians who participate in the registry are more likely to be interested in management and CV prevention, and we cannot guarantee that they are representative of Portuguese medical practice. Finally, the included population was small and the follow-up short, and the loss of many patients further limits the generalizability of the results, which need to be further validated.
ConclusionsLipid abnormalities were highly prevalent in statin-treated patients. This study highlights the need for better treatment, particularly among high CV risk patients. Titration of statin therapy, the use of combination LLT, and optimization of treatment adherence are vital to consolidate the application of guidelines and to achieve major health gains.
Conflicts of interestDISGEN-LIPID was promoted by Challenges in Cardiology and was financed by JABA Recordati SA, a subsidiary of Recordati S.p.A., with scientific consultancy services from Grupo Keypoint.
PMS has received lecture honoraria or consulting fees from Bayer, JABA-Recordati, Merck Sharp and Dohme Portugal, Kowa Pharmaceuticals, Novartis, Daiichi Sankyo, Amgen, Sanofi-Regeneron, and Tecnimede. CA has received lecture honoraria or consulting fees from Abbott, AstraZeneca, Bial Portela, Jaba Recordati, Merck-Sharp and Dohme, Mylan, and Tecnimede Group. JM has received personal fees for consulting and lectures from Bayer Healthcare, Astra Zeneca, Lilly Company, Daiichi Sankyo, Merck Sharp and Dohme, BMS, and Pfizer.
The authors wish to express their gratitude to all the investigators and to Dr Ana Macedo, PhD, from Grupo Keypoint, whose statistical assistance was invaluable to this document.
| Adelino CorreiaAlexandre AntunesAna Rita Medina PaulosAntónio TeixeiraEduarda ComendaElisabete RodriguesEmília BarbosaEstevão PapeFilipe Gonçalo Leitão Marques VilãoFrancisco Caetano Correia JúniorGraça Ferreira da SilvaJoão CoutinhoJoão RoloJorge Manuel Rodrigues Maia AlcaravelaJorge MimosoJorge Manuel Chambel Aguiar | Luís BastoLuís Manuel Queiroz ValérioMaria José FerreiraMiguel CostaNuno FonsecaPalmira DiasPaula GagoPaulo SilvaPedro CardosoPedro CunhaPedro FreitasPedro Manuel Patrício MatosRegina RibeirasSílvia Luísa S. Gonçalves LourençoSusana Castela da CostaTiago FreitasVeloso Gomes |
| Variable (mean ± SD) | Total | Men | Women | p (men vs. women) |
|---|---|---|---|---|
| Diabetes | ||||
| TC, mg/dl | 187.3±45.4 (n=114) | 181.7±46.2 (n=68) | 195.7±43.3 (n=46) | 0.105 |
| LDL-C, mg/dl | 109.7±40.6 (n=68) | 108.7±41.3 (n=43) | 111.4±40.0 (n=25) | 0.790 |
| HDL-C, mg/dl | 50.2±14.1 (n=105) | 45.9±10.8 (n=64) | 57.0±16.0 (n=41) | <0.001 |
| TG, mg/dl | 160.4±75.6 (n=109) | 170.2±83.0 (n=64) | 146.4±61.7 (n=45) | 0.106 |
| nHDL-C, mg/dl | 137.9±40.9 (n=22) | 139.8±41.6 (n=16) | 132.8±42.2 (n=6) | 0.731 |
| ApoB, mg/dl | 99.6±34.9 (n=9) | 102.4±37.8 (n=6) | 94.0±34.9 (n=3) | 0.759 |
| LDL-C≤70 mg/dl | 12 (14.3%) | 8 (15.7%) | 4 (12.1%) | |
| CHD | ||||
| TC, mg/dl | 176.1±43.0 (n=96) | 168.6±40.5 (n=66) | 192.5±44.5 (n=30) | 0.011 |
| LDL-C, mg/dla | 102.2±36.9 (n=62) | 100.7±35.0 (n=48) | 107.4±43.6 (n=14) | 0.553 |
| HDL-C, mg/dl | 49.3±15.3 (n=91) | 46.2±15.2 (n=64) | 56.6±13.1 (n=27) | 0.003 |
| TG, mg/dl | 127.7±73.4 (n=91) | 128.4±69.5 (n=63) | 126.3±82.8 (n=28) | 0.902 |
| nHDL-C, mg/dl | 117.1±30.2 (n=22) | 110.1±23.4 (n=17) | 141.0±40.9 (n=5) | 0.041 |
| ApoB, mg/dl | 103.4±47.2 (n=7) | 90.4±33.8 (n=5) | 136.0±76.4 (n=2) | 0.286 |
| LDL-C≤70 mg/dl | 11 (16.9%) | 8 (16.0%) | 3 (20.0%) | |
| Stroke/TIA | ||||
| TC, mg/dl | 182.9±48.6(n=35) | 178.9±56.7(n=19) | 187.7±38.3(n=16) | 0.604 |
| LDL-C, mg/dlb | 109.7±37.7 (n=21) | 107.4±41.1 (n=9) | 111.4±36.7 (n=12) | 0.814 |
| HDL-C, mg/dl | 51.2±14.0 (n=31) | 44.5±10.9 (n=15) | 57.4±13.9 (n=16) | 0.008 |
| TG, mg/dl | 125.8±42.7 (n=32) | 135.4±41.6 (n=17) | 115.0±42.7 (n=15) | 0.182 |
| nHDL-C, mg/dl | 134.1±59.7 (n=7) | 154.5±75.3 (n=4) | 107.0±15.4 (n=3) | 0.341 |
| ApoB, mg/dl | 82.5±4.9 (n=2) | 79.0 (n=1) | 86.0 (n=1) | |
| LDL-C≤70 mg/dl | 3 (14.3%) | 2 (22.2%) | 1 (8.3%) | |
| Atrial fibrillation | ||||
| TC, mg/dl | 169.6±34.7 (n=41) | 163.7±36.6 (n=26) | 179.8±29.6 (n=15) | 0.154 |
| LDL-C, mg/dl | 95.3±31.8 (n=29) | 92.9±33.3 (n=19) | 99.7±29.8 (n=10) | 0.596 |
| HDL-C, mg/dl | 49.8±13.6 (n=40) | 45.7±11.6 (n=25) | 56.4±14.3 (n=15) | 0.013 |
| TG, mg/dl | 126.6±52.6 (n=40) | 124.7±57.0 (n=25) | 129.7±46.0 (n=15) | 0.775 |
| nHDL-C, mg/dl | 134.1±59.7 (n=7) | 154.5±75.3 (n=4) | 107.0±15.4 (n=3) | 0.341 |
| ApoB, mg/dl | 123.3±40.1 (n=3) | 154.0 (n=1) | 108.0±42.4 (n=2) | 0.539 |
| CKD | ||||
| TC, mg/dl | 186.3±56.4 (n=17) | 180.5±60.4 (n=13) | 205.2±41.8 (n=4) | 0,460 |
| LDL-C, mg/dl | 132.7±36.1 (n=10) | 136.2±36.4 (n=9) | 101.0 (n=1) | 0,385 |
| HDL-C, mg/dl | 45.1±11.0 (n=16) | 40.9±7.8 (n=12) | 57.7±10.2 (n=4) | 0.004 |
| TG, mg/dl | 137.3±66.6 (n=15) | 132.5±70.0 (n=11) | 150.7±63.6 (n=4) | 0.656 |
| LDL-C≤70 mg/dl | 0 | 0 | 0 | |
CHD: coronary heart disease; CKD: chronic kidney disease; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SD: standard deviation; TC: total cholesterol; TG: triglycerides; TIA: transient ischemic attack.
In these patients (n=62/96 with CHD) 17% had LDL-C≤70 mg/dl and 83% had LDL-C>70 mg/dl (chi-square test: p=0.005; Fisher's test: p=0.008 – in small samples, the chi-square error can be high and the test may not be recommended; Fisher's exact test calculates the probability of association of the characteristics under analysis) and 56% had LDL-C ≤100 mg/dl and 44% had LDL >100 mg/dl (chi-square test: p=0.002; Fisher's test: p=0.003).