Structural and electrophysiological changes play a critical role in the development of atrial fibrillation (AF). Although the pathophysiology of paroxysmal AF (PAF) has not been fully elucidated, oxidative stress (OS) and DNA damage appear to be important triggers. Thus far, no studies have investigated the relationships among total oxidant status (TOS), DNA damage, and PAF. The goal of this study was to assess TOS and DNA damage in patients with PAF.
MethodsThis cross-sectional study included 56 patients with PAF and 31 healthy controls. OS was assessed based on TOS, total antioxidant capacity (TAC), and oxidative stress index (OSI). The level of DNA damage was assessed using 8-hydroxy-2′-deoxyguanosine (8-OHdG).
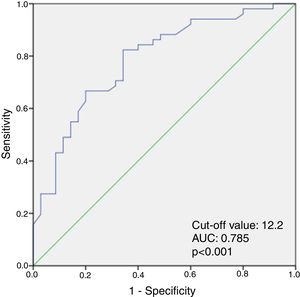
ResultsThere were no significant differences between the groups in terms of baseline characteristics. However, patients with PAF had significantly higher high-sensitivity C-reactive protein (p=0.018), TOS (p=0.001), OSI (p=0.001), and 8-OHdG (p=0.019) levels, compared with the control group. Multivariate logistic regression analysis showed that serum TOS level (odds ratio: 1.608; 95% confidence interval [CI]: 1.188-2.176, p=0.002) was the only independent predictor of PAF. TOS ≥12.2 predicted PAF with a sensitivity of 82% and specificity of 76% (AUC: 0.785, 95% CI: 0.687-0.883, p<0.001).
ConclusionWe found that TOS and DNA damage were significantly greater in patients with PAF than in the control group. Therefore, we propose that TOS and DNA damage can be used to detect patients at higher risk of AF.
As alterações estruturais e eletrofisiológicas têm um papel crítico no desenvolvimento de fibrilhação (FA). Embora a fisiopatologia da FA paroxística (FAP) não seja ainda totalmente conhecida, o stress oxidativo (SO) e a lesão do DNA parecem ser causas relevantes. Até agora, nenhum estudo investigou a relação entre o estado oxidante total (EOT), a lesão do DNA e a FAP. O objetivo deste estudo consisitiu na avaliação do EOT e da lesão do DNA em doentes com FAP.
MétodosEste estudo prospetivo caso-controlo incluiu 56 doentes com FAP e 31 voluntários saudáveis. O SO foi avaliado com base no EOT, no estado antioxidante total (EAT) e no índice do stress oxidativo (ISO). O nível de lesão do DNA foi avaliado através de 8-hidroxi- -2’ deoxiguanosina-2’ (8-OHdG).
ResultadosNão se observaram diferenças significativas entre os grupos relativamente às características basais. No entanto, os doentes com FAP revelaram níveis mais elevados de proteína C reativa (P=0,018), EOT (P=0,001), ISO (P=0,001) e 8-OHdG (P=0,019), quando comparados com o grupo controlo. A análise multivariada por regressão logística mostrou que o nível sérico do EOT (odds ratio: 1,608, intervalo de confiança (IC) 95%: 1,188–2,176, P=0,002) foi o único fator preditivo da FAP. O EOT≥12,2 previu a FAP com uma sensibilidade de 82% e uma especificidade de 76% (AUC: 0,785, IC 95%: 0,687-0,883, P<0,001).
ConclusãoO EOT e a lesão do DNA foram significativamente mais elevados nos doentes com FAP do que no grupo controlo, pelo que sugerimos que o EOT e a lesão do DNA possam ser utilizados para detetar doentes com risco elevado de FA.
Atrial fibrillation (AF) is the most common cardiac arrhythmia and its incidence increases with age. Paroxysmal atrial fibrillation (PAF), one of four types of AF, typically ends within 48 hours. The presence of AF increases the likelihood of heart failure, hospitalization, thromboembolic events, and death.1–3 AF is associated with electrophysiological, contractile, and structural remodeling. In most patients, atrial remodeling begins in sinus rhythm long before the onset of AF. Despite numerous studies, the pathophysiology of PAF has not been fully elucidated; however, inflammation, oxidative stress (OS) and DNA damage are potential substrates for the progression of AF.
OS is caused by an increase in oxidative agents or decrease in antioxidant levels, especially in pathological conditions.4 Previous studies have shown that OS is involved in the pathogenesis of many diseases.5–8 Additionally, experimental studies have demonstrated that elevated OS is associated with chronic AF.9 However, data in humans regarding the relationship between PAF and TOS are limited.
Measurement of the overall oxidation state of the body gives a parameter known as total oxidant status (TOS).10 Assessment of the body's ability to scavenge free radicals gives total antioxidant capacity (TAC).11 The antioxidant system neutralizes oxidants including reactive oxygen species. Oxidants often have a damaging effect on cell nuclei, mitochondria, and membranes. In addition, antioxidant deficiency or elevated OS increases DNA damage. This can lead to cellular death, degeneration, or aging; it also contributes to disease processes. The best indicator of damaged DNA is 8-hydroxy-2′-deoxyguanosine (8-OHdG).12
Thus far, no studies have investigated the relationships among TOS, DNA damage, and PAF. The aim of this study was to fill this gap in the literature by investigating these relationships.
MethodsStudy designThis cross-sectional study included 56 consecutive patients with PAF (33 men; median age 46 years [45-52]) and 31 healthy controls (26 men; median age 46 years [42-52]). The diagnosis of PAF was defined in accordance with the 2016 European Society of Cardiology guidelines in patients who presented to the cardiology clinic or emergency department with complaints of palpitations.13 Patients with a history of diabetes, hypertension, known heart failure, moderate or severe heart valve disease, coronary artery disease, active infection, malignancy, rheumatic or hematological disease, liver disease, renal failure, or sleep apnea were excluded from the study. The study protocol was approved by the local ethics committee and conducted in full accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients enrolled in the study.
Electrocardiographic and echocardiographic analysisAfter a 15-min rest period, 12-lead electrocardiographic data were recorded for all participants at 25 mm/s and a height of 10 mm/mV. Echocardiography was performed using a Vivid 5 ultrasound imaging system (GE Medical Systems, Waukesha, WI, USA), with participants in the left lateral decubitus position. All measurements were performed in accordance with the standards published by the American Society of Echocardiography.14 Left atrial diameter and left ventricular end-systolic and end-diastolic diameters were measured. Left ventricular ejection fraction was calculated based on Simpson's rule. Echocardiographic examinations were performed while participants were in sinus rhythm.
Determination of oxidant status and DNA damageBlood samples were taken from all patients at the time of hospital admission while in AF rhythm. Blood samples were centrifuged at 3000 rpm for 10 min; serum was then separated and stored at -80°C until analysis. TOS and TAC were assessed with commercially available kits (Rel Assay Diagnostics, Turkey) using a microplate reader (Multiskan Go, Thermo Fisher, Waltham, MA, USA). TOS and TAC levels were expressed in μmol H2O2 equivalent/l and mmol Trolox equivalent/l, respectively.10,11 OSI was defined as the ratio of TOS to TAC ×10. Quantification of 8-OHdG was performed using an OxiSelect™ Oxidative DNA Damage ELISA kit (Cell Biolabs, San Diego, CA, USA). All protocols were performed in accordance with the manufacturers’ instructions.12
Statistical analysisIBM SPSS for Windows, version 22.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Continuous variables were presented as mean ± standard deviation or median (interquartile range), while categorical variables were presented as percentages. The Kolmogorov-Smirnov test was used to assess normality of distribution. Comparisons of continuous variables between groups were performed using the Student's t test for parametric variables, and the Mann-Whitney U test for nonparametric variables. Chi-square tests were used to compare categorical variables. Correlation analysis was performed to determine the correlation of TOS with other continuous variables. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off value for TOS. Univariate analysis was performed to identify potential risk factors for PAF. Multivariate logistic regression analysis was performed to determine independent predictors of PAF. Differences with p<0.05 were considered statistically significant.
ResultsA total of 56 patients with PAF and 31 healthy controls were included in this study. The baseline characteristics of the study population are presented in Table 1. There were no significant differences between the groups with respect to baseline clinical and echocardiographic characteristics, except for body mass index (BMI), which was significantly higher in patients with PAF compared to the control group (25 [23.4-26.9] vs. 23.1 [22.5-24.5] kg/m2, p=0.001).
Baseline clinical and echocardiographic characteristics of patients with paroxysmal atrial fibrillation and healthy controls.
| Patients with PAF (n=56) | Control group (n=31) | p | |
|---|---|---|---|
| Age, years | 46 (45-52) | 46 (42-52) | 0.312 |
| Male, n (%) | 33 (56) | 26 (44) | 0.506 |
| Smoking, n (%) | 11(20) | 9 (25) | 0.818 |
| SBP, mmHg | 130 (120-130) | 120 (110-130) | 0.560 |
| DBP, mmHg | 80 (70-80) | 75 (70-80) | 0.404 |
| Heart rate, bpm | 65 (60-69) | 65 (60-72) | 0.524 |
| BMI, kg/m2 | 25 (23.4-26.9) | 23.1 (22.5-24.5) | 0.001 |
| LA diameter, mm | 36 (31-37) | 35 (30-36) | 0.968 |
| LVESD, mm | 51 (51-52) | 50 (50-51) | 0.386 |
| LVEDD, mm | 36 (36-37) | 35 (35-36) | 0.578 |
| IVS thickness, mm | 8±1.5 | 9±1.0 | 0.720 |
| PW thickness, mm | 9±1.6 | 8±1.7 | 0.402 |
| LVEF, % | 59 (55-60) | 60 (55-62) | 0.063 |
BMI: body mass index; DBP: diastolic blood pressure; IVS: interventricular septal; LA: left atrial; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; PW: posterior wall; SBP: systolic blood pressure.
Baseline laboratory parameters are listed in Table 2. Patients with PAF had significantly higher high-sensitivity C-reactive protein (hs-CRP) levels than the control group (2.4 [1.0-7.2] vs. 1.1 [1.0-2.0] mg/dl, p=0.018).
Baseline laboratory characteristics of patients with paroxysmal atrial fibrillation and healthy controls.
| Variables | Patients with PAF (n=56) | Control group (n=31) | p |
|---|---|---|---|
| Glucose, mg/dl | 91±5.9 | 86±6.5 | 0.842 |
| Urea, mg/dl | 32 (27-46) | 32 (25-38) | 0.206 |
| Creatinine, mg/dl | 0.9 (0.7-1.0) | 1 (0.9-1.1) | 0.986 |
| Potassium, mmol/l | 4 (3.8-4.4) | 4 (3.7-4.3) | 0.778 |
| Sodium, mmol/l | 137 (135-138) | 137 (136-138) | 0.793 |
| Hemoglobin, g/dl | 14±1.7 | 13.8±1.8 | 0.914 |
| WBC, 103/μl | 10 (9.3-13.1) | 10.5 (9.0-14.2) | 0.735 |
| PlateletS, 103/μl | 231(196-268) | 230 (196-326) | 0.705 |
| ESR, mm/hour | 16 (11-36) | 15 (10-37) | 0.960 |
| hs-CRP, mg/dl | 2.4 (1.0-7.2) | 1.1 (1.0-2.0) | 0.018 |
| Total cholesterol, mg/dl | 169 (149-211) | 176 (151-211) | 0.885 |
| HDL cholesterol, mg/dl | 44 (33-54) | 40 (31-49) | 0.496 |
| LDL cholesterol, mg/dl | 113 (92-154) | 112 (87-158) | 0.860 |
| TSH, mlU/l | 3.1±2.1 | 3.4±1.7 | 0.443 |
ESR: erythrocyte sedimentation rate; HDL: high-density lipoprotein; hs-CRP: high-sensitivity C-reactive protein; LDL: low-density lipoprotein; TSH: thyroid-stimulating hormone; WBC: white blood cell count.
OS parameters of the study population are shown in Table 3. Patients with PAF had significantly higher TOS (13.7 [12.2-15.9] vs. 11.2 [10.5-13.1] μmol H2O2 equivalent/l, p=0.001), OSI (1.5±0.37 vs. 1.2±0.35 arbitrary units, p=0.001), and 8-OHdG (2.32 [1.54-3.22] vs. 1.74 [1.50-2.22] pg/ml, p=0.019) levels, compared to the control group.
Oxidative stress parameters in the study population.
| Variable | Patients with PAF (n=56) | Control group (n=31) | p |
|---|---|---|---|
| TAC, Trolox equivalent/l | 0.96 (0.87-1.04) | 0.10 (0.87-1.22) | 0.285 |
| TOS, μmol H2O2 equivalent/l | 13.7 (12.2-15.9) | 11.2 (10.5-13.1) | 0.001 |
| OSI, arbitrary units | 1.5±0.37 | 1.2±0.35 | 0.001 |
| 8-OHdG, pg/ml | 2.32 (1.54-3.22) | 1.74 (1.50-2.22) | 0.019 |
8-OHdG: 8-hydroxy-2′-deoxyguanosine; OSI: oxidative stress index; TAC: total antioxidant capacity; TOS: total oxidant status.
In correlation analysis, serum TOS levels were positively correlated with 8-OHdG (r=0.348, p=0.001), OSI (r=0.822, p=0.001), and hs-CRP (r=0.325, p=0.002), whereas they were negatively correlated with TAC (r=-0.300, p<0.005).
When ROC curve analysis was performed to identify the optimal cut-off value of TOS for predicting PAF, TOS ≥12.2 was predictive of PAF with a sensitivity of 82% and specificity of 76% (area under the curve: 0.785, 95% confidence interval [CI]: 0.687-0.883, p<0.001) (Figure 1).
Univariate regression analysis showed that BMI, hs-CRP, 8-OHdG, TOS, and OSI levels were possible predictors of PAF. Multivariate logistic regression analysis revealed that serum TOS level (odds ratio: 1.608; 95% CI: 1.188-2.176; p=0.002) was an independent predictor of PAF (Table 4).
Univariate and multivariate logistic regression analysis identifying independent predictors of paroxysmal atrial fibrillation.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| BMI | 1.181 (1.000-1.396) | 0.050 | 1.201 (0.987-1.426) | 0.057 |
| 8-OHdG | 1.076 (1.020-1.135) | 0.008 | 1.059 (0.996-1.125) | 0.065 |
| TOS | 1.653 (1.283-2.131) | 0.001 | 1.608 (1.188-2.176) | 0.002 |
| OSI | 10.088 (2.613-38.946) | 0.001 | 0.393 (0.030-5.113) | 0.476 |
| hs-CRP | 1.267 (1.040-1.544) | 0.019 | 1.132 (0.944-1.358) | 0.181 |
| WBC | 0.967 (0.838-1.116) | 0.645 | ||
| ESR | 1.001 (0.976-1.026) | 0.960 | ||
| Creatinine | 1.173 (0.024-1.262) | 0.084 | ||
8-OHdG: 8-hydroxy-2′-deoxyguanosine; BMI: body mass index; ESR: erythrocyte sedimentation rate; hs-CRP: high-sensitivity C-reactive protein; OR: odds ratio; OSI: oxidative stress index; TAC: total antioxidant capacity; TOS: total oxidant status; WBC: white blood cell count.
In this study, we found that TOS and DNA damage levels were significantly higher in patients with PAF than in healthy controls. In addition, we demonstrated that TOS was an independent predictor of PAF. To the best of our knowledge, this is the first study to investigate TOS and DNA damage in patients with PAF.
PAF is defined as AF that typically ends within 48 hours. Structural and electrophysiological changes play an important role in the development of AF. This is a time-dependent process that can be explained as adaptive changes of myocytes to protect the atrium from external stress. The amount and duration of exposure to external stress are the most critical factors. Although short- and medium-term exposure (30 min and 1 week) affects myocytes and the extracellular matrix, changes in this period are generally reversible. However, long-term exposure (≥5 weeks) leads to irreversible changes.15,16 In addition, atrial remodeling begins in sinus rhythm long before the onset of AF in patients with recurrent PAF. It is therefore important to identify patients with PAF at an early stage.
OS is one of the principal external stresses during the AF process. It involves an increase in oxidative agents and a decrease in antioxidant levels, and contributes to the progression of atrial remodeling. In addition, OS can lead to DNA damage. In a healthy body exposed to OS, antioxidant mechanisms are activated to overcome oxidative damage. There are two types of antioxidant defense systems in the body: the enzymatic system, comprising various antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, and catalase; and a non-enzymatic system that includes various proteins, vitamins (especially C and E), beta-carotene, and reduced glutathione.4 Previous studies have demonstrated significant increases in OS and DNA damage in various cardiac diseases.17–20 OS is thus presumably associated with the progression of cardiac disease. We aimed to further investigate the roles of OS and DNA damage in patients with PAF.
TOS and TAC, which can be measured more easily and economically than oxidants and antioxidants separately, are generally used as markers of OS levels, while 8-OHdG is a strong marker of DNA damage.10,11 Previous studies have demonstrated the negative effects of oxidative damage on the atrial myocardium in patients with chronic AF.9 However, the precise onset of these changes is unknown.
To the best of our knowledge, no study has investigated the effects of oxidative damage on atrial remodeling in patients with PAF. In this study, we found that serum TOS and 8-OHdG levels were significantly higher in patients with PAF than in the control group. In addition, TOS levels were positively correlated with 8-OHdG levels. These findings suggest that DNA damage due to elevated OS begins early in the PAF process. OS and DNA damage are presumably important factors that contribute to the development of atrial remodeling in patients with PAF. Therefore, early diagnosis of elevated OS and DNA damage may help delay progression from PAF to chronic AF by enabling early preventive interventions, including antioxidant treatments such as lifestyle changes or vitamin supplements. Recent studies have demonstrated that lifestyle interventions and dietary factors may affect the risk of AF. For example, it has been reported that high intake of fish-derived n-3 polyunsaturated fatty acids from diet or supplements may prevent AF episodes following cardiovascular events.21 In addition, a randomized controlled trial assessing the impact of the Mediterranean diet on incident AF showed that a Mediterranean diet including olive oil reduced the risk of AF.22 Moreover, the Routine versus Aggressive risk factor driven upstream rhythm Control for prevention of Early atrial fibrillation in heart failure (RACE 3) study confirmed that targeted treatment of underlying conditions, including physical activity and dietary restrictions, improved maintenance of sinus rhythm in patients with persistent AF.23 All of these results suggest that diet is an important factor in the prevention and management of AF.
Inflammation plays a critical role in cardiovascular disease.24 Previous studies have shown that inflammation is important in predicting the development of PAF.25,26 In the present study, we investigated inflammatory markers including hs-CRP, erythrocyte sedimentation rate and white blood cell count, and found that only hs-CRP was significantly higher in patients with PAF than in the control group. Nonetheless, when inflammatory markers and OS parameters were included in multivariate regression analysis, we found that TOS was the only independent predictor of PAF development. These results suggest that TOS has greater clinical significance than other inflammatory markers in predicting the development of PAF.
The most important limitations of our study were its small sample size and its single-center nature. In addition, this study only compared patients with PAF to healthy individuals; it would also have been valuable to compare patients with PAF to patients with permanent and chronic AF. Moreover, we measured only the baseline levels of oxidative parameters and did not perform serial measurements. Measurement of OS and DNA damage parameters after conversion of AF to normal sinus rhythm could have provided additional information to our study. Finally, the duration of AF may be associated with substrate remodeling, and longer durations may reflect different levels of TOS and DNA damage. However, the duration of AF from the initial diagnosis was not recorded in this study. Larger prospective studies are needed to better determine the associations of oxidative parameters with PAF.
ConclusionThe present study showed that TOS and DNA damage were significantly greater in patients with PAF than in the control group. Therefore, we propose that TOS and DNA damage can be used to detect patients at higher risk of AF.
Conflicts of interestThe authors have no conflicts of interest to declare.