Percutaneous coronary intervention is currently the most common form of revascularization for symptomatic coronary artery disease. In elderly, diabetic and renal patients, there is an increased prevalence of calcified coronary disease. Rotational atherectomy (RA) can be useful in the treatment of these lesions.
Plaque removal was initially proposed as an alternative to balloon angioplasty, hence RA required high-velocity protocols with large-sized burrs (over 2.0 mm). With a high incidence of acute complications and disappointing restenosis rates, the use of RA dwindled. However, the advent of drug-eluting stents, which significantly decreased the rate of restenosis, led to the repositioning of RA as an adjunctive technique in the preparation of densely calcified lesions, improving stent delivery and expansion.
In recent years, a better understanding of the mechanism of action of RA has changed it from a plaque debulking to a compliance modifying technique. As a result, RA has become less aggressive, using smaller size burrs and lower rotational speeds. This conservative approach has improved immediate results, with increased safety and better long-term outcomes.
In this review paper, the technique of RA is explained in the light of current knowledge.
A angioplastia coronária tornou-se a forma mais habitual de revascularização miocárdica na doença coronária sintomática. Na população mais idosa, nos diabéticos e nos doentes com insuficiência renal crónica, a prevalência de lesões calcificadas tende a aumentar. A aterectomia rotacional (AR) é uma técnica de grande utilidade no tratamento destas situações.
Tendo-se considerado inicialmente que a erosão da placa podia constituir uma alternativa à angioplastia de balão, a técnica de aterectomia rotacional utilizava protocolos de alta velocidade com olivas que ultrapassavam os 2,0 mm de diâmetro. A ocorrência de complicações agudas e resultados insuficientes a longo prazo limitaram a sua difusão. O aparecimento dos stents com fármaco com redução significativa das taxas de reestenose, levou ao reposicionamento da aterectomia rotacional como técnica adjuvante na preparação das lesões permitindo uma melhor entrega e expansão dos stents, no contexto de doença aterosclerótica calcificada.
Nos últimos anos, tem-se assistido a uma melhor compreensão do mecanismo de acção da aterectomia rotacional que passou de debulking para modificação de compliance da placa. Consequentemente, a técnica tem-se tornado menos agressiva: utilizam-se olivas de menor calibre, com menor velocidade de rotação, alcançando-se elevadas taxas de sucesso e de segurança no procedimento.
Neste artigo procuramos reunir o conhecimento atual sobre como executar a técnica de aterectomia rotacional.
Percutaneous coronary intervention (PCI) is an essential tool for the treatment of obstructive coronary atherosclerotic disease.1 Over 36 years since its inception,2 the technique has evolved, with greater operator expertise and device innovation. Guide catheters, guidewires, balloons and stents have all developed to allow intervention operators to treat complex coronary lesions with excellent outcomes.
One challenge to successful revascularization is the calcified obstructed coronary vessel.
The process of vascular calcification is poorly understood. It is more common in the elderly, and in those with diabetes and renal impairment, but the precise molecular mechanisms are yet to be unravelled. As the populations ages, with better survival across the medical spectrum, it is now increasingly common for patients with calcific coronary disease to present for intervention.
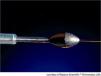
First used in 1989, rotational atherectomy is based on the differential removal of plaque by a rotating diamond-covered burr (Figure 1). The pulverized debris, 7.0–7.5 μm in diameter (the size of a red blood cell), is easily washed away. Additionally, this technique minimizes wall stress.3
Rotational atherectomy has been frequently labelled a calcium-removing technique,4 as this would be the inelastic component with the highest density; however, recent in vivo studies using intravascular ultrasound5 suggest that fibrous tissue is one of the major components to undergo ablation.
The early experience with rotational atherectomy was documented in several trials and registries during the 1990s.6 The first trials compared rotational atherectomy with other plaque-reducing (debulking) techniques such as directional coronary atherectomy (DCA) and laser therapy7,8 that are seldom used today. In these studies, rotational atherectomy achieved good immediate results, but, in the absence of a stent, the restenosis rate was very high and its use dwindled.9
Since the advent of drug-eluting stent (DES) technology, there has been a resurgence in rotational atherectomy.10,11 Its use in modern intervention is not as a stand-alone debulking device, but more as a modifier of calcific lesions12 to allow balloon dilatation and stent deployment.
The indications for this plaque modification technique have widened (Table 1): in addition to the classic indications (densely calcified lesions),13,14 they now include diffuse atheromatous disease in which long stents are required, in-stent restenosis,8,15 calcified ostial lesions16 and chronic total occlusions.17,18 Since rotational atherectomy avoids plaque prolapse or shifting, it could be particularly useful in bifurcation lesions, leading to better preservation of side branches.19–21
Indications for rotational atherectomy.
| Classic indications (plaque debulking) |
| Densely calcified lesions |
| Lesions with inadequate balloon expansion |
| Widened indications (lesion preparation, plaque modification) |
| Long diffuse disease |
| Small vessels (<2.5 mm) |
| Diffuse in-stent restenosis |
| Ostial lesions |
| Bifurcating lesions |
Appropriate lesion preparation is one of the most important determinants of proper stent expansion and apposition, which are themselves protective factors against stent thrombosis and restenosis.22 In the latest guidelines for myocardial revascularization,1 rotational atherectomy is therefore recommended “for preparation of heavily calcified or severely fibrotic lesions that cannot be crossed by a balloon or adequately dilated before planned stenting” (class I indication, level of evidence C).
There have been no randomized trials comparing DES implantation in densely calcified lesions with or without rotational atherectomy, however several publications14,23 suggest good outcomes on follow-up of patients pre-treated with rotational atherectomy. It should be noted that in some cases, the intervention was successful only after rotational atherectomy, which enabled the balloon to cross or the stent to be delivered. The more familiar the intervention cardiologist is with such techniques, and make them part of his/her routine, the more quickly and smoothly they are performed.
In this paper we describe these ‘modern’ rotational atherectomy techniques.
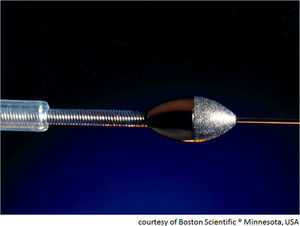
Rotational atherectomy: tools and techniqueThe best studied rotational atherectomy device is the Rotablator® (Boston Scientific, MN, USA). The system includes the Rotablator console and foot pedal, the RotaLink Advancer and burrs (1.25–2.5 mm), RotaWire floppy or intermediate guidewire, RotaLink burr catheter, and compressed air or nitrogen at 7 atm with 140 l/min flow (Figure 2).
The whole system is flushed by continuous saline infusion in order to avoid overheating, lubricate the tube and facilitate debris washout. It is recommended that each 500 ml of flush be prepared with heparin 5000 IU, verapamil 5 mg and isosorbide dinitrate 5 mg. The whole system must be tested before advancing each burr. The console generates the energy to rotate the burr and controls the rotational speed, while the pedal switches burr rotation on and off, and also selects Dynaglide (low speed) or ablation mode.
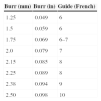
Access site and guide catheter selectionThe French size of the catheter is determined by the largest burr to be used. Table 2 shows the recommendations regarding appropriate caliber for burr size. 6F guides (1.854 mm) allow burrs up to 1.75 mm (1.753 mm). Guides should be 7F for burrs up to 2.0 mm and 8F for larger burrs, for instance in left main lesions.
The guide catheter should have the fewest possible bends and angulations; many operators prefer extra backup catheters (such as CLS, XB, voda or EBU) which provide good coaxial access and support.
Over the recent years, there has been an increase in the experience with the use of radial access for rotational atherectomy.24,25 There are few data on whether femoral or radial access is preferable,26 the choice being according to the operator's preference. The advent of large 7F sheathless guiding catheters27,28 has overcome the previous limitations of the radial approach for rotational atherectomy, and also reduces access site complications.29 This has meant that many operators tend to prefer a radial approach for this frail elderly population.
Guidewire selection and positioningIt is ironic that for the most complex calcified coronary lesions, we have the least forgiving wire. The RotaWire is a 300 cm stainless steel wire with a 0.014″ radio-opaque 22 mm tip and a long shaft, 0.009″ thick. It is fragile and prone to kinking. Extra care is required when handling this delicate spindly wire, as any angulations or bends in the wire facilitate fracture and hinder progression of the burr.
The two wires, either a floppy or an extra support RotaWire, are of limited torquability and push and have proved a hindrance to successful rotational atherectomy outcomes. The use of over-the-wire technology and microcatheters has largely overcome this limitation, allowing for easier access to the distal lumen. The criteria for RotaWire preference are similar to any other coronary intervention. The stiffer RotaWire rectifies sharp proximal angles, which allows better burr progression; this can increase the risk of asymmetric burring due to wire bias30 and threaten arterial wall trauma or perforation. As such, most operators prefer the floppy RotaWire, which follows the anatomy of the artery and keeps the burr better centered.31
Once inside the artery, the distal tip of the RotaWire must be free, non-prolapsing and away from the target lesion. There must be enough leeway for the burr to advance without reaching the radio-opaque end.
All additional wires must be removed before starting ablation, such as a ‘buddy wire’ or in bifurcation lesions where a second wire has been placed in a side branch. The burr will cut any wire in its path.
Burr selectionThe purpose of debulking is to modify the plaque sufficiently to allow balloon and stent use. Modern techniques use smaller burrs, which means there is less plaque pulverization, with less resistance, heat production and platelet activation. The complications of large burr use (impaired microcirculation with accompanying ST elevation, chest pain and bradyarrhythmia, perforation, or ‘stuck’ burrs) are rarely seen in modern practice.
The choice of initial burr should depend on the severity of the lesion, whatever the final diameter of the artery. Previous recommendations to keep within a burr:artery ratio of 0.70,32,33 (Figure 3) are now obsolete. What is more important is to determine whether the rotational atherectomy has caused sufficient plaque modification to allow adequate balloon expansion. If there remains a ‘napkin ring’ of calcium with balloon inflation, a stepwise increase in burr size can be used (in increments no greater than 0.5 mm).
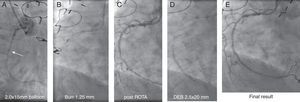
Angiographic images of an 83-year-old woman who underwent coronary artery bypass grafting in 1994, with Canadian Cardiac Society class III angina. A critical mid right coronary artery lesion was the culprit lesion. Radial access and a 6F Amplatzer AR1 guide were used. (A) A Terumo Runthrough floppy wire easily crossed the lesion, but a 2.0 mm×15 mm compliant Mini Trek balloon (Abbott) could not; (B) wires exchanged through the microcatheter and rotational atherectomy with 1.25 mm burr performed; (C) post-rotational atherectomy image; (D) further dilatation with a 2.5 mm×20 mm balloon followed by prolonged inflation of a 2.5 mm×20 mm drug-eluting SeQuent Please balloon (B. Braun); (E) final result.
If, in resistant cases, balloon inflation remains impossible despite the use of appropriately sized burrs, a cutting34 or scoring35 balloon can be used for further lesion preparation.
Burr positioningIt is important to ensure that the wire is very clean and wet, with the flushing solution dripping from the tip. The burr should be advanced with the greatest care to avoid sudden movements and kinking of the RotaWire.
Once in the guide catheter, many operators prefer manual pushing to introduce the burr. Some operators use the Dynaglide facility (rotational speed 70000 rpm) to improve burr deliverability and facilitate distal positioning of the wire. The low spinning velocity reduces friction between the burr, the catheter and the wire, improves sliding and reduces tension. The burr should be positioned around 15 mm proximal to the target lesion, in a segment that allows excellent flow and away from the radio-opaque tip.
AtherectomyIt has been shown that the high spinning rates initially proposed (170–200000 rpm) induce heat production in the plaque and arterial wall endothelium,36 which may lead to activation of inflammatory factors as well as triggering the clotting cascade and platelet activation37 and the release of larger particles, leading to a high incidence of slow-flow and no-reflow phenomena, which are rarely seen with smaller burrs and lower speeds.
While there is little evidence38–40 to support any one particular speed, contemporary rotational atherectomy operators use lower speeds of 130000–160000 rpm, which are equally successful with improved safety.
When about to start atherectomy the operator must ensure that there is enough leeway for the forward and backward motion of the burr. It is recommended to start approximately 15 mm proximal to the lesion. When ablating, the burr must be kept spinning at all times and must never stop distal to the target lesion.
Under continuous vasodilating heparinized infusion, the lesion is addressed by short repetitive movements (‘pecking’) of the RotaLink Advancer®, limiting engagement with the lesion to a minimum (less than 15 seconds) to prevent distal embolization complications.
It is important to be aware that the ablative surface of the burr is only on its distal half (Figure 1), and therefore it can only ablate while moving forward.
The noise produced by the spinning burr is a good key to the resistance the burr is encountering. While a drop in speed when the burr encounters resistance is expected, it should never drop by more than 5000 rpm. If this happens, it means the burr is facing too hard or too tight a lesion, and is an indication to downsize the burr diameter.
After the first burr has been advanced as distal as required and is now moving freely along the whole segment, the burr is frequently upsized for a second passage. It should be borne in mind that arteries taper distally, and hence larger burrs should not be advanced as distally as the first burr has reached.
For each new burr, all steps should be repeated: continuous dripping of flushing solution, pedal test, speed check in both modes and free movement of the RotaLink Advancer®.
ComplicationsOperators should be prepared for the occurrence of bradycardia or even sinus pauses or transient atrioventricular block, particularly when dealing with the right coronary artery or a dominant circumflex circulation. Some centers recommend the use of a temporary pacemaker wire in such cases, but with smaller burrs at lower speeds these phenomena are usually short-lived, with atropine occasionally being required. Some rotational atherectomy operators feel that the potential complications of pacemaker insertion outweigh the complications it is intended to resolve.
Coronary vasospasm is relatively frequent, in spite of continuous intracoronary infusion through the rotational atherectomy catheter and frequent flushing. Again, with good technique and a less aggressive burr strategy, it should be transient and without significant consequences.
Rotational atherectomy has the potential to disrupt a focal coronary dissection, and it is important after rotational atherectomy to ensure this has not taken place. If due care is taken, it is uncommon to have long, spiraling, occlusive dissections of the target vessel.
Abrupt vessel occlusion may be due to spasm, no reflow, dissection or vessel rupture. It usually reflects an aggressive approach.
Although it has been described, coronary artery perforation41,42 is rare following rotational atherectomy. It is more likely to occur with an oversized burr, continuous rather than pecking techniques, and extreme vessel tortuosity. There are a number of ways to deal with this complication depending on its severity, but it usually requires adequate resuscitation, pericardiocentesis, and a call for a cardiac surgeon in case restorative procedures are unsuccessful. In most cases, sustained balloon inflation can control bleeding and covered stents will usually restore vessel integrity.
Impairment of microcirculation can lead to slow flow and no flow. This happens when the burr is too large or the atherectomy was too aggressive or lengthy.43 Larger burrs produce larger particles which can cause microcirculatory obstruction. Once again, smaller burrs, frequent flushing, short pecking ablations and good systemic blood pressure all help to avoid this complication. In these situations, intracoronary verapamil or adenosine can improve flow. In patients with severely impaired left ventricular function, it may be useful to start intravenous inotropes or vasoconstrictors, or to place an intra-aortic balloon pump to prevent blood pressure drop and protect against transient reductions in coronary flow.
A rare but disturbing complication is burr stalling or entrapment, when the burr gets stuck within the vessel and is unable to move either way. This can be due to an oversized burr, lengthy burr displacements, excessively forceful strokes rather than pecking movements, or interruption of ablation with the burr distal to the lesion. Once stalled or trapped the burr should not be spun again, as this can cause vessel dissection by twisting the artery. Several techniques have been suggested to overcome this complication.44,45 One option is to pull firmly and continuously on the burr, being careful of the protrusion of the guide catheter into the artery, which can also cause proximal dissection. Another option is to use a second wire and attempt to inflate a very small balloon, at low pressure, at the site where the burr is stuck. This would displace the plaque away from the burr. If all fails, surgical removal and revascularization are required.
In a multicenter registry,46 complications were described in 6.34% of cases. Minor complications were most frequent (4.71%): focal dissections, transient spasm and flow impairment with ST elevation, transient thrombotic occlusion, slow flow and no reflow, transient second-degree AV block, distal dissections, one stuck burr that was easily pulled out, thrombocytopenia and inexplicable blood pressure drop attributed to vasovagal reaction. In this registry, 1.24% of attempts to cross the target lesion with a RotaWire were unsuccessful.
Major complications were reported in 1.56% rotational atherectomy procedures: one case of vascular rupture, controlled with a graft stent; one case of acute vessel thrombosis that persisted with TIMI 1 flow; extensive dissections in two patients and fracture of a side-branch wire which was retrieved with a snare.
From the clinical viewpoint, the procedures were very well tolerated. However, a mild rise in troponin was present in most cases following the procedure. When there is impairment in flow most patients feel chest pain.
Imaging techniquesIntravascular ultrasound (IVUS)47–49 or optical coherence tomography50–52 are frequently used to plan interventions of severely calcified lesions. When the lesion is very severe the IVUS probe will have difficulty in crossing it before initial rotational atherectomy.
These techniques are a guide to safe decision-making in rotational atherectomy; information on the severity of the residual lesion, the amount of remaining calcium, and its distribution is essential to determine the need for further lesion preparation, in addition to sizing of the vessel and assessing the length to cover with stents. Imaging details increase the safety and long-term success of the procedure.
Removing the burrRemoving the burr requires good coordination between the first and second operators. At Dynaglide spinning velocity, the burr is retrieved by simply advancing the RotaWire, which pushes the burr backwards without the need to pull it out. It is essential to monitor the tip of the wire carefully, and to use smooth, continuous movements, avoiding sudden jerks. Since the wire is barely visible under fluoroscopy, particular attention must be paid to loop formation in the aortic cusps. In an obese patient these loops can go undetected.
ConclusionsRotational atherectomy has re-emerged as an indispensable technique in the preparation of densely calcified vessels. With the aging of the general population, including those with diabetes and renal disease, the need to prepare calcified lesions in advance of stent placement is increasingly common, and this is reflected in wider use of this technology. When used by experienced operators, ‘modern’ rotational atherectomy is safe and effective, minimizing time and complications for patients with the most complex disease.53
As interventional cardiology becomes part of the routine treatment of ischemic cardiomyopathy, it is important for both operators and those who refer patients to be aware of progress in techniques. Only through familiarity with both innovations and limitations can clinical cardiologists appropriately advise their patients on the best treatment. Percutaneous coronary intervention with rotational atherectomy is frequently the only treatment option for some patients, but only those aware of its existence, and where it can be performed well, can change the quality of life of inoperable patients with severely calcified coronary artery disease.
Conflicts of interestThe authors have no conflicts of interest to declare.