Acute severe mitral regurgitation (MR) because of secondary left ventricular impaired regional contractility can present with severe acute heart failure, associated with a high risk for rapid decompensation, pulmonary edema and cardiogenic shock. Frequently, in these highly unstable patients, surgical risk can be prohibitive. Evidence for percutaneous repair of acute MR is scarce, but a few case series show that this approach could be safe and effective for bailing out hemodynamically unstable patients.
We report a case of an 84-year-old man with acute ischemic severe MR post-acute myocardial infarction (MI), who remained hemodynamically unstable despite coronary revascularization, positive pressure non-invasive ventilation, vasodilator therapy and intra-aortic balloon pump (IABP) support. In heart team discussions, he was considered a high risk surgical candidate. We decided on rescue off-label percutaneous mitral valve repair with a MitraClip device (Abbott Vascular, Santa Clara, California), with good clinical result, allowing weaning from the supports and discharge seven days after the procedure. At one-year follow-up, the patient maintained a MV repair results and had a good functional status. In unstable patients with acute ischemic MR, percutaneous MV repair could be a rescue therapeutic option to consider, allowing hemodynamic compensation with potential persistent MR improvement up to one-year follow-up.
A insuficiência mitral (IM) aguda grave causada por alterações isquémicas secundárias loco-regionais do ventrículo esquerdo associa-se a elevado risco de descompensação hemodinâmica com edema pulmonar e choque cardiogénico. Frequentemente, nestes doentes instáveis, o risco cirúrgico pode ser proibitivo. É escassa a evidência existente relativamente à abordagem percutânea da IM aguda neste contexto, existindo apenas algumas séries de casos que parecem mostrar segurança e eficácia no resgate de doentes instáveis.
Reportamos o caso de um doente de 84 anos, género masculino, com IM aguda grave isquémica após enfarte agudo do miocárdio, que permaneceu com instabilidade hemodinâmica apesar de revascularização coronária, ventilação não invasiva com pressão positiva, terapêutica com vasodilatadores e suporte de balão intra-aórtico. Após discussão em reunião de Heart Team, o doente foi considerado de elevado risco cirúrgico, tendo sido orientado para tratamento percutâneo da válvula mitral com dispositivo MitraClip. O procedimento decorreu sem intercorrências imediatas, permitindo redução com critérios de sucesso da IM. A melhoria do perfil hemodinâmico com a correção percutânea da IM permitiu rápido desmame da terapêutica de suporte em curso, com evolução clínica favorável subsequente e alta ao sétimo dia após procedimento. No seguimento clínico e ecocardiográfico a um ano verificou-se a persistência do resultado do tratamento percutâneo da IM e o doente apresentava boa capacidade funcional.
Em pacientes instáveis com MR isquémica aguda, a reparação percutânea da VM poderia ser uma opção terapêutica de salvamento a considerar, permitindo uma compensação hemodinâmica com potencial melhoria persistente da MR até 1 ano de seguimento.
Ischemic mitral regurgitation (MR) is caused by changes in left ventricular (LV) structure and function related to ischemia.1 It is usually associated with a chronic disease process that involves LV regional or global remodeling and consequently a combination of asymmetric or symmetric leaflet tethering and reduction of closing forces. Annular dilatation is frequently associated with global LV remodeling and contributes to MR progression.2
Following myocardial infarction (MI), acute MR may be seen in 8–74% of patients and can be considered a mechanical complication because of papillary muscle rupture or stretching.3 Albeit usually transient, it can persist if the cause is MI, and has a dismal prognosis.4 Importantly, among patients presenting with acute MI complicated by cardiogenic shock, moderate or severe functional MR occurs in 39.1% of cases, which was found to be associated with worse one-year survival. In these patients, impaired regional contractility, LV dilatation and incomplete MV leaflet closure seems to be the main drivers of significant MR.5
Acute MR post-MI can present with severe acute heart failure, with a high risk for rapid decompensation due to pulmonary edema and cardiogenic shock.6 The therapeutic options are somehow limited because of the usual high risk profile of these patients.7 It includes myocardial revascularization, afterload reduction with intra-aortic balloon pump (IABP), inodilators and non-invasive ventilation with positive pressure, to allow time for LV reverse remodeling and improvement of the MR.
Surgical valve replacement or repair is the cornerstone of ruptured papillary muscle treatment and also in the case of persistent severe acute ischemic functional MR. Perioperatively, mortality is high approximately 27–40%, but if left untreated it can be as high as 71–80% in-hospital.7,8 Frequently, in these highly unstable patients, surgical risk can be prohibitive.7
Percutaneous mitral valve repair with MitraClip device (Abbott Vascular, Santa Clara, California) has emerged as an alternative to surgery for the treatment of chronic severe primary and secondary MR.9 Evidence for percutaneous repair of acute MR is scarce, but a few case series have shown that this approach could be safe and effective for bailing out hemodynamically unstable patients.10,11
We present a challenging case of rescue percutaneous MV repair with the MitraClip device for ischemic acute MR as a complication of acute MI.
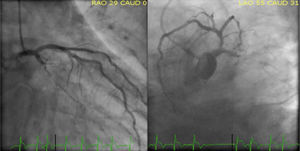
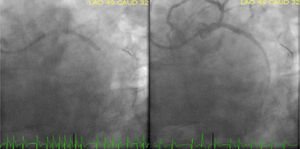
Case reportAn 84-year-old male patient with the cardiovascular risk factors obesity (body mass index 36 kg/m2), dyslipidaemia, arterial hypertension, type 2 diabetes mellitus, and a past medical history of permanent atrial fibrillation (AF) on anticoagulation with acenocoumarin, presented to our emergency department because of persistent oppressive chest pain and shortness of breath nine hours in duration. At admission, he was conscious (Glasgow Coma Scale 15) and symptomatic. On physical examination, he was normotensive (SAP∼118 mmHg) and normocardic (87 bpm), without signs of tissue hypoperfusion. Cardiac sounds were irregular on auscultation and he had an apical blowing holosystolic murmur, radiating to the axilla, grade IV/VI (Levine grading scale). Lung auscultation showed bilateral lower half rales. His electrocardiogram showed AF rhythm, with ST-segment elevation in leads II, III, aVF and ST-segment depression in leads V2-V4. Acknowledged inferoposterior acute myocardial infarction and a loading dose of 600 mg clopidogrel, 300 mg acetylsalicylic acid and an IV bolus dose of unfractionated heparin (5000 IU) were given. The patient was immediately referred for primary percutaneous coronary intervention (PCI). As we can see in Figure 1, emergent coronary angiography (CAG) revealed a dominant left coronary artery, showing total occlusion of the proximal segment of left circumflex artery (LCX) and a moderate lesion (40%) in the proximal left anterior descending artery. Right coronary artery was a small vessel with diffuse irregularities. Because of high thrombotic burden, we performed manual thrombus aspiration of LCX as an initial strategy using a 6 Fr aspiration catheter with multiple passages across the lesion. Thereafter, CAG revealed Thrombolysis in Myocardial Infarction (TIMI) 3 flow in the culprit vessel. A protection guidewire was advanced to the left obtuse marginal artery and we performed a PCI of LCX proximal lesion with deployment of a 4.0×24 mm everolimus-eluting stent (Synergy). The procedure was complicated by no reflow phenomenon after stent implantation, with slow spontaneous improvement until a final TIMI 2 flow in the LCX artery (Figure 2).
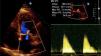
At the end of the procedure the patient developed dyspnea, diaphoresis, cyanosis and peripheral vasoconstriction. He was normotensive (SAP∼100 mmHg), tachycardic (AF with rapid ventricular rate, 160 bpm), with signs of respiratory distress. Arterial blood gases revealed type I respiratory failure (pO2 66 mmHg with Venturi Mask at a 40% FiO2 setting; PaO2/FiO2 ratio 165 mmHg), and a metabolic acidosis (pH 7.32, lactate 7.2, HCO3- 17 mmol/L). He was admitted to the intensive cardiac care unit after the procedure, presenting in cardiogenic pulmonary edema. The patient was started on intravenous (IV) isosorbide dinitrate perfusion, IV furosemide repeated bolus and positive airway pressure ventilation with slow response. A transthoracic echocardiography was performed, showing mild LV dilatation (left ventricular end-diastolic volume index 79 mL/m2), akinesia of the LV lateral, posterior and inferior walls; LV systolic function was moderately depressed (left ventricle ejection fraction (LVEF)) 37% by biplane Simpson method) and there were no signs of right ventricle compromise. There was an evident a restriction of the left posterior mitral valve leaflet, originating an eccentric severe MR jet (color Doppler grade 4+, effective regurgitant orifice area 0.4 cm2, regurgitant volume 75 mL), without signs of posteromedial papillary muscle rupture (PMR). To reduce afterload, an intra-aortic balloon pump (IABP) was inserted via the right femoral artery. The patient slowly progressed, improving their hemodynamic state, with a reduction in lung congestion. Nevertheless, we were unable to remove him from non-invasive ventilation because of refractory pulmonary edema after five days of medical therapy and hemodynamic support with IABP.
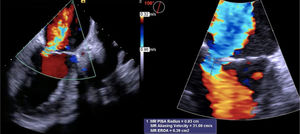
Being a high surgical risk patient (EuroSCORE II 22.26%), the patient was refused for surgery. Despite the ominous prognosis of his clinical condition and the advanced age, considering that the patient was previously independent, with well-preserved cognition and no relevant past medical conditions, in the heart team we discussed the possibility of percutaneous MV repair. A transesophageal echocardiogram (under IABP support) was performed (Figure 3), which showed severe transcomissural, holosystolic, high velocity MR, without signs of significant MV leaflet degeneration and stenosis; posterior leaflet length was 14 mm, with sufficient leaflet tissue for mechanical coaptation (coaptation depth 9.5 mm, coaptation length 3 mm).
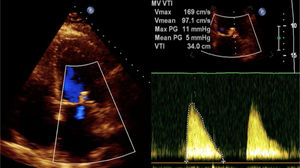
On day seven, percutaneous MV repair was performed, using a right femoral vein approach (24Fr), with deployment of 3 clips (MitraClip NT), successfully (final MR 1+), without complications (transmitral mean gradient in the end of the procedure 5 mmHg) (Figure 4). Within 24 h of the procedure we successfully removed IABP support, and we managed to wean off non-invasive ventilation.
During the hospital stay, the patient developed the following complications: acute non-oliguric renal failure (worse serum creatinine 2.74 mg/dL), with full recovery at discharge; nosocomial Enterococcus faecalis and Proteus mirabilis urinary tract infection, with good antibiotic response; transient ischemic attack on day five, without computed tomography image of cerebral infarction or bleeding, and with full recovery of neurologic deficits. The patient was discharged seven days after MitraClip intervention (day 14). At 30-day follow-up he was in New York Heart Association class II and mild MR (1+) persisted, SPAP<40 mmHg, without signs of significant reverse remodeling (LVEF 35% using the biplane Simpson method and a slight reduction in LV end-diastolic volume index to 68 mL/m2). Clinical and echocardiographic parameters were stable up to one-year follow-up.
DiscussionAcute MI complicated by acute ischemic MR and hemodynamic decompensation is associated with high mortality, and is responsible for ∼5% of deaths after MI.7 It is more frequently associated with inferior territory MI or large infarct area and LV dysfunction.6 PMR requires emergent surgical repair, despite the high peri-operative mortality associated with this intervention.12 In the absence of PMR, functional ischemic MR may improve with complete revascularization.13
In our patient, the delayed presentation to the emergency department increased the infarct area and significantly reduced the probability of functional recovery. Another determining factor was the proximal LCX occlusion with high thrombotic burden, which contributed to the occurrence of no reflow phenomenon due to distal embolization and microvascular occlusion. Additionally, the patient had left-dominant coronary artery circulation, which is associated with increased post-PCI acute MR because of impaired regional contractility, resulting in tethering and systolic restriction of the posterior mitral valve leaflet, increased mitral annulus area and dimensions, mitral valve tenting, and subvalvular apparatus displacement.14–16
Despite coronary revascularization, continuous positive pressure non-invasive ventilation, vasodilator therapy and IABP for afterload reduction, hemodynamic instability persisted with impossibility to wean the patient from the given support. Surgical risk was prohibitive. A successful bailout percutaneous MV repair with MitraClip device enabled significant reduction in MR, with subsequent fast clinical recovery and weaning from supports. Up to one-year follow-up, our patient showed persistently good result from MR treatment, reduced SPAP and improved functional status, although we did not observe any significant LV reverse remodeling, which was probably due to delayed primary PCI and no reflow phenomenon, increasing the infarct area.
Few small case series show feasibility and efficacy of MitraClip implantation in unstable patients with acute MR post-MI in whom hemodynamic instability persists, despite complete revascularization.10 However, this is an off-label approach and we agree that in non-unstable patients, we should delay MV repair to enbable LV reverse remodeling and consequently MR improvement. Although percutaneous treatment for acute functional MR in this context has been showing signs of safety, an already reported matter of concern, particularly after cardiac surgery, is the potential occurrence of afterload mismatch with transient severe LV dysfunction after MitraClip insertion, requiring diligent and effective diagnosis and treatment.17 Data on treating patients with acute MR are still scarce. With increasing experience and evidence, acute MR could also become an indication for percutaneous MV repair.
ConclusionPost-MI acute MR is considered a mechanical complication that adversely affects short- and long-term prognosis. In unstable patients, percutaneous MV repair could be a rescue therapeutic option to consider, enabling hemodynamic compensation and withdrawal of suportwithdrawal of support, showing persistent MR improvement up to one-year follow-up.
Conflicts of interestThe authors have no conflicts of interest to declare.