Even with improvements in lifestyle interventions, better control of cardiovascular (CV) risk factors, and improvements in CV outcomes, cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in Portugal and Europe. Atherogenic dyslipidemias, particularly hypercholesterolemia, have a crucial causal role in the development of atherosclerotic CVD. The clinical approach to a patient with dyslipidemia requires an accurate diagnosis, based on harmonized and standardized lipid and lipoprotein laboratory assessments. Results and reports of these tests, together with assessment of total CV risk and the respective therapeutic targets, will help ensure that clinical guidelines and good clinical practices are followed, increasing the reliability of screening for lipid disorders, producing more accurate diagnoses and CV risk stratification, and improving CV prevention. To this end, this consensus aims to provide clinicians with practical guidance for the harmonization and standardization of laboratory lipid tests, focusing on the most recent dyslipidemia management guidelines.
Apesar da melhor intervenção nos estilos de vida, do melhor controlo dos fatores de risco cardiovascular (CV) e da melhoria dos resultados CV, a doença cardiovascular (DCV) continua a ser a principal causa de morbilidade e mortalidade em Portugal e na Europa. A dislipidemia aterogénica, nomeadamente a hipercolesterolemia, tem um papel causal no desenvolvimento de DCV aterosclerótica. A abordagem clínica de um doente com dislipidemia preceitua um diagnóstico atento, sustentado em procedimentos laboratoriais harmonizados e padronizados. Os resultados e relatórios dos testes de lipídios se ajuntarem o risco CV total e os respetivos alvos terapêuticos garantem que as diretrizes clínicas e as boas práticas clínicas estão a ser seguidas e respeitadas, o que aumenta a segurança no rastreio e no diagnóstico das alterações lipídicas e da estratificação de risco e melhora a prevenção CV. Nesse sentido, este consenso tem como objetivo fornecer aos clínicos orientações práticas para a harmonização e padronização dos testes laboratoriais lipídicos, com foco nas diretrizes mais recentes da abordagem das dislipidemias.
Cardiovascular disease (CVD) remains one of the leading causes of morbidity and mortality worldwide.1,2 In Portugal, diseases of the circulatory system accounted for 29.5% of deaths recorded in 2013, with a mortality of 54.6 per 100000 for cerebrovascular disease and of 32.9 per 100000 for ischemic heart disease.3
Atherogenic dyslipidemias, particularly hypercholesterolemia, play an unquestionable role in the development of atherosclerotic CVD. Accurate and timely diagnosis of dyslipidemia is of crucial importance. For this diagnosis, it is essential to obtain an accurate laboratory assessment of the patient's lipid profile. This information, combined with thorough clinical history collection and physical examination, can be used to determine the patient's CV risk, a key tool in therapeutic management.4 The intensity of risk-reduction therapy should generally be adjusted to the patient's absolute risk for a CVD event. Appropriate screening, prevention, diagnosis, monitoring and treatment, combined with an accurate and standardized laboratory diagnosis, are essential to the management of dyslipidemias and CVD prevention in clinical practice.
In Portugal, a need has been identified to harmonize various aspects of laboratory lipid measurements. A meeting with specialists in clinical pathology, laboratory medicine, clinical analysis, cardiology, internal medicine and endocrinology was held with the purpose of preparing nationwide recommendations for lipid profile assessment and reporting in adult patients, based on the latest guidelines for CVD prevention and treatment. The recommendations presented herein reflect the debate and consensus reached by this expert panel. This proposal reflects the most recent European guidelines on CVD prevention5 and the management of dyslipidemias.4 They should serve as a foundation for standardizing lipid assessment strategies, as well as laboratory lipid assessment reports, in all national clinical analysis laboratories. These recommendations are divided into four main topics: CVD prevention and treatment guidelines; dyslipidemia screening; lipid biomarker assessment; and reporting of laboratory lipid assessments.
Cardiovascular disease prevention and treatment guidelinesThere are various national and international guidelines on the prevention and treatment of CVD. This expert panel recommends adopting the recommendations of the European Atherosclerosis Society (EAS) and European Society of Cardiology (ESC) for the management of dyslipidemias4 and for CVD prevention in clinical practice,5 and following the three specific standards of good clinical practice (GCP) published by the Portuguese Directorate-General of Health (DGS) on this matter,6–8 specifically in terms of CVD risk stratification and target lipid values. These GCP standards from the DGS should be sources of guidance and instruments of clinical decision support in the National Health Service to promote the development of excellence in health care and its evaluation in the hospital network, health centers, family health units and continuous care.
We advocate appropriate screening, diagnosis, monitoring and treatment of dyslipidemias, as a crucial part of CVD prevention in clinical practice. For assessment of total CV risk, we support the SCORE risk chart for determination of 10-year risk of a first fatal atherosclerotic CV event (e.g. myocardial infarction, stroke or other occlusive arterial disease, including sudden cardiac death), in apparently healthy people with no recognized CVD. Individuals with a history of a CV event, type 1 or 2 diabetes, very high levels of individual risk factors (e.g. familial hypercholesterolemia or blood pressure ≥180/110 mmHg), or chronic kidney disease, have very high or high total CV risk and no further risk estimation is required.
The SCORE data indicate that total risk for CV events is about three times higher than the risk of fatal CVD in men, and four times higher in women, but somewhat less in the elderly. In older patients the likelihood of a first fatal CV event is naturally higher. In older people (>60 years of age), the SCORE risk threshold should not be applied strictly, because their age-specific risk is normally around these levels, even when other CV risk factor levels are normal.
Young people with high levels of risk factors deserve particular consideration. Lifetime risk is probably the best approach to evaluate the impact of risk factors in this population, but there are still insufficient epidemiological cohort data to support its application. In young people, an estimate of their relative risk – rather than their absolute risk, which is presumably low – or the use of ‘CV risk age’ may be helpful.4
Recommendations for the screening of dyslipidemiaScreening for dyslipidemia is indicated in all adults (men aged ≥40 years and women aged ≥50 years or postmenopausal), particularly in the presence of other classic CV risk factors; in patients with clinical CVD (secondary prevention) or with clinical conditions associated with increased CV risk (primary prevention), especially in patients with obesity, metabolic syndrome and/or diabetes; and in HIV-infected patients (Table 1, adapted from9). It is also recommended to screen offspring of patients with severe dyslipidemia and family members of patients with premature CVD.
Who to screen for dyslipidemia in adults at risk.
| All patients with these conditions regardless of age: | Men aged ≥40 years of age and women aged ≥50 years (or postmenopausal) |
| • Clinical evidence of CVD | |
| • Abdominal aortic aneurysm | |
| • Diabetes | |
| • Hypertension | |
| • Current cigarette smoking | |
| • Stigmata of dyslipidemiaa | |
| • Family history of premature CVDb | |
| • Family history of dyslipidemia | |
| • CKD | |
| • Obesity (BMI ≥30 kg/m2) | |
| • IBD and other inflammatory disorders | |
| • HIV infection | |
| • Erectile dysfunction | |
| • COPD | |
| • Hypertensive disease or diabetes in pregnancy |
The rationale of CVD risk assessment is to convince individuals without treatable risk factors and low CV risk to maintain a healthy lifestyle, to recommend individuals with treatable CV risk factors or unhealthy behaviors to modify their attitudes and to treat and manage modifiable risk factors, and to identify subjects who will likely derive most benefit from pharmacotherapy and concomitant lifestyle interventions.
We recommend that CV risk reduction should be individualized and treatment goals should be identified. Low-density lipoprotein cholesterol (LDL-C) is the primary treatment target, but total cholesterol (TC) can be accepted if other lipid parameters are not available. Non-high-density lipoprotein cholesterol (non-HDL-C) and/or apolipoprotein B (ApoB) can be used as secondary treatment targets. We strongly suggest that lipid treatment goals take total CV risk into consideration. LDL-C treatment targets should be:
- •
For low- and moderate-risk individuals: <115 mg/dl (<3.0 mmol/l)
- •
For high-risk patients: <100 mg/dl (<2.6 mmol/l), or a reduction of at least 50% if baseline LDL-C is between 100 and 200 mg/dl (2.6-5.2 mmol/l).
- •
For very high-risk patients: <70 mg/dl (<1.8 mmol/l) or a decrease of at least 50% if baseline LDL-C is between 70 and 135 mg/dl (1.8-3.5 mmol/l).
The diagnosis of dyslipidemia should always be confirmed by subsequent laboratory assessment of lipid profile, carried out in a minimum of four weeks, prior to the beginning of any pharmacological therapy (in line with the DGS's standard no. 019/2011).6
The frequency of testing depends on the person's CV risk profile. After 4-12 weeks from the start of drug treatment a second fasting lipid panel should be performed. Subsequently, assessments should be performed every three months until the lipid goals are achieved, and thereafter every 12 months, as clinically indicated. However, when LDL-C lowering therapy is adapted (e.g. any intensification of lifestyle interventions, titration of statin therapy, or adding of non-statin therapies), we recommend a new lipid panel, again 4-12 weeks after treatment adjustment, followed by resumption of the above regimen.
Recommendations for lipid biomarker assessmentWe recommend that a baseline lipid assessment should include estimation of TC, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), LDL-C (calculated with the Friedewald formula, based on fasting plasma TC, TG, and HDL-C values, or determined directly), and non-HDL-C. Fasting and non-fasting lipid levels have similar prediction strength and should be regarded as complementary. Non-fasting lipid levels can be applied in screening and risk estimation; however, in general, a 12-hour fasting period is still considered optimal when lipoprotein measurements are used in CV risk screening and estimation and for characterizing dyslipidemias before treatment.
We are aware of the significant limitations of the Friedewald formula.10,11 Its estimates are not valid when TG >400 mg/dl (>4.5 mmol/l), in patients with type III hyperlipoproteinemia (dyslipidemia with accumulation of cholesterol-rich remnants) or chylomicronemia, or in nonfasting specimens. Recently, novel LDL-C estimation formulas such as (non-HDL-C) - (TG/adjustable factor mg/dl) have been proposed,12 in which an adjustable factor was established as the strata-specific median TG:very low density lipoprotein cholesterol (VLDL-C) ratio. Although this new method appears to provide a better estimate of LDL-C (particularly in patients with LDL-C ≤70 mg/dl in the presence of high TG levels), it needs to be more widely validated, and the formula selected in each laboratory determination should be clearly stated.
If available, and in particular clinical circumstances, ApoB and lipoprotein(a) (Lp(a)) can also be estimated. Non-HDL-C is an umbrella term for all plasma atherogenic lipoproteins (VLDL, VLDL remnants, intermediate-density lipoproteins, LDL, and Lp(a)). It is calculated as TC minus HDL-C, and is associated with ApoB levels. A powerful CV risk predictor, non-HDL-C is simple to compute and does not need fasting conditions.13 This is the reason for including non-HDL-C, unlike ApoB, in the standard lipid profile assessment.
Measurement of Lp(a) is not currently recommended, and should only be performed in specific patients4,14,15 (Table 2). CV risk is significant when Lp(a) >50 mg/dl. In patients at risk with high Lp(a) levels the treatment of modifiable CV risk factors should be intensified, particularly LDL-C (with intensive lipid-lowering therapy). The effect on CVD events of targeting Lp(a) has not been established and Lp(a) should not be a lipid target in CV prevention.
Indications for Lp(a) screening.
| • Premature CVD |
| • Familial hypercholesterolemia |
| • Family history of premature CVD and/or elevated Lp(a) |
| • Recurrent CVD despite optimal lipid-lowering therapy |
| • ≥5% 10-year risk of fatal CVD according to SCORE |
| • Hemodialysis and CKDa |
| • Intermediate (3-5%) 10-year risk of fatal CVD according to SCOREa |
The expert panel recommends including the methods used for lipid biomarker quantification, as well as the specific equipment employed to that end and the associated variation coefficients, in all laboratory lipid reports.
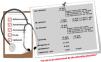
We strongly emphasize the importance of lipid test reports including specific information on target LDL-C values, according to the different CV risk levels (<70 mg/dl, <100 mg/dl and <115 mg/dl), in line with the DGS's standard no. 005/2013.8 This should be accompanied by a supporting statement, such as “CV risk to be determined by the attending physician” (Figure 1). We also propose that laboratory reports should flag nonstandard lipid values based on desirable concentration cut-points, defined by guidelines and consensus statements.4,5,9,16
Final wordAtherosclerotic CVD is preventable. We and our scientific societies wish to promote the best health care for individuals of both sexes and all ages who are at CV risk. We are aware that effective CV prevention is frequently overlooked in our daily practice. CV prevention is in fact an ethical obligation. It is important to recognize CV risk, to stress the importance of laboratory reports, and to identify clinical situations that warrant judicious intervention.
FundingNo external funding was used in the preparation of this manuscript.
Conflicts of interestPMS has received lecture honoraria or consulting fees from Bayer, Jaba Recordati, Merck Sharp and Dohme Portugal, Kowa Pharmaceuticals, Novartis, Daiichi Sankyo, Amgen, Sanofi-Regeneron, and Tecnimede. JSD has received lecture honoraria or consulting fees from Novo-Nordisk, Merck Sharp and Dohme Portugal, Sanofi-Regeneron, Novartis Oncology, Boehringer-Ingleheim, and Tecnimede. VG has received honoraria from AstraZeneca, Merck Sharp Dohme Portugal, Bial, Jaba Recordati, and Amgen. The other members have no conflicts of interest to declare.
The consensus panel wishes to express its gratitude to Anabela Farrica and Diogo Ribeiro of Eurotrials, Scientific Consultants, S.A., whose assistance was invaluable in preparing the first draft of this document.