Single coronary artery (SCA) with no associated congenital heart disease is a rare congenital anomaly. Most cases are asymptomatic and incidental findings, but SCA can cause ischemia, congestive heart failure, and sudden cardiac death (SCD).
Case reportA 44-year-old woman presented with Takotsubo cardiomyopathy and cardiogenic shock. Selective cannulation of the left coronary artery (LCA) was not possible on coronary angiography (CA); an SCA was revealed arising from the right sinus, continuing distally as the circumflex artery and thereafter as the left anterior descending artery. Coronary computed tomography angiography (CCTA) confirmed left main atresia and no coronary stenosis. Cardiac magnetic resonance imaging (MRI) showed diffuse myocardial edema and no perfusion defects. The patient's clinical course was favorable under conservative management.
DiscussionOur paper describes an incidental finding of right SCA. We report a Lipton type R-I, in which a dominant right SCA supplies the entire myocardium. It is the rarest SCA presentation, with an incidence of 0.0008%; only 15 cases have been reported in the literature, all of which were studied by CA. Of these 15, one had SCD, five angina, one ventricular arrhythmia and one complicated acute coronary syndrome. CCTA confirmed the diagnosis in seven patients, MRI in one and transesophageal echocardiography in another. Nine patients had coronary lesions. Two underwent coronary artery bypass grafting, one percutaneous intervention and 11 conservative treatment.
ConclusionRight SCA with congenital absence of the LCA is one of the rarest coronary artery anomalies. In a significant percentage of patients it is associated with ischemia and can be life-threatening. CCTA and MRI are the modalities of choice for diagnosis and risk stratification.
Artéria coronária única (ACU) sem cardiopatia congénita associada é uma entidade rara. Maioria dos casos é assintomática e achados acidentais, mas podem causar isquémia, insuficiência cardíaca congestiva e morte súbita (MS).
Caso clínicoMulher, 44 anos, com cardiomiopatia Takotsubo e choque cardiogénico. Canulação seletiva da artéria coronária esquerda (ACE) não possível na coronariografia, tendo-se revelado uma ACU a emergir do seio direito, que se continuava distalmente como artéria circunflexa e, posteriormente, como artéria descendente anterior. Angio-tomografia computorizada coronária (ATCC) confirmou atrésia tronco comum, sem estenoses coronárias. A ressonância magnética cardíaca (RMN) mostrou edema miocárdico difuso, sem defeitos perfusão. Evolução favorável sob tratamento conservador.
DiscussãoOs autores descrevem um achado acidental de uma ACU direita. Reportam um tipo R-I de Lypton, onde a ACU direita dominante irriga todo o miocárdio. É a apresentação de ACU mais rara, com uma incidência de 0,0008%. Apenas 15 casos foram descritos na literatura. Todos realizaram coronariografia. De salientar que um teve MS, cinco angina, um arritmias ventriculares e um síndrome coronária aguda complicada. ATCC confirmou o diagnóstico em sete doentes, RMN em um e ecocardiograma transesofágico noutro. Nove doentes tinham lesões coronárias. Dois foram submetidos a cirurgia de revascularização miocárdica, um a intervenção percutânea e 11 a tratamento conservador.
ConclusãoACU direita com ausência congénita da ACE é uma das anomalias das artérias coronárias mais raras. Cursa numa percentagem significativa de doentes com isquémia e pode constituir uma ameaça à vida. ATCC e RMN são as modalidades de escolha para diagnóstico e estratificação do risco.
coronary artery anomalies
coronary artery bypass grafting
coronary artery disease
coronary angiography
coronary computed tomography angiography
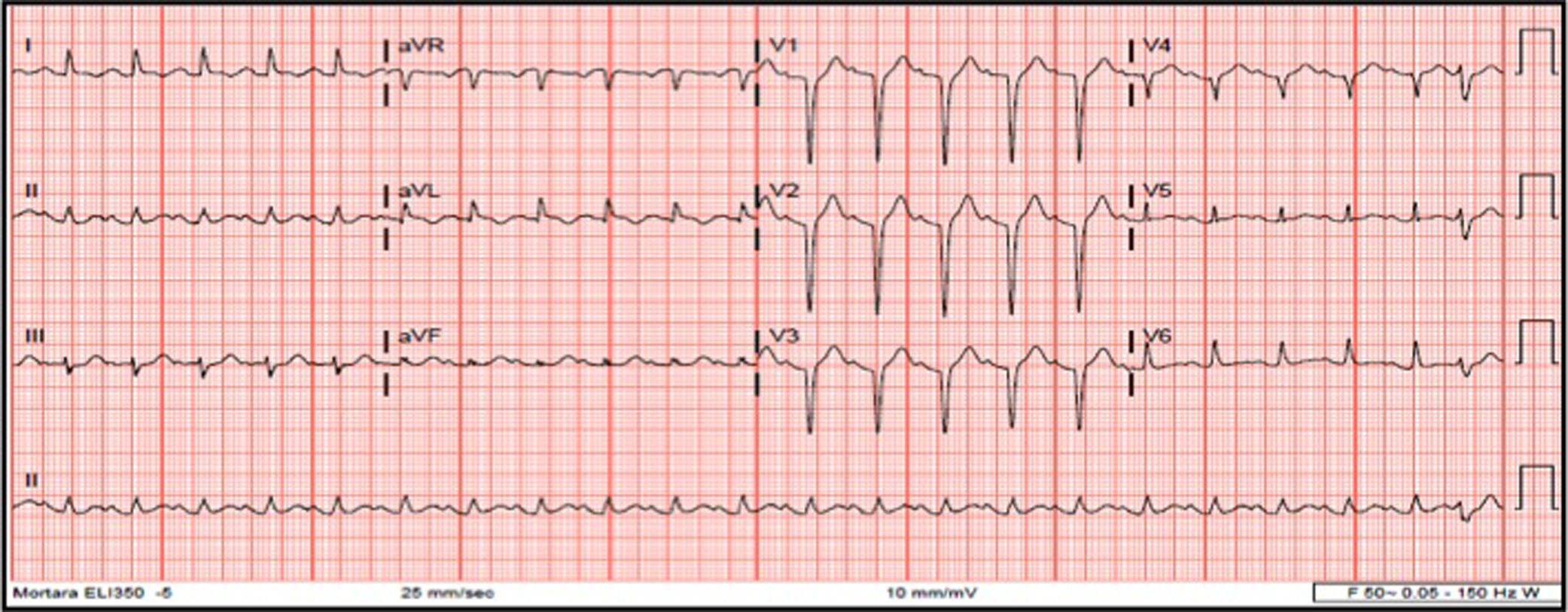
electrocardiogram
left anterior descending artery
left coronary artery
left circumflex artery
left ventricular
magnetic resonance imaging
percutaneous coronary intervention
posterior descending artery
right coronary artery
right sinus of Valsalva
single coronary artery
sudden cardiac death
transthoracic echocardiography
A 44-year-old woman with no known cardiovascular risk factors or other relevant medical history and not under medication presented at our emergency department after experiencing six hours of chest tightness radiating to the shoulder, following emotional stress, with no relieving or aggravating factors. There were no other associated symptoms or clinical context (e.g. infection).
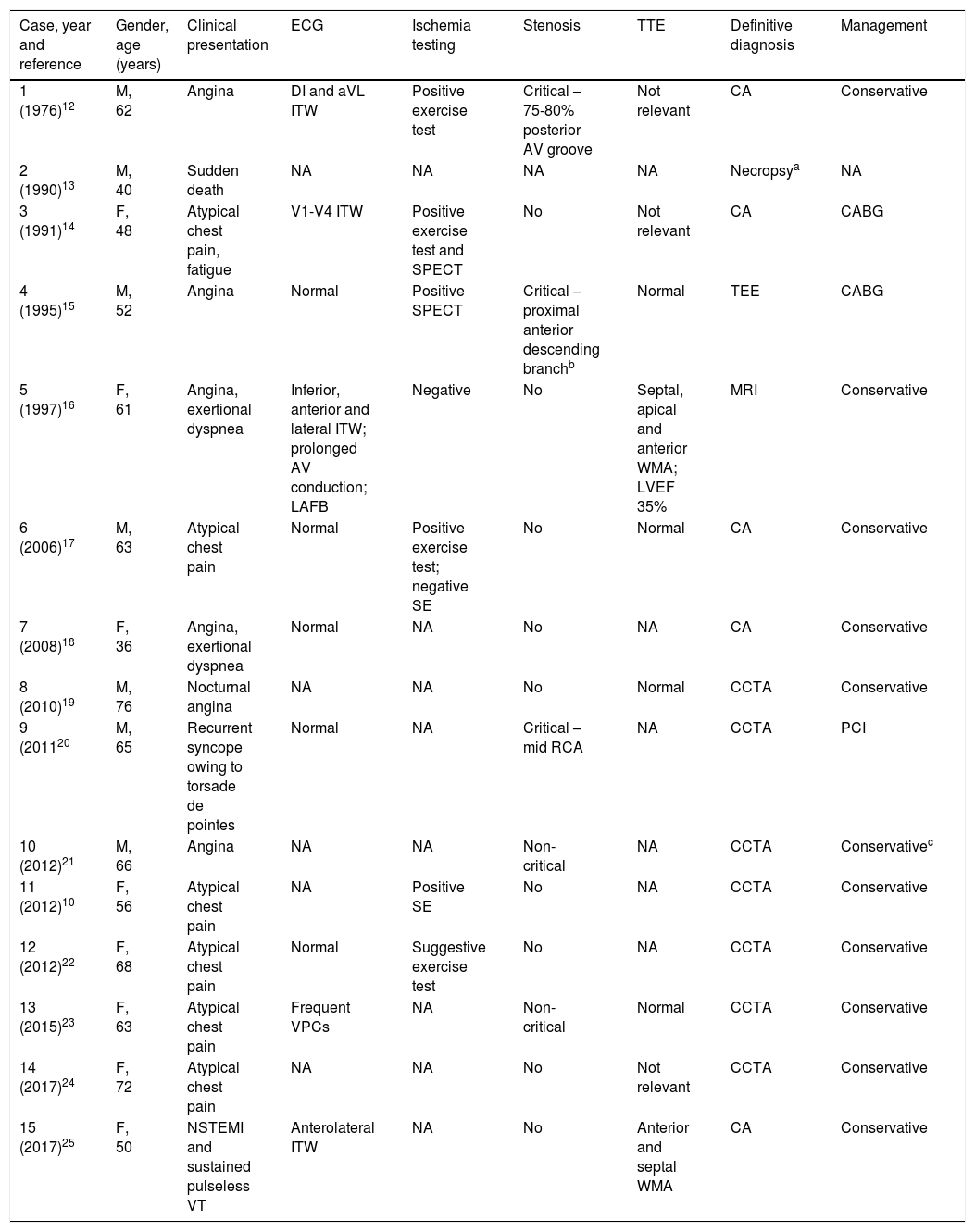
At admission, she still had chest pain, and blood pressure of 140/74 mmHg, tachycardia (140 ppm), slight polypnea but with no need for supplementary oxygen, and bibasilar rales; there was no peripheral edema. Laboratory findings were leukocytosis, normal C-reactive protein, mild elevation of troponin T (112 ng/ml; cut-off <14 ng/l), N-terminal pro-brain natriuretic peptide 950 pg/ml and dyslipidemia. The electrocardiogram (ECG) revealed sinus tachycardia, QS pattern from V1-V4, and first-degree atrioventricular block following one premature ventricular contraction (Figure 1). Arterial blood gas analysis showed hyperlactacidemia (maximum 3 mmol/l). The chest X-ray showed signs of pulmonary congestion. Transthoracic echocardiography (TTE) revealed akinesia of the mid-distal walls, hyperkinesia of the basal segments, severe left ventricular (LV) dysfunction and no LV outflow tract obstruction.
She was admitted to the coronary care unit with a provisional diagnosis of Takotsubo (stress) cardiomyopathy.
Within three hours she progressed to cardiogenic shock with need for mechanical ventilation, and inotropic and vasopressor support with dobutamine and noradrenaline was begun. Sinus tachycardia continued but there were no changes on the ECG.
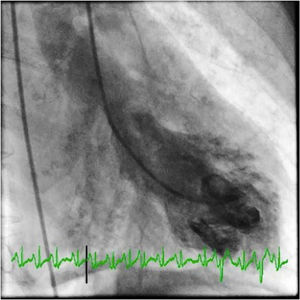
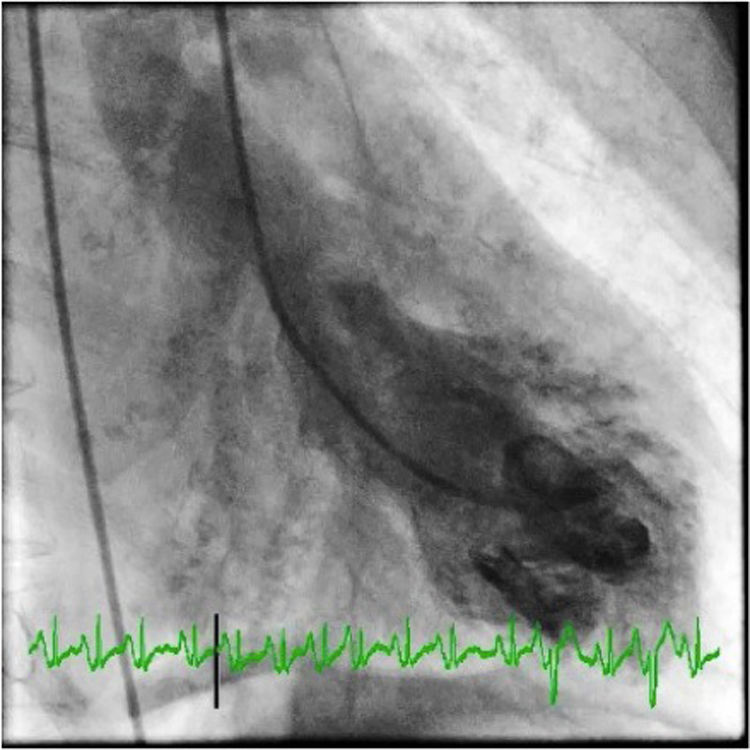
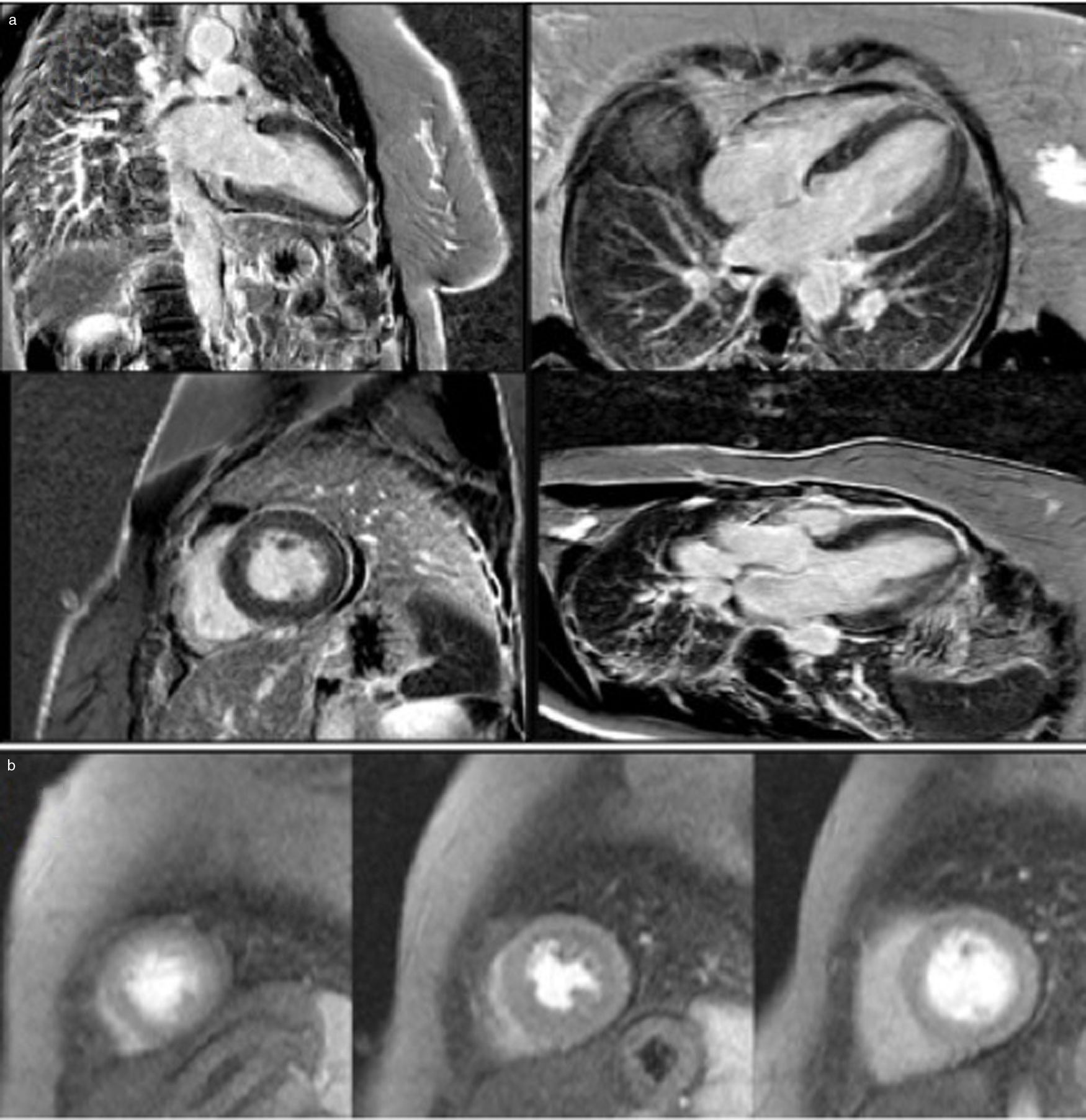
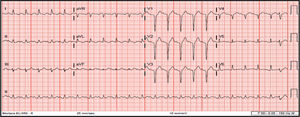
She underwent coronary angiography (CA) during which selective cannulation of the left coronary artery (LCA) was not possible, and a non-selective injection disclosed no coronary artery arising from the left coronary sinus. On selective injection of the right coronary sinus, a single ostium was visualized. The right coronary artery (RCA) was a great vessel, with no stenosis. A hypoplastic left anterior descending artery (LAD) and left circumflex artery (LCx) were perfused from RCA collateral vessels, both without lesions (Figure 2). Left ventriculography revealed apical ballooning akinesis, basal hyperkinesis and severe LV dysfunction (Figure 3).
Coronary angiography in multiple projections disclosing no coronary artery arising from the left coronary sinus (a); a single coronary artery originating from the right ostium – a large caliber right coronary artery – with no lesions (b); antegrade flow through a posterior lateral branch to the left circumflex artery (LCx) and to the hypoplastic left anterior descending artery (LAD) (b-d); the posterior descending artery gives off collaterals to the distal segment of the LAD (c). Both LCx and LAD had no lesions (c, d).
The most likely hypothesis was anomalous coronary artery origin, but an ostial left main lesion could not be excluded.
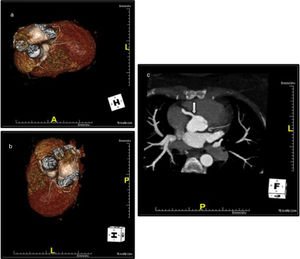
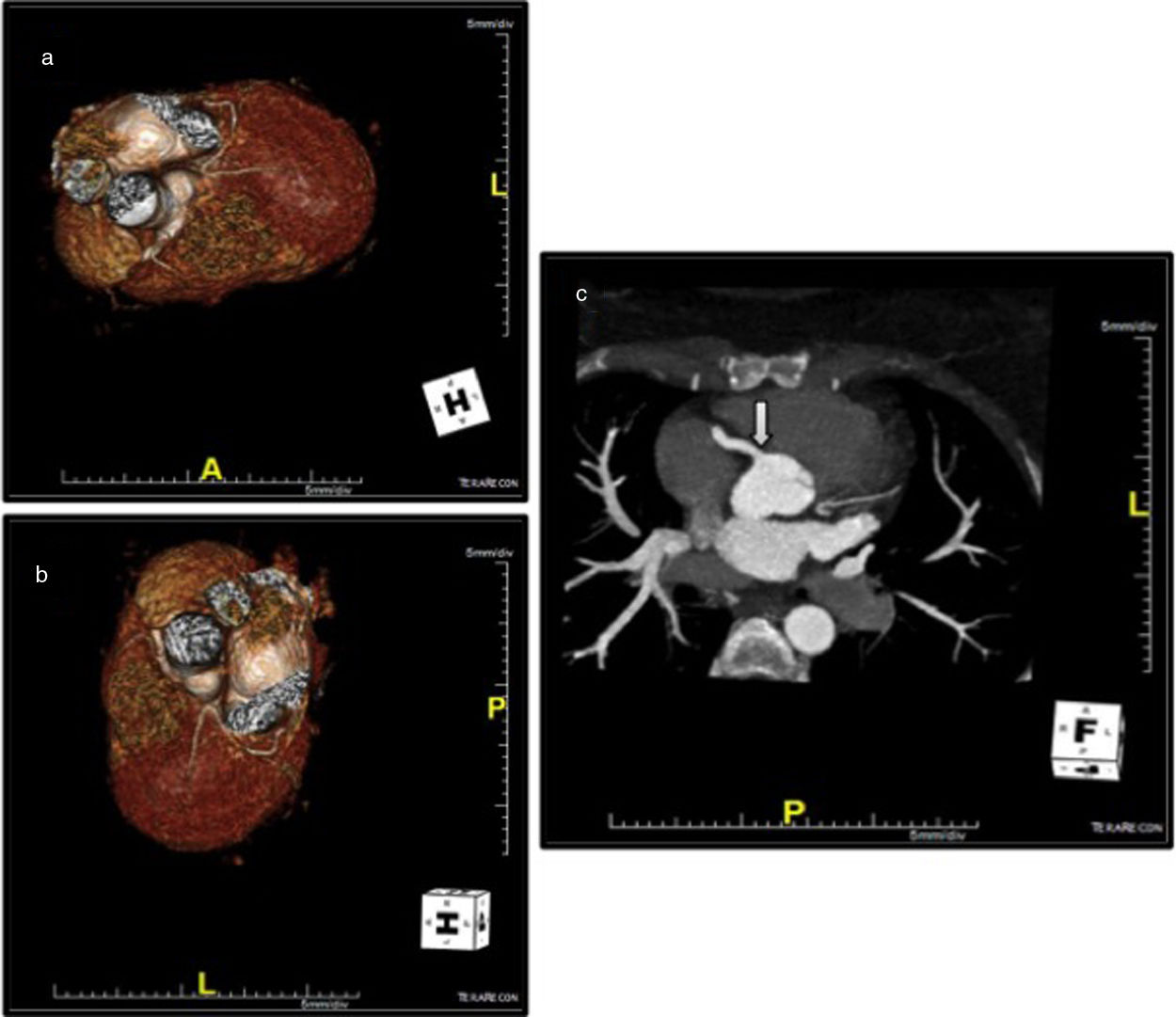
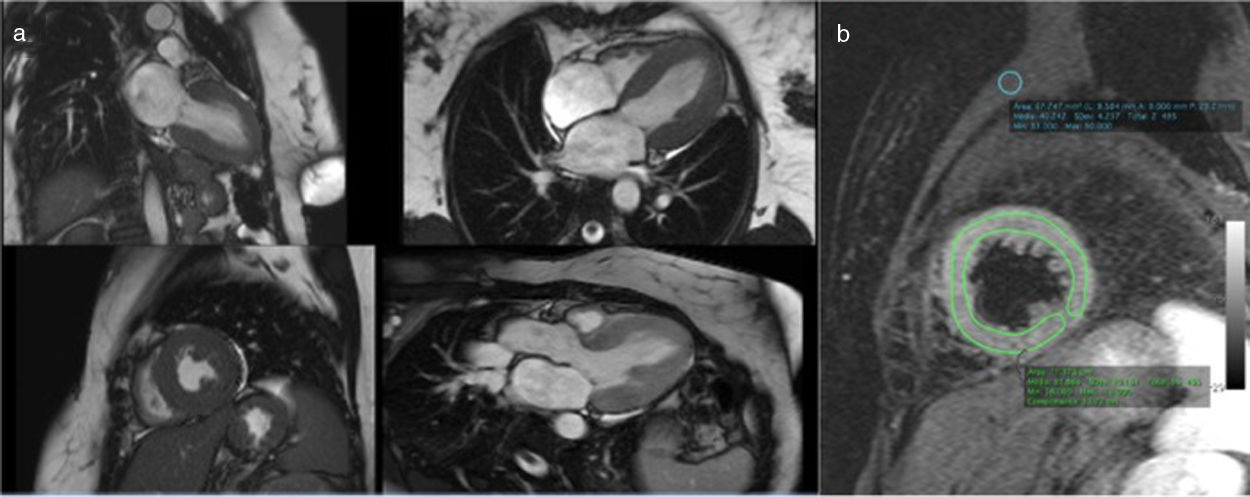
Coronary computed tomography angiography (CCTA) revealed a single coronary artery with its ostium in the right sinus of Valsalva (RSV), agenesis of the left main artery and a hypoplastic LAD, the RCA with a normal course, antegrade flow through a posterior lateral branch (PLB) to the LCx and LAD, and collaterals originating from the posterior descending artery (PDA) to the distal segment of the LAD. All coronary arteries were patent and with no evidence of atherosclerotic plaque (Figure 4).
Coronary computed tomography angiography depicting a single coronary artery with its ostium in the right sinus of Valsalva (arrow), agenesis of left main artery and hypoplastic left anterior descending artery; right coronary artery with a normal course. All coronary arteries were patent with no evidence of atherosclerotic plaque.
The patient's clinical course was favorable, with spontaneous ventilation and withdrawal of inotropic and vasopressor support at 48 hours, optimal diuretic response, and gradual recovery of global and segmental LV function.
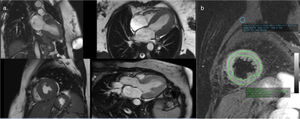
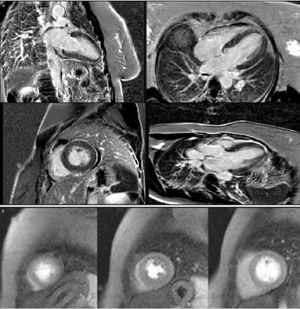
Cardiac magnetic resonance imaging (MRI), performed six days after admission, showed mild hypokinesia of the mid segments of both anterior and anteroseptal walls, preserved global LV systolic function, and diffuse myocardial edema (Figure 5), with no significant late enhancement and no perfusion defects (Figure 6), which was a clue to the diagnosis of Takotsubo cardiomyopathy.
A diagnosis of Takotsubo syndrome was made with an incidental finding of a right single coronary artery.
The patient's clinical course was uneventful and she was discharged on day 7, medicated with low-dose beta-blockers and angiotensin-converting enzyme inhibitors.
At two-month follow-up she was in New York Heart Association class I. TTE revealed normal global and segmental LV function and the ECG was normal.
IntroductionCoronary artery anomalies (CAAs) are rare congenital disorders, mostly clinically silent, that are usually diagnosed incidentally during CA, as seen in our patient, or at post-mortem.
Single coronary artery (SCA) has been known since 1903.1 It is an anomalous condition in which the SCA originates from a single aortic orifice, either the left or the right aortic ostium, and gives rise to the entire coronary circulation.
Around 40% of SCAs are associated with other congenital heart defects.2 Isolated congenital SCA is a very rare condition, occurring in 0.024-0.098% of the general population,2–5 and with an incidence of 0.066% in patients undergoing cardiac catheterization3 and an incidence of 3.32% of all coronary anomalies.5
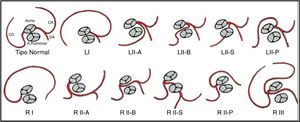
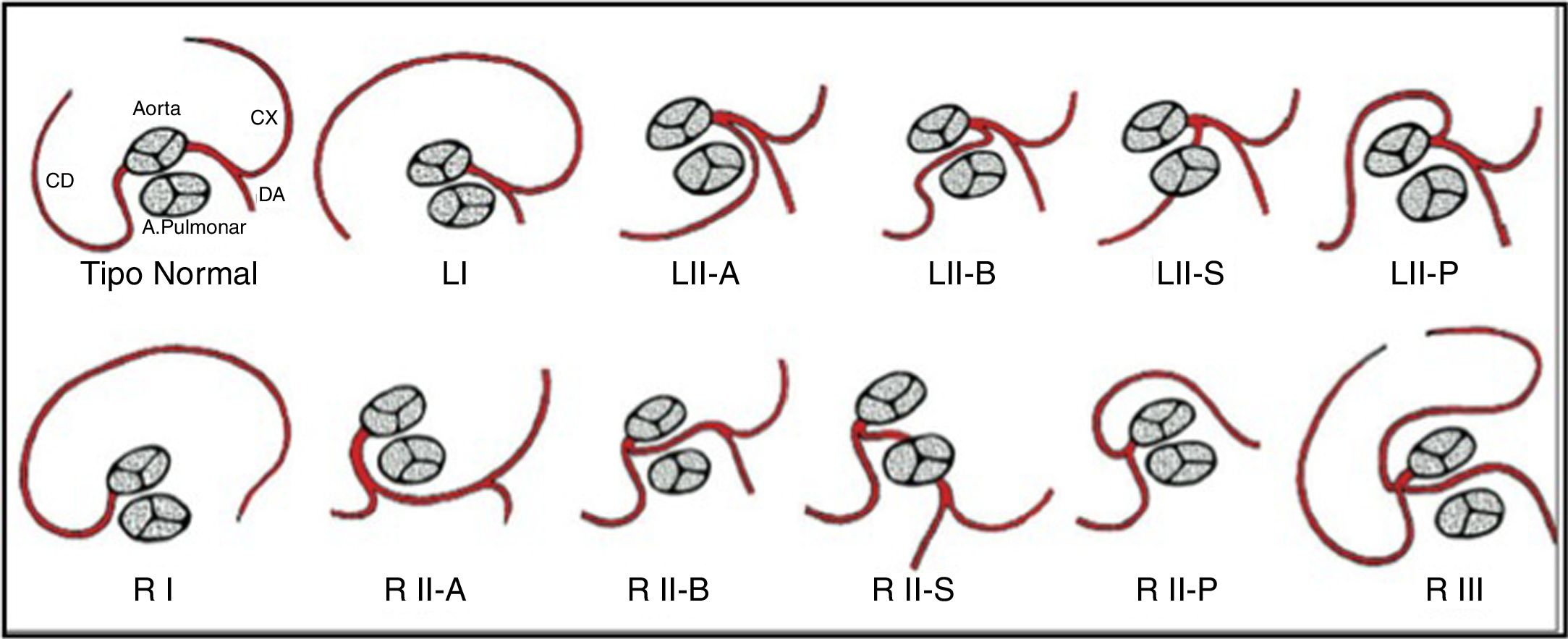
In the 20th century, different classification systems for SCAs based on necropsy findings and angiographic variants were suggested.6 The current system is the modified Lipton classification,5 based on features such as anatomical distribution, ostial location and the course of the transverse trunk (Figure 7). It divides patients into three groups: Group I has an anatomical course of either a right or a left coronary artery; in Group II the SCA arises from the right or left coronary sinus and from this a very large trunk crosses the base of the heart to arrive in the vicinity of the normal contralateral coronary artery; while Group III describes both LAD and LCx arteries arising separately from the proximal part of the normal RCA. There are five anatomical subtypes according to the relationship of the anomalous coronary artery with the great vessels.1,2,7 Half of SCAs arise from the RSV and half originate from the left sinus of Valsalva.6
Lipton's classification of single coronary artery. Adapted from Guérios et al.7
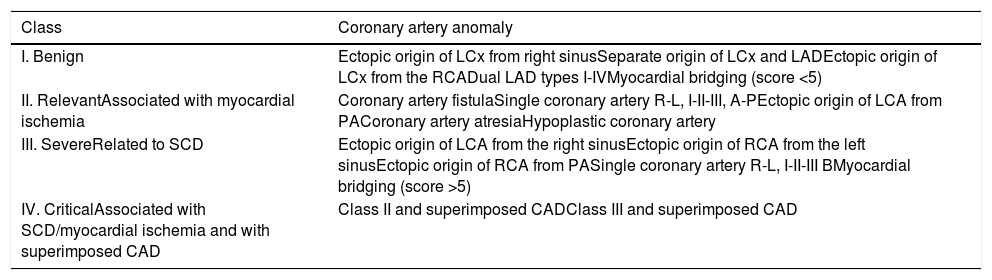
In 2005, Rigatelli et al.4 based their classification of CAAs on the clinical significance of the anomaly, which depends essentially on its course in relation to the great vessel and the presence of concomitant atherosclerotic coronary artery disease (CAD). SCA is not seen as a benign entity and is considered as relevant, associated with fixed myocardial ischemia (class II), in subtypes R or L, I-II-III, A-P; as severe, related to sudden cardiac death (SCD) (class III), in subtype R or L, I-II-III B; or as critical (class IV), associated with superimposed CAD (Table 1).
Rigatelli's classification of coronary artery anomalies. Adapted from Rigatelli et al.4
| Class | Coronary artery anomaly |
|---|---|
| I. Benign | Ectopic origin of LCx from right sinusSeparate origin of LCx and LADEctopic origin of LCx from the RCADual LAD types I-IVMyocardial bridging (score <5) |
| II. RelevantAssociated with myocardial ischemia | Coronary artery fistulaSingle coronary artery R-L, I-II-III, A-PEctopic origin of LCA from PACoronary artery atresiaHypoplastic coronary artery |
| III. SevereRelated to SCD | Ectopic origin of LCA from the right sinusEctopic origin of RCA from the left sinusEctopic origin of RCA from PASingle coronary artery R-L, I-II-III BMyocardial bridging (score >5) |
| IV. CriticalAssociated with SCD/myocardial ischemia and with superimposed CAD | Class II and superimposed CADClass III and superimposed CAD |
CAD: coronary artery disease; LAD: left anterior descending artery; LCA: left coronary artery; LCx: left circumflex artery; PA: pulmonary artery; SCD: sudden cardiac death.
Most cases of SCA are asymptomatic,1 but they may present with angina, arrhythmias such as non-sustained ventricular tachycardia, syncope, or SCD.1,2,6
The likelihood of SCD is higher when the anomalous artery has a slit-like orifice, an acute angle takeoff or an interarterial course. The malignant variety occurs when the artery courses between the aorta and the pulmonary trunk, mostly when the LCA originates from the RSV (accounting for 50% of SCD in one series).6 The risk of SCD is significantly higher in young athletes, one reason for which is compression between the pulsating vessels during heavy exercise.6
This risk highlights the importance, for diagnostic and therapeutic purposes, of recognizing not only the route taken by the anomalous artery, but also any malignant characteristics associated with the artery. CA is usually the first-line diagnostic tool in the detection of an SCA, but identifying its origin, determining its exact course (mainly proximal) and visualizing the relationships are often difficult. Precise delineation of anatomical and functional characteristics requires further complementary diagnostic modalities such as CCTA or MRI. CCTA with reformulated three-dimensional images is the preferred tool for diagnosing SCA and for identifying its origin and entire course, particularly a proximal course relative to the great vessels.2,6,8 It is also valuable in clinical decision-making. Cardiac MRI is also useful in determining the anatomy and functional significance of SCA.9
Management of patients with an SCA includes observation, medical treatment and percutaneous or surgical intervention. Revascularization is only recommended if there is significant atherosclerosis and documented ischemia.
Various degrees of ischemia may be observed, either because the SCA becomes unable to support the entire coronary circulation, or due to critical or non-critical lesions in the proximal vessel that may make distal coronary arterial lesions more hemodynamically significant. Fifteen percent of isolated SCAs may be associated with myocardial ischemia directly caused by the abnormal anatomy of the arteries and not by coronary artery disease.10 An unusual takeoff angle and tortuous course predisposes to accelerated atherosclerosis.11
Studies have suggested that the incidence of CAD is no different in patients with SCA compared with the general population.6 It should be treated the same way as CAD in native coronaries, with routine interventions such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).6
The prognosis of individuals with SCA is unclear and there are no guidelines for treatment of this condition.
DiscussionRight SCA with congenital absence of the LCA (type R-I, the anomaly in the case presented) is the least common type of SCA, and is extremely rare, with a reported incidence of 0.0008%.5 In this variant, the right coronary artery supplies the left system, and follows the usual pathway to the right atrioventricular groove, giving off the posterior descending artery in the normal fashion near the crux and then continuing in the left atrioventricular groove to give off posterolateral left ventricular branches. According to Rigatelli et al.’s classification, our patient is described as class II, since there were no coronary lesions.
A careful review of the literature revealed that only 15 cases with a similar anomalous coronary origin and pattern have been reported (Table 2).
Congenital right single coronary artery (Lipton R-I subtype) in adults with no associated congenital heart disease.
| Case, year and reference | Gender, age (years) | Clinical presentation | ECG | Ischemia testing | Stenosis | TTE | Definitive diagnosis | Management |
|---|---|---|---|---|---|---|---|---|
| 1 (1976)12 | M, 62 | Angina | DI and aVL ITW | Positive exercise test | Critical – 75-80% posterior AV groove | Not relevant | CA | Conservative |
| 2 (1990)13 | M, 40 | Sudden death | NA | NA | NA | NA | Necropsya | NA |
| 3 (1991)14 | F, 48 | Atypical chest pain, fatigue | V1-V4 ITW | Positive exercise test and SPECT | No | Not relevant | CA | CABG |
| 4 (1995)15 | M, 52 | Angina | Normal | Positive SPECT | Critical – proximal anterior descending branchb | Normal | TEE | CABG |
| 5 (1997)16 | F, 61 | Angina, exertional dyspnea | Inferior, anterior and lateral ITW; prolonged AV conduction; LAFB | Negative | No | Septal, apical and anterior WMA; LVEF 35% | MRI | Conservative |
| 6 (2006)17 | M, 63 | Atypical chest pain | Normal | Positive exercise test; negative SE | No | Normal | CA | Conservative |
| 7 (2008)18 | F, 36 | Angina, exertional dyspnea | Normal | NA | No | NA | CA | Conservative |
| 8 (2010)19 | M, 76 | Nocturnal angina | NA | NA | No | Normal | CCTA | Conservative |
| 9 (201120 | M, 65 | Recurrent syncope owing to torsade de pointes | Normal | NA | Critical – mid RCA | NA | CCTA | PCI |
| 10 (2012)21 | M, 66 | Angina | NA | NA | Non-critical | NA | CCTA | Conservativec |
| 11 (2012)10 | F, 56 | Atypical chest pain | NA | Positive SE | No | NA | CCTA | Conservative |
| 12 (2012)22 | F, 68 | Atypical chest pain | Normal | Suggestive exercise test | No | NA | CCTA | Conservative |
| 13 (2015)23 | F, 63 | Atypical chest pain | Frequent VPCs | NA | Non-critical | Normal | CCTA | Conservative |
| 14 (2017)24 | F, 72 | Atypical chest pain | NA | NA | No | Not relevant | CCTA | Conservative |
| 15 (2017)25 | F, 50 | NSTEMI and sustained pulseless VT | Anterolateral ITW | NA | No | Anterior and septal WMA | CA | Conservative |
AV: atrioventricular; CA: coronary angiography; CCTA: coronary computed tomography angiography; ECG: electrocardiogram; F: female; ITW: inverted T waves; LAFB: left anterior fascicular block; LVEF: left ventricular ejection fraction; M: male; MRI: magnetic resonance imaging; NA: not available; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; RCA: right coronary artery; SE: stress echocardiography; SPECT: single-photon emission computed tomography; TEE: transesophageal echocardiography; TTE: transthoracic echocardiography; VPCs: ventricular premature contractions; VT: ventricular tachycardia; WMA: wall motion abnormalities.
Of these 15 patients, seven were male and eight were female, and their mean age was 58.5±11 years (range 36-76). Angina was found in five patients, two had exertional dyspnea, six atypical chest pain, one nocturnal angina, one recurrent syncope owing to sustained torsade de pointes, one presented with acute myocardial infarction complicated by sustained pulseless ventricular tachycardia and one (case 2) suffered SCD (post-mortem examination revealed evidence of left ventricular dilation and hypertrophy).
On the resting ECG, four patients had non-specific ventricular repolarization changes, one had prolonged PR interval and left axis deviation, and one had frequent ventricular premature contractions.
TTE was obtained in nine patients, in three of whom there was evidence of wall motion abnormalities, one with evidence of moderate systolic LV dysfunction.
All patients underwent CA. A definitive diagnosis was made without other imaging modalities in five patients; CCTA was performed and confirmed the diagnosis in seven patients, MRI in one and transesophageal echocardiography in another.
Most of the patients were referred for conservative treatment; two underwent CABG and one underwent PCI.
CA showed no coronary artery lesions in nine patients. In two cases (10 and 13) there was an intermediate lesion, no ischemia test result was available, and they were managed medically; patient 10 underwent repeat CA a year later for acute coronary syndrome that revealed significant disease progression and underwent PCI. Three patients had critical stenosis. Patient 1 had a positive exercise test and CA showed a critical stenosis at the level of the posterior atrioventricular groove, which was managed medically. Patient 9, the one with recurrent syncope owing to torsade de pointes, had a critical stenosis in the mid segment of the RCA, and underwent PCI with implantation of a drug-eluting stent. Patient 4 had symptoms and signs of ischemia but no clear evidence of lesions on CA; he underwent surgery and intraoperatively a significant stenosis was observed in a proximal segment of the anterior descending branch, and CABG was performed using the left interior mammary artery.
In patient 3 there was no stenosis, but both exercise testing and myocardial perfusion scintigraphy revealed evidence of ischemia. In view of her poor response to medical management, she underwent atrial pacing studies, in which she developed 2:1 atrioventricular block with concomitant chest pain and flat ST depression. A saphenous vein graft was used to reconstruct the left main coronary artery.
Three patients (6, 11 and 12) had a positive exercise test but no evidence of coronary lesions; they were managed medically. Patient 15 was admitted with non-ST-elevation myocardial infarction complicated by sustained pulseless ventricular tachycardia; since there were no lesions on CA, she was managed medically. Despite wall motion abnormalities and LV systolic dysfunction, patient 5 had a negative ischemia test and no CAD; she underwent conservative treatment.
Although SCA is described in most cases as having a benign course, our literature review showed that right SCA is associated with angina in a significant proportion of patients and may present with life-threatening ischemic complications.
Our patient's history and clinical course, coupled with complete recovery of segmental and global LV systolic function and the absence of significant late enhancement or perfusion defects, supported the diagnosis of Takotsubo cardiomyopathy. We believe her right SCA anomaly was an incidental finding, since no association between the two entities is known, nor could we find any. CCTA and MRI excluded the presence of anatomic or functional high-risk features, which led us to adopt a conservative approach.
SCA has been reported in association with atherosclerotic disease, coronary artery fistulas, mitral valve prolapse, bicuspid aortic valves, tetralogy of Fallot, transposition of the great arteries and hypertrophic cardiomyopathy,2 but an association with Takotsubo syndrome has never previously been reported.
ConclusionWe present the case of an SCA originating from a single ostium in the RSV, with a normal course, and absence of the LCA, which is an extremely rare type of SCA.
Despite the very low incidence and usually benign course of this entity, the possibility of SCA should be borne in mind during the diagnostic process, especially when both ostia cannot be identified on CA, because of its prognostic implications, placing the patient at increased risk for future events such as ischemia, infarction, heart failure or SCD.
CCTA is an unequaled noninvasive imaging modality for diagnosis and for identification of high-risk anatomic characteristics, aiding in risk stratification.
Conflicts of interestThe authors have no conflicts of interest to declare.



 LCx) and to the hypoplastic left anterior descending artery (
LCx) and to the hypoplastic left anterior descending artery (