Sepsis is a systemic inflammatory response syndrome of suspected or documented infectious origin, whose outcome is multiorgan failure. Sepsis-induced myocardial dysfunction (SIMD), present in more than 50% of septic patients, is characterized by (i) left ventricular (LV) dilatation with normal or low filling pressure, (ii) right and/or LV (systolic and/or diastolic) dysfunction and (iii) reversibility. Since the first definition proposed by Parker et al. in 1984, attempts have been made to define SIMD. Many parameters are used to assess cardiac function in septic patients, sometimes making it more difficult to measure due to the intrinsic hemodynamical changes in this condition. Nevertheless, with advanced echocardiographic techniques, such as speckle tracking analysis, it is possible to diagnose and assess systolic and diastolic dysfunction, even in the earliest stages of sepsis. Cardiac magnetic resonance imaging brings new insights into the reversibility of this condition. Many uncertainties still remain regarding the mechanisms, characteristics, treatment and even prognosis of this condition. There are also inconsistent conclusions from studies, therefore this review attempts to summarize our current knowledge of SIMD.
Sépsis é uma síndrome de resposta inflamatória sistémica de origem infecciosa, suspeita ou documentada, cujo resultado é falência multiorgânica. Disfunção miocárdica induzida por sépsis (SIMD), presente em mais de 50% dos doentes com sépsis, é caracterizada por (i) dilatação ventricular esquerda com pressão de preenchimento normal ou baixa, (ii) disfunção ventricular direita e/ou esquerda (sistólica e/ou diastólica) e (iii) reversibilidade. Ao longo dos anos, houve tentativas de definir SIMD, desde a primeira definição proposta por Parker et al. em 1984 até à atual era tecnológica. Há vários parâmetros utilizados para avaliar a função cardíaca em doentes sépticos, por vezes difíceis de medir devido às alterações hemodinâmicas intrínsecas desta condição. No entanto, com técnicas ecocardiográficas avançadas, como speckle tracking, é possível diagnosticar e avaliar a disfunção sistólica e diastólica, mesmo em fases precoces da sépsis. A ressonância magnética cardíaca traz novos conhecimentos acerca da reversibilidade desta condição. Existem muitas incertezas acerca dos mecanismos, características, tratamento e prognóstico desta condição, com conclusões inconsistentes entre os estudos, logo, esta revisão tenta sumarizar o nosso conhecimento atual sobre SIMD.
The Third International Consensus Definitions for Sepsis and Septic Shock1 defines sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock is defined by a vasopressor requirement to maintain a mean arterial pressure (MAP) ≥65 mmHg and serum lactate level ≥2 mmol/L (≥18 mg/dL), after adequate fluid resuscitation. Mortality lies between 30% and 50% in developed countries.1–3
Sepsis-induced myocardial dysfunction (SIMD) or septic cardiomyopathy is a reversible myocardial dysfunction that occurs as part of multi-organ failure caused by sepsis.4 Forty to fifty percent of septic shock patients have SIMD, depending on the definition used, and this seems to be one of the major predictors of unfavorable prognosis.5–7 According to Flynn et al., patients with SIMD have 70–90% mortality, compared to a 20% in septic patients without SIMD.5 In septic patients, cardiac dysfunction manifests itself with hemodynamic instability or end-organ hypoperfusion, associated with raised cardiac biomarkers and myocardial dysfunction documented on multiple imaging techniques.8 The complexity of the cardiovascular system, the myriad methods of assessment, and variations in the pre-septic state of the heart makes elucidating a cause-and-effect relationship difficult.9
Septic cardiomyopathy was first described by McLean et al.10 in 1967, where the diagnostic criteria to identify heart failure (HF) was a low cardiac index (CI). Subsequently, in 1984, Parker et al.,11 using radionuclide cineangiography, characterized SIMD as reversible myocardial depression due to sepsis and septic shock, defined by decreased initial LV ejection fraction (LVEF) <40% with increased mean end-diastolic and end-systolic volumes (EDV, ESV), that usually occur within two to three days of the onset of sepsis and resolves in seven to 10 days.3,11–13
The current definition characterizes SIMD as:14
- I.
Ventricular dilatation with an increase in ventricular compliance and normal to low filling pressure, unlike the pattern of cardiogenic shock where ventricular pressures are elevated.15
- II.
Decreased EF, without a decrease in stroke volume (SV).
- III.
Diminished response to fluid resuscitation and catecholamines.16
- IV.
Reversibility in seven to 10 days. Cardiac magnetic resonance imaging (CMRI) detects changes suggestive of myocardial edema or an altered metabolic state, a pattern distinct from that seen in ischemia and necrosis consistent with irreversibility. Some theorize that SIMD may represent a protective “hibernating” state of the heart.9
- V.
Exclusion of acute coronary syndrome as etiology.
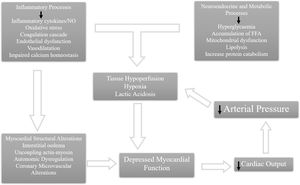
In 1947, Wiggers17 described the presence of a circulating myocardial depressant factor responsible for myocardial dysfunction for the first time. The host immune system identifies infection through recognition of pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) and lipoteichoic acid of bacteria, which bind to pattern-recognition receptors, such as toll-like receptors (TLRs), expressed on the surface of host cells.3,15,18 This binding activates intracellular pathways that culminate in the expression of the nuclear translocation of nuclear factor-κB and the increased transcription of inflammatory mediators,19 such as TNFα and IL-1β. These cytokines induce myocyte apoptosis, with consequent ventricular dilatation, and act directly in the peripheral vasculature. This affects myocardial performance via alterations in systemic vascular resistance and venous return.9,20–22 TNFα can also block β-adrenergic effects on contractility.18 Early infection control with adequate antibiotic care is important during septic shock to decrease PAMPs arising from invasive microorganisms. TLRs are also activated after binding to endogenous danger-associated molecular patterns (DAMPs). Through TLRs, PAMPs and DAMPs have a direct cardiodepressant effect by downregulating β-receptors, and therefore impairing the adrenergic response of the cardiomyocytes. They also trigger cardiomyocyte apoptosis and impair myofibril function due to the disruption of calcium liberation.18 On the other hand, they also have an indirect cardiodepressant effect by causing endothelial dysfunction, microcirculatory dysfunction and fluid maldistribution.18 LPS activates TLRs in cardiomyocytes, activating intracellular pro-apoptotic patterns and increasing caspase-3 activity, which may be associated with decreased sensitivity of the myofilaments to calcium, or even structural breakdown of the sarcomere itself.18,23 Clinically, there is depressed myocardial contraction.23
High mobility group box 1 (HMGB1), a circulating histone, is a DAMP released during tissue damage. It acts by amplifying oxidative stress through HMGB1–TLR4 interactions, reducing mitochondrial membrane potential and adenosine triphosphate levels and by impairing cardiac excitation-contraction coupling.18,19 Mitochondrial dysfunction, one of the major pathways of sepsis-induced cardiomyopathy, is a result of an unbalanced production of reactive oxygen species (ROS) and reactive nitrogen species, which impairs oxidative phosphorylation and culminates in mitochondrial apoptosis.14,18
The production of excess antimicrobial products and inflammatory mediators elicits the generation of ROS and nitric oxide (NO), causing adjacent tissue damage and an amplified inflammatory reaction.19 Cytokines increase the activity of inducible nitric oxide synthase, enhancing NO production, and decreasing myofibril response to calcium, which contributes to focal rupture of actin-myosin interactions, resulting in ventricular dilatation.7,12,14,20–22 As in the case of cytokines, NO and ROS cause down-regulation of β-adrenergic receptors and depression of post-receptor signaling pathways, further depressing myocardial function.18
Finally, despite the usual increase of coronary blood flow characteristic of sepsis,13 there is an imbalance between oxygen delivery and consumption.12,15,21 Cardiac microcirculation undergoes major changes during sepsis with endothelial disruption and blood flow maldistribution, which can worsen cardiac function, contributing to the multi-organ failure of sepsis.24
However, therapies aimed at targeting a single mechanism or pathway have not been effective in improving outcomes in these patients.7Figure 1 summarizes the pathophysiology of SIMD.
DiagnosisClinical features suggestive of a diagnosis of SIMD include: a prior history of HF, a “septic cool extremities phenotype” on clinical exam, hemodynamic instability despite vasopressor therapy, failure to respond to a preload challenge, an abnormal cardiac function and elevated cardiac biomarkers. For the diagnosis of SIMD, it is essential to use imaging techniques and cardiac biomarkers.
Imaging techniquesThe diagnosis of SIMD can be based on several cardiac parameters (such as CI, ejection fraction and EDV), which over the years have been obtained using different methods.
Pulmonary artery catheter (PAC) has been proven to be inadequate to diagnose SIMD and, currently, there is no endorsement for its use in sepsis.25 LV filling pressures do not reflect ventricular preload in this population because of the underlying changes in ventricular compliance and the reduced systemic afterload, meaning cardiac index measurement by PAC are sensitive in detecting myocardial intrinsic depression (e.g., CI can remain high despite SIMD).20 Furthermore, intense catecholaminergic stimulation, that sustains a frank hyperdynamic state, tends to cover up depressed ventricular function in septic shock patients.21
Using a portable radionuclide cineangiography technique, Parker et al.11 detected decreased biventricular EF (with LVEF<40%), despite normal to high CI in 50% of patients included. The author11 and Weisel et al.26 introduced the concept of intrinsic ventricular contractile dysfunction for the first time, concluding that a substantial portion of the hypotension observed in septic patients was due to primary alterations in systolic cardiac function, rather than the consequence of dysfunction originating from the peripheral circulation and the absolute or relative intravascular volume depletion. Parker also demonstrated a paradoxical relationship between decreased LVEF and lower mortality.3,11 However, subsequent studies, using echocardiography, have not confirmed these results.3,27 After correction of arterial vasoplegia, which occurs in sepsis, cardiomyopathy was fully unmasked.28
Echocardiographic variables have been shown to have a diagnostic as well as prognostic importance in SIMD. Advanced echocardiography provides greater detail regarding myocardial function and hemodynamics, and includes modalities such as three-dimensional echocardiography, color or spectral Doppler imaging, tissue Doppler imaging (TDI) and myocardial strain studies using speckle tracking echocardiography (STE).5
Left ventricular ejection fraction has been used to categorize the severity of septic systolic ventricular dysfunction, using semi-quantitative techniques: mildly (LVEF 41–51%), moderately (30–40%) or severely abnormal(<30%).29 However, LVEF is an imperfect measure of LV systolic function in SIMD, since it is dependent on LV contractility, preload and afterload conditions, which are in constant variation in sepsis.5,21,25,30 In the hyperdynamic phase of septic shock, despite high LVEF (typically >55%), SV is low because of insufficient cardiac preload due to a high vascular permeability and vasodilation.19 Later, we observe low EF with normal SV, due to the reduced afterload.
As stated, the adaptive cardiac response to an acute reduction in LV contractility is ventricular dilatation, which preserves cardiac output (CO) via the Starling mechanism.18 The presence or absence of this adaptive response may be more important than LVEF itself and it appears to have major prognostic implications.5,31 Indeed, during the late phase of sepsis, non-survivors had lower LV EDV, suggestive of a persistent preload deficiency.19 Systolic function, assessed by LVEF, is overestimated in cases of severe septic vasodilatation,4 and, therefore, it fails to detect early changes of septic cardiomyopathy, and may have neither diagnostic nor prognostic purposes in septic patients.26,32,33
Left ventricular diastolic dysfunction (DD) occurs frequently during severe sepsis and septic shock.34 Mitral annular early diastolic peak velocity obtained by TDI (e′ wave), that reflects LV relaxation, is one of the most load independent measures of intrinsic DD.3 The ratio of early mitral inflow velocity (E), recorded with pulsed-wave Doppler, to the e′ wave (E/e′) correlates with LV end-diastolic pressure (EDP), and high E/e′ ratio represents low LV compliance (elevated EDP), which can be seen in SIMD.3 However, in this condition, E/e′ ratio does not correlate with compliance as accurately as it does in congestive HF, where it is much higher, and, therefore E/e′ is an imperfect surrogate of LV EDP in sepsis and septic shock.35
Landesberg et al.31 concluded that DD (defined as mitral e′ <8 cm/s) was the strongest predictor of mortality in SIMD (OR=0.7, p<0.001), even when adjusted to strong variables including Acute Physiology And Chronic Health Evaluation II (APACHE II) score, low urine output, low CI and hypoxemia. The reason for the worse prognosis in patients with DD, which has yet to be confirmed, could be their poor tolerance to fluids.36
Peak systolic velocity measured at the mitral annulus (mitral s′) is another adequate surrogate to estimate LV systolic function, since it reflects LV contractility, and it correlates with LV EF.32,37 Mitral s′ seems to be less preload dependent than EF, which is important in the context of septic shock.34,38 Septic survivors have lower values of mitral s′ versus non-survivors, with a cut-off of 9 cm/s predicting 90-day mortality.32 However, Golowenko et al.39 found that the correlation between s′ and CO was stronger in HF patients (R=0.48, p=0.021) than in the septic group (R=0.34), not supporting the use of an individual's s′ to estimate CO in sepsis. Also, low vascular resistance in sepsis reduces afterload and hence potentially increases mitral s′.39
Speckle tracking echocardiography, first described in 2004, is one of the most recent and accurate echocardiographic methods to assess myocardial function, in multiple cardiac events, including SIMD, since it is less affected by changes in ventricular loading conditions and myocardial compliance.5
The most consistently reproducible measurement of cardiac function, using STE, is global longitudinal strain (GLS).5 STE enables the detection of subtle subclinical myocardial dysfunction early during illness.9,24
Chang et al.,40 in a cohort of 111 septic patients, found that LV GLS can detect early cardiac dysfunction even in patients with preserved LV EF (>50%) and a value of LV GLS ≥−13% was an independent predictor of intensive care unit and hospital mortalities (hazard ratio (HR)=4.34 and HR=4.21, respectively). In this study, there were no significant differences in LVEF, mitral e′, or tricuspid s′ between non-survivors and survivors. Furthermore, LV GLS appeared to add incremental prognostic information over the APACHE II score, enabling early identification of high-risk septic patients.40
Using STE measurements, Dalla et al.,41 found that LV GLS was 18% lower in patients with sepsis than in trauma patients (controls). Fifty percent of septic patients with normal LVEF (>50%) had decreased LV function (GLS>−15%) compared to 8.7% of non-septic patients,41 concluding that STE could detect early SD in septic patients, before EF and CO changes could be appreciated by conventional imaging.
Finally, in an attempt to find a difference in the severity of myocardial dysfunction between sepsis and septic shock, Ng et al.25 demonstrated that patients with septic shock have more myocardial impairment compared to patients with sepsis only (GLS of −14.5% versus −18.3%, respectively). LVEF failed to detect this difference. The same authors also demonstrated the reversibility of SIMD, comparing GLS at diagnosis and upon recovery (−14.5% versus −16.0%, p=0.010).25 Opposing this finding, De Geer et al.4 found, for the first time, that LV GLS remained unchanged over time, unlike LVEF and e′, which speaks against SIMD reversibility. Decreased GLS can represent more subtle changes in the myocardium in septic shock that persist even after clinical recovery and these changes may be detected with more sensitivity with cardiac MRI. Further studies are needed.
Regarding right ventricular (RV) dysfunction, a condition seen in up to 60% of all patients presenting with septic shock, is often present in association with LV SD and/or DD.5 RV dysfunction involves decreased RVEF and RV dilatation and, in sepsis, it is multifactorial. It can be a manifestation of the underlying infectious process, a response to aggressive volume expansion during the early management of sepsis; or due to an increase in RV afterload due to hypoxemia, hypercapnia and aggressive positive pressure mechanical ventilation for acute respiratory failure, which also decreases preload and thereby worsens RV performance.42 Assessment of RV DD in septic patients and its prognostic implications remains an area for future research.5
Reduced tricuspid annular plane systolic excursion (TAPSE), <17 mm, indicates abnormal RV function, and correlates with increased mortality in critical illness as well as in SIMD.9,43 Gajanana et al.,44 in a cohort study with 120 critically ill patients, found that tricuspid annular plane systolic excursion <2.4 cm was the best cut-off for predicting in-hospital and long-term mortality. However, this study did not include patients with sepsis or septic shock nor patients with SIMD.
In contrast in a cohort study conducted by Vallabhajosyula et al.,42 with 388 septic patients, 55.2% with RV dysfunction and 25.8% with isolated RV dysfunction (defined by TAPSE<16 mm and tricuspid s′<0.15 cm/s), it was shown that RV dysfunction was not an independent predictor of one-year survival; however, isolated RV dysfunction was (HR=1.6).42
Finally, CMRI is a promising technique in SIMD. One of the first reports including two patients with SIMD studied with CRMI45 describes wall motion abnormalities with decreased LV contractility (LVEF of 24 and 40%, respectively), mild and moderately increased size of LV EDV (58 and 88 mL/m2, respectively), homogeneous myocardial enhancement on T2-weighted images (compatible with edema), and viability on all analyzed myocardial segments, with no evidence of myocarditis. After gadolinium injection, there was no myocardium late enhancement on the LV wall, reflecting the presence of viable myocardium.45 Within a few days, myocardial dysfunction and dilatation had been completely reversed in these patients. Control CMRI was performed six weeks after the first imaging exam in the first patient and it showed a complete recovery of cardiac contractility.45 Myocardial perfusion and viability were also normal, with no evidence of scar or inflammation.
Once disrupted, the endothelium can cause heterogeneous microvascular flow and myocardial edema. CMRI and histology show that myocardial edema may in part explain the rise of cardiac troponin seen in SIMD.16
Cardiac biomarkersBrain natriuretic peptide (BNP), troponin-T and I (TnT and TnI, respectively) are three of the most studied cardiac markers in the context of multiple heart diseases, including SIMD.
Brain natriuretic peptide is a cardiac biomarker of LV dysfunction and increased LV filling pressure. Plasma BNP concentration can be elevated in patients with septic shock in cases of systolic myocardial dysfunction and may serve as a useful biochemical index of myocardial depression early in sepsis.13,22 In addition to LV or RV pressure and/or volume overload, other stimuli may be involved in the release of this peptide, mainly the intensity of inflammation, vasopressors use and acute/chronic renal failure. In SIMD, BNP plasma level >190 ng/L can differentiate survivors from non-survivors, with a sensitivity of 70% and a specificity of 67%.7 BNP values should be obtained not only at admission but also throughout a hospital stay, due to the dynamic changes in their plasma concentrations during sepsis and septic shock.46 Brueckmann et al.22 found a correlation between compromised LV function based on echocardiographic data obtained from 29 septic patients on day two and NT-proBNP values (R=0.41, p<0.05).
The cardiac-specific contractile protein TnI is elevated in septic patients, probably due to non-ischemic mechanisms, such as cell apoptosis, permeability changes and loss of integrity in myocyte cell membrane with consequent cytosolic TnI leakage,8,22 direct cellular toxicity due to excessive catecholamine levels, increased myocardial wall stress by pressure or volume overload and renal dysfunction.47 Cardiac troponins correlate with the presence of LV SD, DD and RV dysfunction on echocardiography of septic patients.8
Landesberg et al.31 showed that both high sensitive-TnT (hs-TnT) and NT-proBNP are significantly higher in septic patients with reduced and with preserved LVEF and only DD (e′<8 cm/s), than in septic patients with normal systolic and diastolic function. Their results showed that both hs-TnT and NT-proBNP predicted in-hospital mortality, even after adjustments for highest creatinine levels. TnT values were only minimally elevated whereas NT-proBNP values were markedly elevated, suggesting that myocardial wall stress and dilatation can be very significant in sepsis and septic shock, but myocardial necrosis or apoptosis is minimal.31
Other investigations failed to detect enough specificity in NT-proBNP and hs-cTnT to diagnose SIMD. In fact, there is a high heterogeneity among studies results8,22,31,47 regarding cardiac biomarkers in sepsis and it may be because their concentrations above the reference range are common and nonspecific to this condition.9
Secretoneurin is a recent biomarker, whose production is augmented in states of myocardial dysfunction by neuroendocrine and myocardial tissues.48 It seems to improve risk prediction in patients critically ill with myocardial dysfunction.48 Currently, there are no studies on the use of secretoneurin in patients with SIMD, and it is unlikely that it can be used as a surrogate marker for echocardiographic indices in patients with severe sepsis or septic shock; but rather, it provides additional information alongside echocardiographic indices of myocardial function.48
Neutrophil gelatinase-associated lipocalin (NGAL) is an endogenous bacteriostatic protein secreted by neutrophils, macrophages and renal tubular cells, mostly known as the novel early marker of acute kidney injury.49 Even though plasma NGAL is elevated in the presence of coronary artery disease and HF, its role in patients with SIMD has not yet been identified, since there are no studies in this population.
TreatmentThe mainstream of sepsis management is fluids, non-specific organ support (vasopressors, inotropes, renal replacement techniques, artificial ventilation) with optimization of hemodynamic parameters and antibiotic therapy according to the infection origin. No specific treatment exists for SIMD.20
Fluid therapy with crystalloids is the cornerstone of early sepsis management, as recommended by Surviving Sepsis Campaign (SSC)50 and it should be initiated with an intravenous fluid challenge of 20 mL/kg to increase preload and thereby CO.19,51 Recent research suggests that hypodynamic shock is a mere manifestation of inadequate volume resuscitation and may be prevented by appropriate early volume loading.23 However, it is being increasingly recognized that over fluid resuscitation, in the days following initial sepsis resuscitation, can have detrimental effects. The risk of pulmonary edema is particularly elevated due to increased permeability of the pulmonary microcirculation and vasodilation, causing prolonged artificial ventilation.19,51 Positive fluid balance is a poor prognostic factor, and this effect is likely to be more dramatic in patients with septic cardiomyopathy because of the abnormal Frank–Starling relationships.17
Current SSC Guidelines recommend continuous hemodynamic monitoring and the use of dynamic variables predictive of “fluid responsiveness” to guide fluid resuscitation.50,51
The role of echocardiography in this scenario is crucial. First, it can evaluate or exclude the presence of SIMD, for which it is mandatory to have a prior (pre-sepsis) evaluation. Second, fluid responsiveness can be predicted by echo variables, such as the collapsibility index of the superior vena cava, by transesophageal echocardiography,36 or the collapsibility or distensibility index of the inferior vena cava, by transthoracic echocardiography. Third, after initial resuscitation, echocardiography identifies patients with persistent hypovolemia or fluid responsiveness, and so, it can help to optimize volume status and to avoid fluid overload,36 including the monitoring of LV filling pressure with E/e′.
The loss of vascular tone secondary to arterial vasodilation is among the main factors behind hemodynamic instability in septic patients and contributes to fluid resuscitation resistance. Norepinephrine (NE), an α and β-agonist, is the first-choice vasopressor in patients suffering from hypotension, despite adequate volume resuscitation, to restore adequate organ perfusion.19,50
Norepinephrine has a stronger α-adrenergic profile compared with β-1 which results in increased afterload more than increased myocardial function.17 While this is essential for the treatment of distributive shock, increasing afterload in SIMD causes CO to drop and could result in the “unmasking” of cardiac dysfunction. Repeated echocardiography could unmask LV systolic failure caused by sepsis after correction of LV afterload with norepinephrine.35 Epinephrine and dopamine have more favorable α to β-1 adrenergic activity profiles, consequently associated with more arrhythmias and increased afterload. The risk of arrhythmias and potential increased mortality has led to the recommendation against the use of dopamine in septic shock.50 The risk of isolated α-vasoconstriction without any β-1 of both vasopressors could cause increased afterload and decreased CO potentially worsening hemodynamics.17 Although catecholamines are the recommended first-line therapy for septic shock, high doses of administered catecholamines are associated with poor outcomes and severe side effects, including myocardial injury and peripheral ischemia.
In patients diagnosed with SIMD, with low CI and EF and persistent signs of hypoperfusion, despite the optimization of volume status, an inotropic agent is recommended to augment CO.50 Dobutamine is the first-line inotropic for SIMD.
Dobutamine is a synthetic catecholamine that acts on α-1, β-1, and β-2 adrenergic receptors.52 The SSC Guidelines support the use of dobutamine in the presence of myocardial dysfunction, indicated by elevated cardiac filling pressures and low CO or ongoing signs of hypoperfusion, despite achieving adequate intravascular volume and MAP.50 Although dobutamine leads to an increase in CI, myocardial oxygen demand also increases, thus increasing the risk of myocardial ischemia and tachyarrhythmias.52
The use of dobutamine in septic patients may also not be effective in patients with severe HF since myocardial adrenergic responsiveness is depressed among septic patients.52
Vieillard-Baron et al.53 found, in a cohort of 67 septic patients, that echocardiography could be used to guide inotropic treatment. Global hypokinesia was present in 60% of patients, and the administration of dobutamine plus a reduced dose of NE partially corrected LV hypokinesia. Hemodynamic improvement were observed within 24 hours. In contrast, the administration of NE (alone) in the absence of hypokinetic state in septic shock caused global LV hypokinesia within 24 or 48 hours of continuous NE infusion.53 This can be explained by the fact that, by increasing afterload, NE unmasks potential myocardial failure.53 Increasing norepinephrine dosage in a patient exhibiting global LV hypokinesia associated with a drop in arterial pressure is logically expected to worsen hypokinesia. For this reason, the authors considered that, when global hypokinesia is found, hemodynamic support should be modified, reducing norepinephrine dosage and adding an inotropic agent.53
Levosimendan, a calcium-sensitizing drug with both inotropic and vasodilatory properties,50,54 was also proposed in sepsis. Currently there are no studies about the use of levosimendan in patients with SIMD. The LeoPARDS54 study assessed the efficacy of levosimendan to reduce acute organ dysfunction, including the heart, in patients with septic shock (regardless of EF) versus standard of care (e.g., fluids, NE and dobutamine). The study showed no differences between both arms and there was no difference in terms of 28-day mortality. In a meta-analysis of seven RCTs, Bhattacharjee et al. found that levosimendan had no benefit in terms of mortality at the longest follow-up and length of ICU stay in comparison to dobutamine in patients with sepsis and septic shock with myocardial dysfunction. Norepinephrine requirements were similar in both groups.
However, due to the higher cost of levosimendan, dobutamine remains the inotropic treatment of choice.50
In conclusion, judicious fluid resuscitation and a balanced infusion of inotropic and vasopressor agents can reverse tissue hypoperfusion and attenuate sympathoadrenergic stress. Potential therapies to reduce excessive adrenergic stress comprise temperature and heart rate control, adequate use of sedative/analgesic drugs and supplementary hydrocortisone and/or vasopressin infusion.55,56
Finally, extracorporeal life support, commonly referred to as extracorporeal membrane oxygenation (ECMO), provides cardiopulmonary support to patients with refractory cardiovascular failure of various etiologies and should be considered for septic cardiomyopathy with intractable circulatory failure.57
Sepsis-induced cardiogenic shock, presumed due to SIMD, is considered a potential indication for venous-arterial (VA) ECMO, as a bridge to recovery, because SIMD can be reversed in seven to 10 days; and appears to be associated with better prognosis.57 VA- ECMO increases afterload and decreases SV but provides systemic perfusion in the place of an injured heart.57
In a cohort of 14 refractory septic shock patients, VA-ECMO was indicated in case of acute refractory cardiovascular failure defined as the presence of tissue hypoxia (such as extensive skin mottling or elevated blood lactate), despite adequate intravascular volume, severely altered LV EF (<25%), low CI (<2.2 L/min/m2) and sustained hypotension despite infusion of very high-dose catecholamines.58 Results showed that the median time on ECMO was short (5.5 days (2–12) days), and median LV EF was 60% (40–70%) when VA-ECMO was removed, compatible with the reversibility of SIMD.58 Based on this assumption, the authors hypothesized that VA-ECMO could help salvage septic shock patients by restoring adequate perfusion to vital organs to reverse multiple organ failures buying time to achieve infection control by antibiotics.58
Veno-arterio-venous extracorporeal membrane oxygenation (VAV-ECMO) is another feasible rescue strategy for a small proportion of adult patients with combined respiratory and cardiac failure secondary to septic shock with septic cardiomyopathy.59 In a small cohort of patients supported with VAV-ECMO59 with 12 septic patients, all had cardiac dysfunction with median LV EF of 16% at time of cannulation. It was shown that, compared with ischemic cardiomyopathy, the rate of improvement was quicker and the need for additional support was lower in patients with SIMD,59 with better survival (reported as 75%). Currently, there are no studies comparing the use of VA-ECMO and VAV-ECMO in patients with SIMD. However, it is known that VAV-ECMO has a higher rate of thrombotic events, therefore VA-ECMO should be used as first-line in SIMD until further studies arrive.
Finally, in a very recent retrospective, multicenter, international, controlled trial60 that included 82 patients with sepsis-induced cardiogenic shock refractory to conventional treatment, it was shown a large and significant improvement in survival at 90 days in septic patients using VA-ECMO compared with controls not receiving VA-ECMO (mortality 60% vs. 25%, RR for mortality was 0.54, p=0.0029). All patients had severe myocardial dysfunction (EF<35%) and had severe hemodynamic compromise (severe lactic acidemia despite receiving high doses of catecholamines and aggressive fluid therapy). VA-ECMO permitted a rapid decrease in vasopressor dose and restored adequate perfusion of vital organs, as indicated by relatively rapid decreases in serum lactate. Improvement of cardiac function was also relatively quick and permitted weaning from ECMO support after six days in survivors; a shorter time than reported in patients who receive ECMO for primary cardiogenic shock.60 Therefore, early identification of this specific group of patients, which might represent up to 10% of all patients with septic shock, and the early application of VA-ECMO support, could be crucial to improving their outcomes.60 However, this study was not randomized, and thus is open to potential biases. Prospective, randomized, controlled trials in this field are needed.
PrognosisPrognosis in SIMD is not clear, probably because of the multiple variabilities involved, such as age, patient history, type of microorganisms and time to resuscitation.15,61
Over the years, since Parker's studies, different parameters, from imaging techniques to laboratory biomarkers have been investigated as potential SIMD prognostic markers; however their results have been inconsistent and their prognostic value remains debatable. Table 1 summarizes the principal studies and their results on prognosis.
Summary of prognostic factors among sepsis-induced myocardial dysfunction studies.
| Reference | Population | Measure | Results and conclusions |
|---|---|---|---|
| Parker et al.11 | N=20 septic patients | LVEF by radioisotopic ventriculography | 50% of patients with LVEF<40%Low LVEF was associated with lower mortality. |
| Brueckmann et al.22 | N=57 patients with severe sepsis | NT-proBNP and NT-proANP measured on second day of sepsis, LVEF by TTE, performed in 29 patients | High NT-proBNP was associated with higher mortality (R=3.9, p<0.01) and severity of disease (higher APACH II, R=0.42, p<0.05), NT-proANP did not associate with mortality (p=0.29).NT-proBNP values correlated with the compromise of LV function (R=0.41, p<0.05). |
| Huang et al.27 | Meta-analysis of 14 studies (N>750 patients with sepsis or septic shock) | LVEF, RVEF, LV end diastolic diameter (LVEDD) or volume (LVEDV) and RV end diastolic diameter (RVEDD), volume (RVEDV) by radionuclide, PAC-TD, TTE and TEE | Low EF had no correlation with mortality.RVEF and RV dimensions had also no correlation with mortality. |
| Palmieri et al.30 | N=115 septic patients | LVEF and GLS by TTE with speckle tracking analysis | Mortality was higher in patients with GLS close to 0, in a 28-days follow-up.GLS showed correlation with mortality at 28-days follow-up (HR 1.16, p=0.05).LVEF had no prognostic significance. |
| Landesberg et al.31 | N=262 septic patients | LV volume, LVEF, mitral e′ and E/e′ by TTE with TDI measurements hs-TNT and NT-proBNP | LV dilation rather than LV SD had prognostic significance.E/e′ (OR=1.16, p<0.001) and mitral e′ (OR=0.7, p<0.001) were the strongest predictors of in-hospital mortality.DD (e′<8 cm/s) or SD+DD associated with higher mortality compared to those with no SD or DD.Both hs-TNT and NT-proBNP predict in-hospital mortality. |
| Brown et al.34 | N=78 septic patients | E/e′, E/A, e′ for DD and EF for SD using spectral Doppler of mitral inflow by TTE | Grade I DD was associated with 28-days mortality (OR=1.33, p=0.03).Grade II and III DD did not associate with mortality.Within the group of patients with grade I DD, those with low filling pressures had worse outcome than those with higher filling pressures. |
| Santos et al.37 | N=63 septic patients | s′ velocity, e′ wave and E/e′ by TTE with TDI measurements | Mean mitral s′ velocity and e′ wave were lower among non-survivors (s′: 13.25 vs. 7.33 cm/s, p<0.0001; e′: 16.4 vs. 9 cm/s, p=0.0025).Mean E/e′ was higher among non-survivors (10.85 vs. 5.63 cm/s, p<0.0001).Higher E/e′, s′ and e′ correlated with higher severity scoring systems, mainly SOFA (R=0.46, R=−0.36 and R=−0.40, respectively p<0.005). |
| Sanfilippo et al.38 | Meta-analysis of 7 studies with 636 septic patients | Septal or lateral e′ and LVEF by TTE with TDI measurements | DD was correlated with higher mortality (R=1.82, p=0.02), but SD had no correlation with mortality (R=0.93, p=0.73).However, SD+DD was associated with higher mortality (R=2.06, p=0.05). |
| Chang et al.40 | N=111 septic patients | LVEF, GLS of LV, RV s′ and e′ wave by TTE with TDI and speckle tracking analysis | LV GLS≥′13% predicted in-hospital mortality with a sensitivity of 76% and a specificity of 82%.No significant difference in LVEF, mitral e′, or tricuspid s′ between non-survivors and survivors. |
| Landesberg et al.47 | N=106 patients with severe sepsis and septic shock | E/e′, e′ and RVESV index by TTE with TDI analysishs-cTNT measurements | RV ESV index (OR=1.05, p=0.038) and mitral e′ (OR=0.02, p=0.004) were independent predictors of in-hospital mortality.Concomitant, hs-cTNT also predicted in-hospital mortality in univariate analysis (OR=4.8, p=0.004). |
| Sturgess et al.62 | N=21 septic patients | E/e′ measured by TTE with TDI measurementsTNT, BNP and NT-proBNP | E/e′ was higher among non-survivors (9.05±2.75 cm/s vs. 15.32±2.74 cm/s, p=0.0002).E/e′ was an independent and better predictor of hospital outcome compared to cardiac biomarkers (NT-proBNP and TNT). |
| Orde et al.63 | N=60 septic patients | RV free wall strain, LVEF and GLS of LV by TTE, with speckle tracking analysis | Severe RV free wall dysfunction (RV free wall strain<-13%) was the only measure parameter associated with 6-months mortality (OR=1.1, p=0.02).LV dysfunction had no mortality impact on 30-days or 6-months follow-up. |
| Pulido et al.64 | N=106 septic patients | LVEF, é, ś, E/é and mitral inflow pulsed wave Doppler measurement of peak E and A waves, E/A ratio by TTE with TDI measurements and speckle tracking analysis | There was no difference in LVEF, CO, or E/é between 30-day survivors and non-survivors. There was no significant difference in 1-year mortality between normal cardiac function group vs. DD (E/e′>15) group, LV SD group (LVEF≤50%) and RV dysfunction group (tricuspid s′<15 cm/s). |
| Papanikolaou et al.65 | N=42 septic patients (12 with sepsis and 30 with septic shock) | Serial measurements of BNP over 5 daysLVEF by TTE and PAC-derived RVEF | BNP and maximum dose of NE, on day 1, and RVEF were independent predictors of 28-days mortality (HR=0.999, HR=1.085, HR=0.873, respectively). BNP in serial measurements correlated with greater disease severity (assessed by APACHE II and SOFA scores).BNP values did not predict 28 days mortality, however, BNP >800 pg/mL at day 1 (AUC=0.70, p=0.03) and >840 pg/mL at day 2 (AUC=0.68, p=0.044) did it. Sepsis-induced LV and RV systolic dysfunction were not independent predictors of BNP elevation. |
| Raja et al.66 | N=54 septic patients (50% with myocardial dysfunction, LVEF<55%) vs 54 healthy controls | TNT and NT-proBNP serum concentration | TNT values (0.8±1.5 vs 0.05±0.1 ng/mL, p=0.006) and NT-proBNP values (14991 pg/mL vs 3134 pg/mL, p=0.006) were higher in non-survivors vs. survivors. |
APACHE II: Acute Physiology and Chronic Health Evaluation II score; AUC: area under the curve; CI: confidence interval; DD: diastolic dysfunction; e′-wave: mitral annular early diastolic peak velocity; E/A: ratio of the peak mitral inflow velocity during early diastole (E) to the peak mitral inflow velocity of the atrial phase (A); E/′: ratio of the early mitral inflow velocity (E) to the mitral annular early diastolic peak velocity (é wave); GLPS: global longitudinal peak strain; GLS: global longitudinal strain; HR: hazard ratio; hs-TNT: high sensitive troponin T; LV: left ventricle; LVEF: left ventricular ejection fraction; NT-proANP: N-terminal proAtrial natriuretic peptide; NT-proBNP: N-terminal proBrain natriuretic peptide; OR: odds ratio; PAC(−TD): pulmonary artery catheter (−thermodilution); RV: right ventricle; RVEF: right ventricular ejection fraction; RV ESV: right ventricle end-systolic volume; s′: peak systolic velocity measured at the mitral valve annulus; SD: systolic dysfunction; SIMD: sepsis-induced myocardial dysfunction; SMD: standardized mean difference; TAPSE: tricuspid annular plane systolic excursion; TDI: tissue doppler image; TEE: transoesophageal echocardiography; TTE: transthoracic echocardiography; TNT: troponin T.
Since the early 19802, septic cardiomyopathy has been an increasingly recognized clinical entity. Myocardial dysfunction is only one of the organ dysfunctions that can be present in a patient with sepsis and its presence is associated with worse prognosis. Currently, diagnosis of SIMD is based on echocardiographic variables and the presence of high serum cardiac biomarkers, in a sepsis patient in a state of HF. With the advent of new echocardiographic techniques such as STE and TDI, it is possible to identify cardiomyopathy at an early stage in the natural course of SIMD. As there are no specific treatments for septic cardiomyopathy, the current sepsis guidelines represent the cornerstone of therapy. Physicians are encouraged to use VA-ECMO for patients refractory to conservative therapy, given the reversible nature of this condition. Prognosis associated with this condition is not consistent across all studies, therefore further evidence is needed.
Conflicts of interestNone declared.