Heart failure (HF) is a major health problem with a significant impact on morbidity, mortality, quality of life and healthcare costs. Despite the positive impact of disease-modifying therapies developed over the last four decades, HF mortality and hospitalization remain high.
We aim at reviewing the evidence supporting the use of sodium-glucose co-transporter-2 (SGLT-2) inhibitors, as a novel strategy for HF with reduced ejection fraction (HFrEF) treatment.
The consistent observation of a reduction in HF hospitalizations in type-2 diabetes cardiovascular safety trials EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI 58 and VERTIS raised the hypothesis that SGLT-2 inhibitors could have an impact in HF treatment.
This hypothesis was first confirmed in 2019 with the DAPA-HF publication showing that dapagliflozin on top of optimized HFrEF therapy, reduced HF-hospitalizations and cardiovascular mortality. This was reinforced by the EMPEROR-Reduced publication in 2020 showing that empagliflozin on top of optimized HFrEF therapy, reduced HF-hospitalizations. Both studies established SGLT-2 inhibitors as a fourth pillar of HFrEF prognosis-modifying therapy, in addition to the gold standard triple neurohormonal modulation/blockade.
A insuficiência cardíaca (IC) é um importante problema de saúde, com impacto significativo na comorbidade, mortalidade, qualidade de vida e nos custos com saúde. Apesar do impacto positivo das terapias modificadoras de prognóstico desenvolvidas nas últimas quatro décadas, a mortalidade e as hospitalizações por IC permanecem elevadas. O nosso objetivo é rever a evidência que apoia o uso de inibidores do cotransportador sódio-glicose (inibidores do SGLT-2) como uma nova estratégia para o tratamento de IC com fração de ejeção reduzida (ICFEr). A observação consistente de uma redução nas hospitalizações por IC nos ensaios de segurança cardiovascular de diabetes tipo-2 EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI 58 e VERTIS levantou a hipótese de que os inibidores do SGLT-2 poderiam ter um impacto no tratamento da IC. Esta hipótese foi confirmada pela primeira vez em 2019 com a publicação do DAPA-HF, mostrando que a dapagliflozina adicionada à terapia optimizada para ICFEr reduziu as hospitalizações por IC e a mortalidade cardiovascular. Isso foi reforçado pela publicação do EMPEROR-Reduced em 2020, mostrando que a empagliflozina, adicionada à terapia optimizada para ICFEr reduziu as hospitalizações por IC. Ambos os estudos estabeleceram os inibidores do SGLT-2 como o quarto pilar da terapia modificadora do prognóstico da ICFEr, adicionada ao gold standard do tratamento da ICFEr – a estratégia de modulação/bloqueio neuro-hormonal triplo.
Heart Failure (HF) is a deadly condition. During the 1980s, five years after first onset of HF symptoms, around 75% of HF patients would be dead. In the four decades that followed, there was significant progress in HF treatment, resulting in the emergence of a series of disease-modifying therapies with a positive prognostic impact in HF with reduced ejection fraction (HFrEF). Despite this, mortality is still high at approximately 50% 5 years after onset of first HF symptoms.
Heart failure with reduced ejection fraction prognosis-modifying pharmacological therapy is currently based on the triple combination of angiotensin-converting enzyme inhibitors (ACEi)/angiotensin-II receptor blockers (ARB)/angiotensin receptor-neprilysin inhibitor (ARNi), with a beta-blocker (BB), and a mineralocorticoid receptor antagonist (MRA).1
In 2015, the EMPA-REG OUTCOME trial publication2 raised the hypothesis that a new class of anti-diabetic drugs – sodium-glucose co-transporter 2 (SGLT-2) inhibitors – could have an impact on HF treatment. This hypothesis was proven correct in 2019, with the publication of DAPA-HF.3 The latter established dapagliflozin as a new additional step in the aforementioned triple prognostic-modifying therapy, by inducing incremental gains in HFrEF prognosis. This was reinforced by the publication in 2020 of the EMPEROR-Reduced4 study, which showed identical results with empagliflozin.
This paper aims at reviewing the evidence that supports the use of SGLT-2 inhibitors as a fourth pillar of contemporary HFrEF treatment.
- 1.
Sodium-glucose co-transporter-2 inhibitors: From anti-diabetic drugs to a hypothetical role in HF treatment
SGLT-2 inhibitors were initially developed for the treatment of type 2 diabetes (T2D).5 SGLT-2 inhibitors currently approved in the European Union include dapagliflozin, canagliflozin, empagliflozin and ertugliflozin.
Sodium-glucose co-transporter-2 inhibitors are responsible for the reabsorption of 80-90% of the glucose filtered by the glomeruli, through the coupling of the electrochemical energy produced by active sodium transport to the co-transport of glucose1. SGLT-2 inhibitors act by selective inhibition of SGLT-2 at the proximal tubule of the nephron, thus improving glycemic control by blocking glucose reabsorption and promoting glucosuria.6 SGLT-2 inhibitors were effective at lowering glycated hemoglobin, as well as reducing bodyweight in a broad spectrum of T2D patients.5
In recent years, several cardiovascular outcome trials have been conducted to demonstrate the cardiovascular safety profile of SGTL-2 inhibitors in high or very-high cardiovascular risk T2D patients. In the EMPA-REG OUTCOME trial, empagliflozin compared to placebo, was shown to reduce the composite primary endpoint of death due to cardiovascular causes, non-fatal myocardial infarction or nonfatal stroke (3-point major cardiovascular events (MACE)).2 Similar results were observed in the CANVAS program,7 in which canagliflozin was compared to placebo. In the DECLARE-TIMI 58 trial, dapagliflozin compared to placebo met the pre-specified criterion for non-inferiority with respect to 3-point MACE, and the same was observed in the VERTIS CV study,8 in which ertugliflozin was compared to placebo.
Somewhat unexpectedly, in all four of the abovementioned studies, a reduction in HF hospitalizations was observed in the drug-study arm as compared with the control arm.2,7–9 Additionally, in the EMPA-REG, the CANVAS and the DECLARE-TIMI 58 trials, a reduction in the composite endpoint of cardiovascular death/HF hospitalizations was also observed in the drug-study arm compared to the placebo arm.2,7,9 This raised the possibility that SGLT-2 inhibitors could have a beneficial effect in the treatment of HF.10,11
- 2.
Randomized clinical trials of SGLT-2 inhibitors in HF.
Consequently, further SGLT-2 inhibitors studies were conducted among patients with HF, with or without T2D, under optimized HF-therapy, to test the possible additional positive effects of SGLT-2 inhibitors, as well as the safety of this strategy. In the case of dapagliflozin, these were the DAPA-HF3 (in patients with HfrEF) and the ongoing DELIVER12,13 (in patients with HF with preserved ejection fraction [HfpEF]) trials. Similar trials were conducted with empagliflozin in HFrEF and HFpEF patients: the EMPEROR-Reduced and the ongoing EMPEROR-Preserved trials.4,14
DAPA-HF was an international, multicenter, phase 3, placebo-controlled trial in patients with chronic HF, with or without T2D, randomized to receive dapagliflozin 10 mg or placebo.3 The study included patients with HFrEF (left ventricular ejection fraction [LVEF]≤40%), New York Heart Association (NYHA) functional class≥II, elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) and under optimal HF therapy.3,15 The latter included the combination of an ACEi/ARB/sacubitril-valsartan (Sac/Val), plus a BB, and a MRA unless contraindicated or not tolerated. Additionally, patients were permitted an implantable cardioverter-defibrillator (ICD), or cardiac resynchronization therapy (CRT), or both, whenever indicated.3,15
The primary outcome was the composite end-point of worsening heart failure (WHF) or cardiovascular death. An episode of WHF was either an unplanned hospitalization due to HF or an urgent visit resulting in intravenous HF therapy.3,15
Overall 4744 patients were randomized: 94% were on an ACEi, or an ARB or on Sac/Val; 96% were on a BB, 71% on MRA and 93% on diuretics. A sizable proportion of patients had an implanted device: 26% had an ICD and 8% were under CRT. T2D was present in 42% of patients.3 During an 18.2-month median follow-up, the primary outcome occurred in 16.3% of patients in the treatment arm vs. 21.2% in the placebo arm (hazard ratio [HR] 0.74; 95% confidence interval (CI), 0.65-0.85; p<0.001) resulting in a 26% WHF risk reduction with dapagliflozin.3 The difference in the frequency of the primary outcome amongst the two therapeutic arms reached statistical significance at one month of follow-up.3
A first WHF event occurred in 10.0% of patients on dapagliflozin vs. 13.7% on placebo (HR 0.70; 95% CI, 0.59-0.83).3 Death from cardiovascular causes occurred in 9.6% patients on dapagliflozin vs. 11.5% patients on placebo (HR 0.82; 95% CI, 0.69 to 0.98). Results of the primary endpoint were similar amongst patients with and without T2D.3
The incidence of the secondary composite outcome of hospitalization for HF or death from cardiovascular causes was lower in the dapagliflozin group than in the placebo group (HR 0.75, 95% CI, 0.65-0.85; p<0.001).3
Compared to the placebo group, the dapagliflozin group showed a higher increase in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 8 months of follow-up vs. baseline (HR 1.18, 95% CI 1.11-1.26; p<0.001).3 This indicates a positive effect on symptom perception and quality of life in patients with HFrEF already under optimal background prognosis-modifying therapy.
Death from any cause, usually interpreted as a safety endpoint, occurred in 11.6% patients on dapagliflozin vs. 13.9% patients on placebo (HR 0.83, 95% CI, 0.71 to 0.97).3
Finally, in the dapagliflozin group compared to the placebo group, there was a significant decrease in NT-proBNP (p<0.001), in systolic blood pressure (p=0.002), in glycated hemoglobin (p<0.001) and in bodyweight (p<0.001), whilst a significant increase in hematocrit (p<0.001) occurred.3
The frequency of serious adverse events and specifically those due to hypoglycemia, hypovolemia or renal insufficiency was similar amongst treatment groups.3
A post-hoc analysis of DAPA-HF showed that the impact of dapagliflozin on the primary outcome was consistent, regardless of baseline therapy, including the presence or absence of sacubitril/valsartan (HR for the primary endpoint of 0.75 (95% CI, 0.50-1.13) and 0.74 (95% CI, 0.65-0.86), respectively).16
Although patients enrolled in the DAPA-HF trial were under optimal neuro-hormonal blockade/modulation (as shown by the high rates of ACEis/ARBs/Sac-Val, BBs and MRAs) before randomization, approximately 25% of those randomized to placebo had a primary event over 18 months, attesting an unmet need requiring further improvement in current standard therapy.3,15 DAPA-HF showed that the addition of dapagliflozin to current optimal medical treatment represents an additional step towards the goal of saving lives, reducing hospitalizations, improving symptoms and quality of life in patients with HFrEF, regardless of the presence of T2D.3,15,16 It also supported the EMA approval of dapagliflozin as a HF therapy in patients without T2D.
DAPA-HF was followed by the DEFINE-HF study which included 263 HFrEF (LVEF≤40%) patients, with NYHA functional class II-III, estimated glomerular filtration (eGFR) rate ≥30 mL/min/1.73 m2, and elevated natriuretic peptides and under optimal neuro-hormonal blockade/modulation.17 Patients were randomized to receive dapagliflozin 10 mg or placebo. Compared to placebo, dapagliflozin did not show a significant improvement in NT-proBNP neither at 6 weeks nor at 12 weeks of follow-up, the first co-primary outcome.17 The second dual co-primary outcome of a ≥5 point improvement in KCCQ-Overall Summary Score (KCCQ-OSS) or a ≥20% reduction in NT-proBNP was met in 61.5% of dapagliflozin-treated patients vs, 50.4% in placebo group (adjusted odds ratio [OR] 1.8, 95% CI 1.03-3.06, nominal p=0.039). This was attributable to both higher proportions of patients with ≥5-point improvement in KCCQ-OSS (42.9 vs. 32.5%, adjusted OR 1.73, 95% CI 0.98-3.05), and ≥20% reduction in NT-proBNP (44.0 vs. 29.4%, adjusted OR 1.9, 95% CI 1.1-3.3) by 12 weeks. Results were consistent among patients with or without T2D, and other pre-specified subgroups (all p values for interaction=non-significant).17
More recently, the EMPEROR-Reduced study with empagliflozin in patients with HFrEF was published.18 This was a phase 3, placebo-controlled study (NCT03057977), which involved 3,730 patients with NYHA class II-IV, HF with ejection fraction ≤40% randomized to placebo or empagliflozin 10 mg daily, added to guideline-directed medical therapy (ACE inhibitors/ARBs/ARNIs, beta-blockers and MRAs).4,18 The baseline clinical characteristics and demographics were relatively similar to the DAPA-HF.4 The primary endpoint was a time-to-first-event analysis of the combined risk of HF hospitalizations or CV death. After a median of 16 months, the primary outcome occurred in 361 of 1863 patients (19.4%) in the empagliflozin group and in 462 of 1867 patients (24.7%) in the placebo group (HR 0.75; 95% CI [0.65-0.86]; p<0.00). This was primarily driven by reduced rates of HF hospitalization in the empagliflozin group (HR 0.70; 95% CI [0.58-0.85]; p<0.001). The trial failed to demonstrate a significant reduction in CV death (HR 0.92; 95% CI [0.75-1.12]) and in all-cause mortality (HR 0.92; 95% CI [0.77-1.10]) compared to placebo.4 No difference in quality of life as measured using KCCQ was observed when comparing both therapeutic arms. A significant reduction was observed in the rate of renal disease progression, as measured by eGFR rate slope over time, in the empagliflozin group compared to patients receiving placebo.4
The adverse events profile for empagliflozin was similar to that reported in previous studies.4
In summary, the results of DAPA-HF and EMPEROR-Reduced trials consolidate a new approach to HFrEF management, through the addition of SGLT-2 inhibitors to current standard triple disease-modifying therapy in patients with and without T2D. These studies showed that dapagliflozin and empagliflozin are effective in reducing HF hospitalizations. DAPA-HF showed that dapagliflozin can reduce CV death in patients with HFrEF.3,4,19
- 3.
The SOLOIST WHF: A SGTL-2 and SGLT1 inhibitor randomized trial in patients with type 2 diabetes and recent worsening heart failure
The recently published SOLOIST WHF in patients with T2D and recent worsening HF showed that sotagliflozin – a SGTL-2 and SGLT1 inhibitor – versus placebo, initiated before or shortly after discharge, reduced cardiovascular mortality, hospitalizations and urgent visits for HF.20
- 4.
Possible mechanisms explaining the benefits of SGLT-2 inhibitors
- a)
Remodeling
The mechanisms underlying SGLT-2 inhibitors benefits in cardiovascular and renal events remain unclear.10,21
Left ventricular (LV) remodeling is a major predictor of CV events and prognosis in HF, therefore the effects of SGLT-2 inhibitors in remodeling21 may, at least in part, explain the benefits observed in HF.22
Many different additional hypotheses have been put forward, such as the effect of SGLT-2 inhibitors on promoting diuresis/natriures,10,11,23–25 on improving cardiometabolic efficiency,22,26,27 blood pressure profile and renal function. Other explanatory hypotheses include the increase in hemoglobin concentration among others.
- b)
The “smart diuretic” hypothesis
By inducing SGLT-2 inhibition at the proximal tubule, SGLT-2 inhibitors promote glycosuria and natriuresis. This, in turn, may lead to a reduction in plasma volume, resulting in a decrease in LV end-diastolic pressure (LVEDP) and congestion. The latter is responsible for 90% of HF hospitalizations.22 This decrease in LVEDP may lead to diminished cardiomyocyte cytosqueletal stimulation, which is a major LV remodeling pathway, and consequently a determinant of bad prognosis in HF.10
Moreover, additional sodium-hydrogen exchanger 3 channel inhibition at the proximal tubule further contributes to increased diuresis and natriuresis, decreased intravascular volume, increased hemoconcentration, and reduced body weight and blood pressure. All of these effects may lead to a reduction in LV wall stress which, in turn, may also lead to diminished deleterious cytoskeletal pro-proliferative signaling and consecutive LV remodeling.10
Sodium-glucose co-transporter-2 inhibitors may selectively reduce interstitial volume with minimal change in intra-vascular volume.28,29 This enables the reduction of congestion without decreasing afferent arteriole pressure, thus not activating the sympathetic nervous system (SNS) drive.28–32
As opposed to conventional diuretics, which can reduce LV end-diastolic pressure at the cost of harmful SNS activation, SGLT-2 inhibitors can reduce cytoskeletal stimulation with no neurohormonal activation.10 SGLT-2 inhibitors also attenuate STAT3 phosphorylation, leading to decreased myocardial extracellular matrix accumulation and cardiac fibrosis.10
Thus, preliminary evidence seems to suggest that the beneficial effects of SGLT-2 inhibitors in HF may involve LV remodeling attenuation10,28,29 via a diuretic-like effect, although without the neuro-hormonal activation associated with conventional diuretics.
- c)
The cardio-renal hypothesis
Heart failure hospitalizations are due to congestion in 90% of the cases. SGLT-2 inhibitors reduce the rate of decline in renal function among diabetic patients.33 This could be relevant in decreasing HF hospitalizations.
- d)
The cardiometabolic efficiency hypothesis
By lowering blood pressure, increasing aortic compliance and improving ventricular-arterial coupling, SGLT-2 inhibitors can reduce cardiac workload and myocardial oxygen consumption.10 These benefits can be amplified by hemoconcentration, leading to increased hemoglobin/oxygen-carrying capacity.10,25
A change in cardiac fuel supply by shifting glucose oxidation to a more efficient fat/ketones oxidation has been hypothesized by some authors.28
Finally, by inhibiting sodium/hydrogen exchanger-1 in cardiomyocytes, SGLT-2 inhibitors improve mitochondrial function in cardiomyocytes, which is crucial for cardiomyocyte function and survival.29
Several studies investigating the impact of SGLT-2 inhibitors are currently ongoing or have recently been completed, including mechanistic studies of empagliflozin and dapagliflozin.29,31 Results of this research will provide more information and details about the potential mechanisms of action of SGLT-2 inhibitors and implications for cardiovascular benefits.
- 5.
Implications for clinical practice
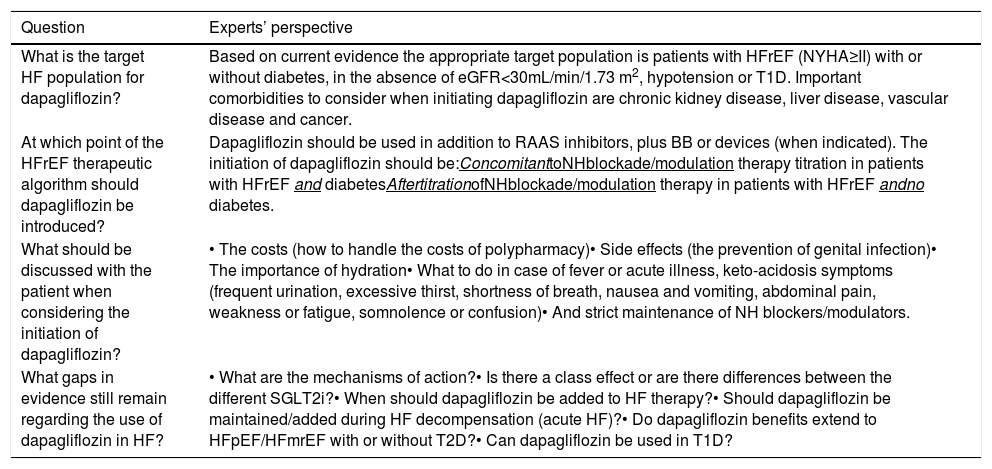
The impact of the abovementioned SGLT-2 inhibitor trials in patients with HFrEF in the soon-to-be published 2021 European Society of Cardiology Heart Failure guidelines remains unknown. However, the unmet need for new disease-modifying therapies that may further improve survival, morbidity, functional capacity and quality of life in HF, as has already been demonstrated with SGLT inhibitors, is well recognized. As a consequence, dapagliflozin recently became the first SGLT-2 inhibitor to be approved by the European Medicines Agency for the treatment of HFrEF patients. In order to incorporate this into clinical practice, Table 1 summarizes the experts’ consensus on the use of dapagliflozin in the treatment of patients with HFrEF, according to DAPA-HF results and the regulatory agency's approval.
Expert consensus on the use of dapagliflozin in heart failure patients with reduced ejection fraction, according to DAPA-HF results and the regulatory agency's approval.
| Question | Experts’ perspective |
|---|---|
| What is the target HF population for dapagliflozin? | Based on current evidence the appropriate target population is patients with HFrEF (NYHA≥II) with or without diabetes, in the absence of eGFR<30mL/min/1.73 m2, hypotension or T1D. Important comorbidities to consider when initiating dapagliflozin are chronic kidney disease, liver disease, vascular disease and cancer. |
| At which point of the HFrEF therapeutic algorithm should dapagliflozin be introduced? | Dapagliflozin should be used in addition to RAAS inhibitors, plus BB or devices (when indicated). The initiation of dapagliflozin should be:ConcomitanttoNHblockade/modulation therapy titration in patients with HFrEF and diabetesAftertitrationofNHblockade/modulation therapy in patients with HFrEF andno diabetes. |
| What should be discussed with the patient when considering the initiation of dapagliflozin? | • The costs (how to handle the costs of polypharmacy)• Side effects (the prevention of genital infection)• The importance of hydration• What to do in case of fever or acute illness, keto-acidosis symptoms (frequent urination, excessive thirst, shortness of breath, nausea and vomiting, abdominal pain, weakness or fatigue, somnolence or confusion)• And strict maintenance of NH blockers/modulators. |
| What gaps in evidence still remain regarding the use of dapagliflozin in HF? | • What are the mechanisms of action?• Is there a class effect or are there differences between the different SGLT2i?• When should dapagliflozin be added to HF therapy?• Should dapagliflozin be maintained/added during HF decompensation (acute HF)?• Do dapagliflozin benefits extend to HFpEF/HFmrEF with or without T2D?• Can dapagliflozin be used in T1D? |
BB: beta-blocker; T1D: type 1 diabetes; T2D: type 2 diabetes; eGFR: estimated glomerular filtration rate; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; NH: neuro-hormonal; NYHA: New York Heart Association; RAAS inhibitors: include angiotensin-converting–enzyme inhibitors (ACEis), angiotensin II-receptor blockers (ARBs), sacubitril–valsartan (Sac/Val) and mineralocorticoid receptor antagonists (MRAs); SGLT2i: sodium-glucose cotransporter-2 inhibitors.
Heart failure is a highly prevalent syndrome and is associated with high morbidity, mortality and costs. Both the DAPA-HF and the EMPEROR-Reduced studies showed that, in addition to optimized contemporary HFrEF therapy, dapagliflozin and empagliflozin were effective in reducing HF hospitalizations. Moreover, dapagliflozin demonstrated a significant reduction in CV death. In both studies the results were observed independently of the presence of T2D. The mechanisms explaining these results are not entirely clear and go beyond controlling blood glucose. They may include the contribution of congestion control, the improvement in cardio-metabolic efficiency and the reduction in ventricular remodeling, among others.
DAPA-HF and EMPEROR-Reduced trials provided evidence for the addition of dapagliflozin or empagliflozin to the present gold standard neurohormonal modulation/blockade strategy in patients with HFrEF. Thus, SGLT-2 inhibitors have emerged as the fourth pillar of the pharmacological disease-modifying therapy in HFrEF patients, regardless of the presence or absence of T2D.
Conflicts of interestJosé Silva-Cardoso has received speaker and consultant fees, advisory board participation fees, or investigational grants from Abbott, AstraZeneca Pharmaceuticals, Bial, Boehringer Ingelheim, Menarini, Merck Serono, Merck Sharp & Dohme, Novartis, Orion, Pfizer, Sanofi, Servier, and Vifor Pharma. Aurora Andrade has received speaker or advisory boards fees from Novartis, AstraZeneca, Servier, Orion and Bial. Dulce Brito has received speaker and consultant fees or investigational grants from AstraZeneca Pharmaceuticals, Boehringer Ingelheim, Novartis, Orion, Pfizer, Roche Diagnostics, Sanofi, Servier, and Vifor Pharma. Jorge Ferreira has received speaker and consultant fees from Amgen, AstraZeneca, Boehringer-Ingelheim, Novartis. Cândida Fonseca has received speaker and consultant fees, or investigational grants, from AstraZeneca Pharmaceuticals, Bayer, Boehringer Ingelheim, Merck Serono, Novartis, Orion, Pfizer, Sanofi, Servier, and Vifor Pharma. Marisa Peres has received speaker or advisory board fees from AstraZeneca, Servier, and Novartis. Fátima Franco has received speaker or advisory board fees from Boehringer, AstraZeneca, Novartis and Servier. Brenda Moura has received speaker or advisory board fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Servier, Novartis, Vifor Pharma.
Financial support for the preparation of this article was provided by AstraZeneca. AstraZeneca had no role in the writing of the report and in the decision to submit the article for publication.