Valvular heart disease (VHD) is increasing worldwide, mostly because of aging. Percutaneous valve intervention is the preferred therapeutic option in high-risk patients.
ObjectiveTo characterize the profiles of patients with VHD admitted to the cardiology ward at a tertiary referral center.
MethodsOn the basis of ICD-9 codes for VHD, the discharge notes of 287 patients hospitalized over a 22-month period were reviewed and analyzed. One hundred characteristics were considered.
ResultsMedian age was 74 (23-93) years, and 145 (51%) were male. The admissions were elective (for valve intervention) in 36%. Heart failure (HF) was the reason for urgent admissions in 29.3%. Multiple comorbidities were observed in 53% of patients. Etiology of VHD was degenerative in 68%, functional in 15.3% and rheumatic (predominantly in women and younger patients) in 8.7%. Aortic valve disease was present in 63% (aortic stenosis in 56%), and was associated with HF (p=0.004), atrial fibrillation (AF) (p=0.01), and left ventricular (LV) dilatation (p=0.003) or hypertrophy (p<0.001). Mitral valve disease (51%), mostly mitral regurgitation (degenerative or functional), predominated in women, and was associated with HF, AF, LV dilatation (p<0.001) and reduced LV ejection fraction (p=0.003). Significant tricuspid regurgitation (34.8%) associated with the presence of previously implanted cardiac devices (p<0.001). Valve intervention (mostly transcatheter aortic valve implantation) was performed in 41% of patients. Mean length of hospital stay was 12±14.3 days and overall in-hospital mortality was 9.8%.
ConclusionsNowadays, the profiles of hospitalized patients with VHD are dominated by the elderly, with degenerative disease and multiple comorbidities, presenting with HF, AF and LV remodeling, who frequently undergo valve intervention, usually via a percutaneous approach. Mortality remains significant in this high-risk population.
A doença cardíaca valvular (DCV) é problema crescente, relacionando-se com o envelhecimento populacional. A intervenção valvular por via percutânea é opção preferencial em alguns contextos, inclusive em doentes de elevado risco.
ObjetivoCaracterização do perfil dominante do doente com DCV admitido em enfermaria de cardiologia (centro terciário de referência).
MétodosRevisão de processos clínicos de 287 doentes (códigos ICD-9 para DCV) internados num período de 22 meses. Foram consideradas para análise 100 características.
ResultadosIdade - 74 (23-93) anos; 145 (51%) homens. Admissões eletivas (intervenção valvular): 36%. Insuficiência cardíaca (IC): causa de admissão urgente em 29,3%. Comorbilidades múltiplas: 53% dos doentes. Etiologias: degenerativa (68%); funcional (15,3%); reumática (8,7%) – predominantemente em mulheres e em doentes mais jovens. Doença valvular aórtica - 63% (estenose em 56%), associou-se à presença de IC (p = 0,004), fibrilhação auricular (FA)- p = 0,014 – e hipertrofia (p < 0,001) ou dilatação ventricular esquerda (VE) – p = 0,003. Doença valvular mitral (51%) - predominantemente regurgitação - degenerativa ou funcional, mais frequente em mulheres; associou-se à presença de IC, FA, dilatação VE – p < 0,001 - e fração de ejeção VE diminuída (p = 0,003). Insuficiência tricúspide (34,8%), associou-se à presença de eletrocatéteres previamente implantados (p < 0,001). Intervenções valvulares: 41% dos doentes, predominantemente TAVI. Duração de internamento: 12 ± 14,3 dias; mortalidade global intra-hospitalar: 9,8%.
ConclusõesO perfil atual do doente hospitalizado com DCV é dominado pelo idoso com doença degenerativa e múltiplas comorbidades, apresentando remodelagem VE, IC e FA, e sendo frequentemente submetido a intervenção valvular (predominantemente por via percutânea). A mortalidade é significativa nessa população de risco elevado.
Valvular heart disease (VHD) has recently been described as “the next cardiac epidemic”,1 in particular due to its increased prevalence in the elderly mainly from degenerative causes in industrialized countries.1,2 However, rheumatic etiology is still the most frequent cause of VHD in developing countries and predominantly affects younger populations.3
The epidemiology of VHD is especially difficult to study as most cases run their natural history without significant symptoms for varying periods of time, and imaging studies (particularly echocardiography) are required for accurate diagnosis and characterization.2,3 Based on data from large population-based epidemiological studies in the USA which included individuals from the general population who had been assessed prospectively with echocardiography,3 the estimated prevalence of significant VHD in developed countries is 2.5%. Mitral regurgitation (MR) was found to be the most frequent VHD, especially in the elderly.3 However, in the Euro Heart Survey, a prospective study conducted in 25 European countries that included inpatients and outpatients with moderate to severe VHD, infective endocarditis, or prior valve intervention,4 the most frequent VHD (43.1%) was aortic stenosis (AS), predominantly of degenerative cause. Rheumatic etiology was found in 22% of cases.4
Published data clearly show a high prevalence of AS and MR in industrialized countries, where VHD mainly affects the elderly population due to increased life expectancy, and in association with functional and structural cardiac abnormalities.2,5 VHD is linked to significant cardiovascular and all-cause mortality, independently of ventricular function and comorbidities.5
Degenerative AS, a consequence of a continuous process of valve sclerosis and calcification,6 increases significantly in prevalence with age,2,3,6,7 and around 40% of individuals aged over 75 years are estimated to have a calcified aortic valve. When intervention is indicated, the decision between surgical aortic valve replacement (SAVR) or transcatheter aortic valve implantation (TAVI) should be made by the heart team according to the individual patient's characteristics.8
Functional MR is the most frequent valve disease in the USA,3,9,10 as a consequence of the left ventricular (LV) remodeling and dilatation commonly seen in both non-ischemic cardiomyopathy (mostly due to hypertensive heart disease or idiopathic dilated cardiomyopathy) and ischemic heart disease.10 As the prevalence of heart failure (HF) is increasing, functional MR is also likely to increase in the future. In patients with severe primary or secondary (functional) MR, in addition to optimal medical management of HF,11 valve repair or replacement may be necessary, and the recent advent of percutaneous techniques has provided an effective and safe way of improving HF symptoms in suitable candidates.8,11,12
In developing countries rheumatic heart disease (which mainly affects the mitral valve) is still the major cause of VHD, due to the persistently high prevalence of rheumatic fever, and is associated with reduced life expectancy.2,13,14 The prevalence of rheumatic heart disease in developed countries has decreased, but as degenerative valve disease affecting the elderly is increasing,14 the burden of VHD will is likely to grow substantially in the future.3
The present study aims to characterize the current profiles of patients with significant VHD admitted over a period of 22 consecutive months to the cardiology ward at a large referral tertiary hospital center in southwestern Europe (Lisbon, Portugal).
MethodsData sourcesPatient data were acquired by searching for electronic discharge notes coded with a diagnosis of valve disease according to the International Classification of Diseases, Ninth Revision (ICD-9) of all patients admitted to the cardiology ward in the Cardiology Department of Santa Maria University Hospital between January 1, 2014 and October 3, 2015 (see Appendix 1 for details).
The search identified 391 patients, whose discharge notes were then manually reviewed. Only patients with significant VHD and those with prior heart valve interventions were selected. Accordingly, only patients who underwent echocardiographic assessment during the hospitalization under study (index hospitalization) were included.
Significant VHD was defined on the basis of clinical plus imaging criteria (mainly echocardiography),15,16 and included moderate to severe valvular stenosis and/or regurgitation of any cardiac valve (or multiple valves) that was responsible in any way for the index hospitalization. Left ventricular ejection fraction (LVEF) was considered to be reduced if <50%.17
Study population and designIn accordance with the above criteria, 66 patients were excluded for presenting only mild valvular disease; additionally, 38 patients were also excluded because of insufficient information available in the system (patients admitted electively to undergo selective examinations only or patients with hospital stay less than 24 hours). The final study population included 287 patients.
Discharge notes of these patients were carefully manually reviewed. In addition to characterization of VHD (valves involved, disease severity and etiology) on the basis of all the available information, the following data were collected and analyzed: demographics (gender, age, ethnicity, place of birth), admission and discharge dates, type of admission to the cardiology ward (elective or urgent), main reason for hospital admission, length of hospital stay (LOS), major cardiovascular risk factors (systemic hypertension, diabetes, dyslipidemia, smoking), presence and type of comorbidities, history of chronic HF and New York Heart Association (NYHA) functional class, presence of advanced atrioventricular (AV) block, bundle branch block (BBB) or atrial fibrillation (AF) (chronic or new-onset), presence of pulmonary hypertension (assessed invasively or by echocardiography), coronary artery disease (CAD) (assessed by coronary angiography), coronary artery bypass grafting (CABG) during the index admission, implanted cardiac devices (during the index hospitalization or in the past), valvular intervention (during the index hospitalization or in the past), hospital admissions due to VHD in the 12 months preceding the index hospitalization, mortality (and cause of death) during the index hospitalization, and destination after discharge. Various echocardiographic parameters and indices (assessed during the index hospitalization before any new valvular intervention) were also collected, including cardiac chamber size and measures of systolic LV and right ventricular (RV) function.
In addition to the descriptive data, we looked for associations between the type and etiology of VHD and demographic, clinical, electrocardiographic and echocardiographic features, in order to improve characterization of the profiles of hospitalized patients with VHD.
Statistical analysisThe statistical analysis was performed with Microsoft Excel 2013® and IBM SPSS® version 22.0. A descriptive analysis of the different variables was performed and possible differences between genders were assessed. Quantitative variables are expressed as means ± standard deviation, and qualitative variables as absolute or relative frequencies (percentages). The Student's parametric t test for independent variables was used to compare quantitative variables as a function of the presence and type of VHD (mitral, aortic, mitral-aortic or tricuspid valve disease). Categorical variables were analyzed using the chi-square test and Fisher's exact test, as appropriate. Possible associations between type of VHD and demographic, clinical, electrocardiographic and echocardiographic features were analyzed using Pearson's correlation test.
A p-value of <0.05 for a confidence interval of 95% was considered statistically significant.
ResultsPopulation characteristics and interventions are displayed in Table 1.
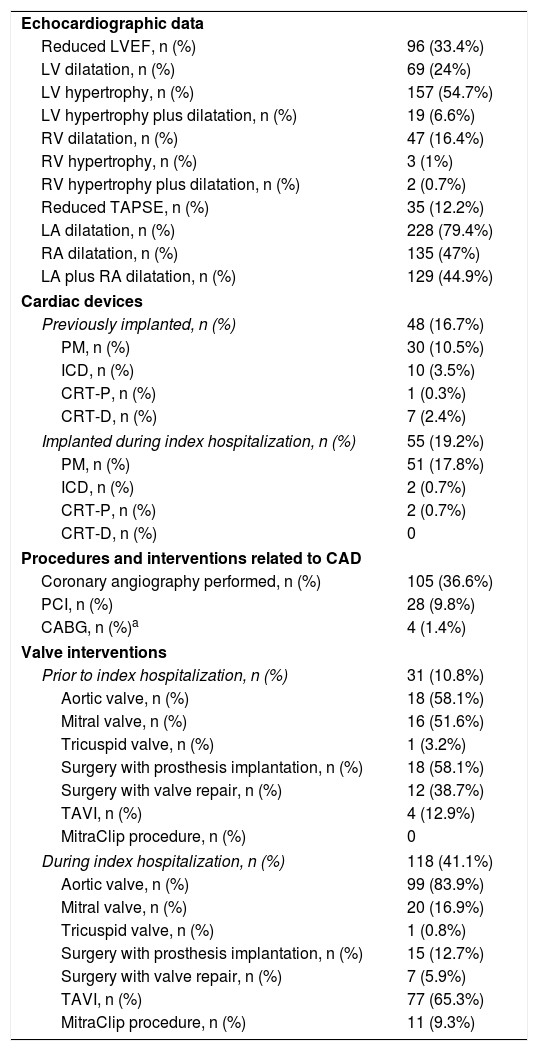
Population with valvular heart disease: characteristics and procedures.
| Echocardiographic data | |
| Reduced LVEF, n (%) | 96 (33.4%) |
| LV dilatation, n (%) | 69 (24%) |
| LV hypertrophy, n (%) | 157 (54.7%) |
| LV hypertrophy plus dilatation, n (%) | 19 (6.6%) |
| RV dilatation, n (%) | 47 (16.4%) |
| RV hypertrophy, n (%) | 3 (1%) |
| RV hypertrophy plus dilatation, n (%) | 2 (0.7%) |
| Reduced TAPSE, n (%) | 35 (12.2%) |
| LA dilatation, n (%) | 228 (79.4%) |
| RA dilatation, n (%) | 135 (47%) |
| LA plus RA dilatation, n (%) | 129 (44.9%) |
| Cardiac devices | |
| Previously implanted, n (%) | 48 (16.7%) |
| PM, n (%) | 30 (10.5%) |
| ICD, n (%) | 10 (3.5%) |
| CRT-P, n (%) | 1 (0.3%) |
| CRT-D, n (%) | 7 (2.4%) |
| Implanted during index hospitalization, n (%) | 55 (19.2%) |
| PM, n (%) | 51 (17.8%) |
| ICD, n (%) | 2 (0.7%) |
| CRT-P, n (%) | 2 (0.7%) |
| CRT-D, n (%) | 0 |
| Procedures and interventions related to CAD | |
| Coronary angiography performed, n (%) | 105 (36.6%) |
| PCI, n (%) | 28 (9.8%) |
| CABG, n (%)a | 4 (1.4%) |
| Valve interventions | |
| Prior to index hospitalization, n (%) | 31 (10.8%) |
| Aortic valve, n (%) | 18 (58.1%) |
| Mitral valve, n (%) | 16 (51.6%) |
| Tricuspid valve, n (%) | 1 (3.2%) |
| Surgery with prosthesis implantation, n (%) | 18 (58.1%) |
| Surgery with valve repair, n (%) | 12 (38.7%) |
| TAVI, n (%) | 4 (12.9%) |
| MitraClip procedure, n (%) | 0 |
| During index hospitalization, n (%) | 118 (41.1%) |
| Aortic valve, n (%) | 99 (83.9%) |
| Mitral valve, n (%) | 20 (16.9%) |
| Tricuspid valve, n (%) | 1 (0.8%) |
| Surgery with prosthesis implantation, n (%) | 15 (12.7%) |
| Surgery with valve repair, n (%) | 7 (5.9%) |
| TAVI, n (%) | 77 (65.3%) |
| MitraClip procedure, n (%) | 11 (9.3%) |
CABG: coronary artery bypass grafting; CAD: coronary artery disease; CRT-D: cardiac resynchronization therapy defibrillator; CRT-P: cardiac resynchronization therapy pacemaker; ICD: implantable cardioverter-defibrillator; LA: left atrium; LOS: length of hospital stay; LV: left ventricular; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; PM: pacemaker; RA: right atrial; RV: right ventricular; SAVR: surgical aortic valve replacement; TAPSE: tricuspid annular plane systolic excursion; TAVI: transcatheter aortic valve implantation.
The study population included 142 females (49.5%) and 145 males (50.5%), mean age 74.86±13.39 years (range 23 to 93), with no difference between genders. Most patients were born in Portugal and 11% were originally from Portuguese-speaking African countries.
The majority of patients were admitted through the emergency room or by transfer from other departments or hospitals (66.9%), while 33.1% were elective admissions. Fifty-six patients (19.51%) were hospitalized for causes not directly related to VHD, primarily with acute coronary syndrome (ACS) (71.4% of these cases and 16.4% of all patients with VHD), and valvular disease was a secondary diagnosis.
Regarding all patients with a diagnosis of VHD, decompensated HF was the most frequent reason for admission (84 patients, 29.3%). This proportion was even higher in the group of 231 patients admitted for VHD (35.5%). Eighty-four (36.4%) were admitted electively for valve intervention, 70 (24.4%) because of a dysrhythmia (AF in 9.1%), 15 (5.2%) in the context of a syncopal episode (four of which were due to complete AV block), and seven (2.4%) due to infective endocarditis.
Forty patients (13.9%) had been hospitalized for reasons related to VHD in the 12 months preceding the index hospitalization.
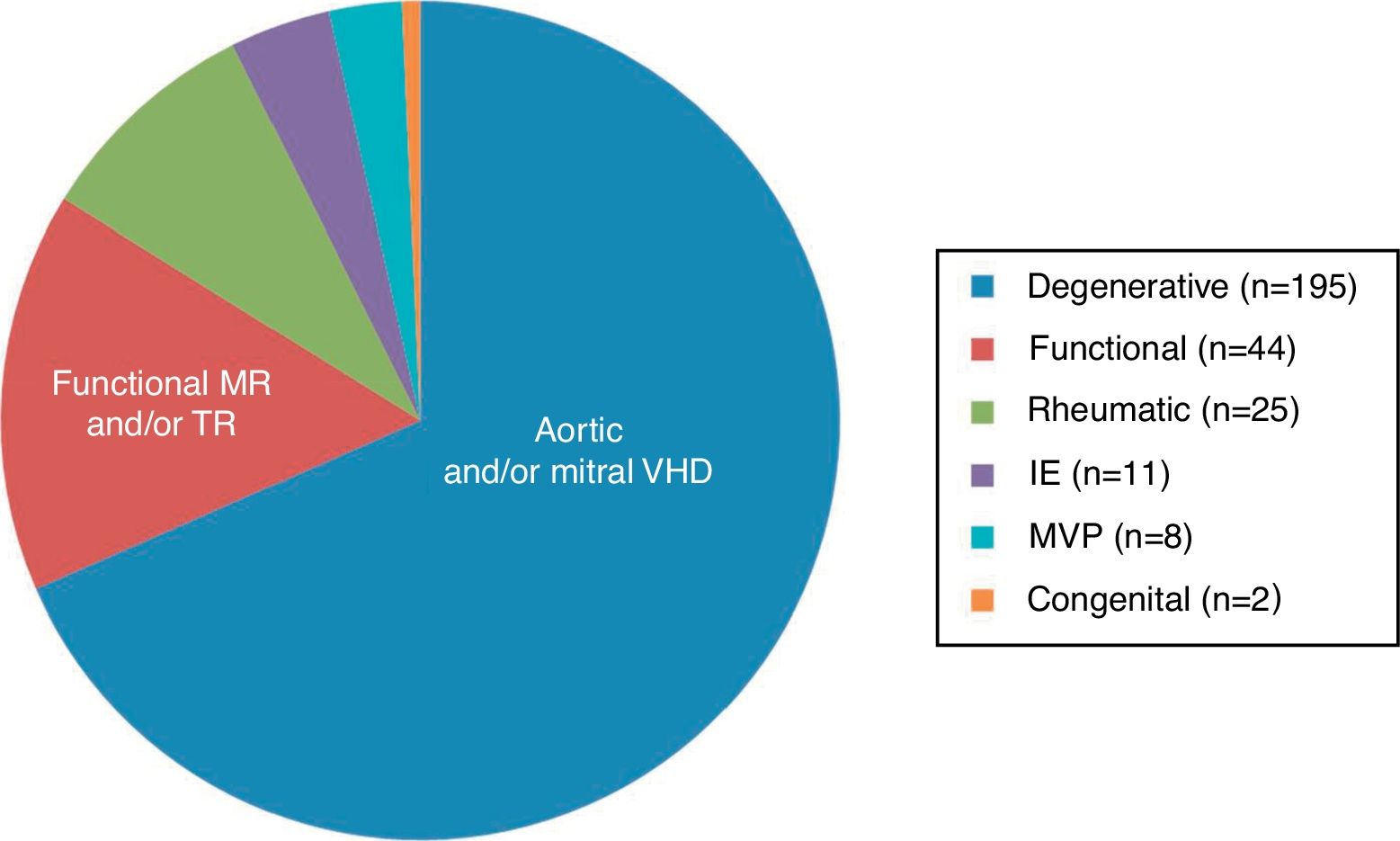
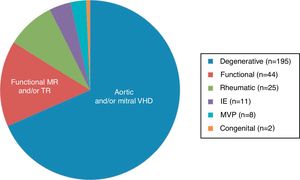
Etiology and type of valvular diseaseEtiologies and types of valvular disease are shown in Figure 1. Most patients (67.9%) had degenerative aortic and/or mitral valve disease, followed in frequency (15.3%) by significant functional mitral and/or tricuspid regurgitation (TR) (secondary to left and/or right ventricular dilatation due to ischemic heart disease or dilated cardiomyopathy). Rheumatic etiology was diagnosed in 8.7% of patients. Other etiologies included infectious endocarditis (3.8%), mitral valve prolapse (2.8%), and congenital VHD (0.7%).
Most patients with rheumatic valve disease were women (84%, p<0.001), with no significant gender differences observed in other etiologies. Age correlated directly with degenerative etiology (r=0.473; p<0.001), and inversely with rheumatic disease (r=-0.36; p<0.001).
Aortic valve disease was documented in most cases (181 patients, 63.1%), of whom 89% (56% of all patients) had AS, and 27.1% (17.1% of all patients) had aortic valve regurgitation. Mitral disease was diagnosed in 146 patients (50.9%), mostly MR (90.4%, 46% of all patients); mitral stenosis was observed in 15% of these patients (7.7% of all patients). Concomitant aortic and mitral valve disease was present in 22.3% of patients.
There were no documented cases of tricuspid valve stenosis, but significant TR was diagnosed in 100 patients (34.8%), and was associated with mitral valve disease in 69 patients (69.7% of patients with TR, 24% of all patients). Isolated moderate to severe TR was observed in 20 cases (6.97%).
Moderate pulmonary valve regurgitation was infrequent (13 patients), and was diagnosed in the context of predominant mitral valve disease and TR; it did not require targeted therapeutic intervention in any patient.
Cardiovascular risk factors and other comorbiditiesA significant proportion of patients admitted had chronic HF (39.4%), and 47.4% had chronic AF. Systemic hypertension was the most frequent cardiovascular risk factor observed, present in 219 (76.3%) patients, while 177 (61.7%) patients had more than one of the classical cardiovascular risk factors. Multiple comorbidities were found in 53% of patients. The most common was CAD (n=81; 28.21%), followed by chronic kidney disease (n=60; 20.9%), chronic anemia (n=76; 26.5%), chronic pulmonary disease (n=43; 15%) and malignancies (n=39; 13.6%).
Coronary angiography was performed in 105 (36.6%) of patients, mostly in the context of ACS, but also due to a previous history of CAD or as a standard procedure before valve intervention. Significant CAD was documented in 55 (52.4%) of cases. Coronary revascularization was performed in 32 (11.1%) patients, by a percutaneous approach in 28 and by CABG in four.
Electrocardiographic dataAF was diagnosed in 136 (47.4%) patients, and was significantly associated with aortic valve disease (p=0.014), mitral valve disease (p<0.001) or TR (p<0.001). Advanced AV block was also frequent, with 29 patients (10.1%) having complete AV block at presentation (16 patients with AS and 13 with MR; AS and MR coexisted in five patients). Complete AV block was the primary reason for pacemaker implantation in the index hospitalization. However, it was not a frequently documented cause of syncope on admission. Other causes of syncope at presentation were sinus node dysfunction and ventricular tachycardia.
Echocardiographic dataReduced (<50%) LVEF was documented in 33.4% of patients and was associated with mitral disease (p=0.003). In 12.2% of patients RV longitudinal systolic function assessed by tricuspid annular plane systolic excursion (TAPSE) was also compromised, coexisting with LV systolic dysfunction in 22 (62.9%) patients.
LV dilatation (24%) and LV hypertrophy (54.7%) were both associated with aortic valve disease, but only the former with mitral disease. The left atrium was dilated in 79.4% of patients, but there was no significant association with aortic or mitral valve disease. RV dilatation was present in 16.4% of cases and right atrial dilatation in 47%; both conditions were associated with the presence of severe TR.
Cardiac devicesForty-eight (16.7%) patients had a cardiac device in place prior to the index admission: a pacemaker (PM) in 30 (10.5%), an implanted cardioverter-defibrillator (ICD) in 10 (3.5%), and a cardiac resynchronization therapy (CRT) device in eight (2.7%) patients. Severe chronic TR was documented in 62.5% of these patients, and was associated with mitral valve disease in 21 (43.8%; significant chronic MR in 50% of these) and with aortic valve disease in 10 patients (20.8%; moderate or severe AS in 41.7%).
In addition, in 55 patients (19.2%) a device was implanted for the first time during the index hospitalization: a PM in 51 (17.8%) patients, an ICD in two (0.7%), and a CRT pacemaker in another two.
Associations between valvular heart disease and demographic, clinical and functional dataAortic valve diseaseDisease of the aortic valve showed no gender predominance, but correlated directly with age (p<0.001), degenerative etiology (p<0.001) and presence of HF (p=0.004), AF (p=0.014), LV dilatation (p=0.003), and LV hypertrophy (p<0.001). There was no significant association between aortic valve disease and rheumatic etiology, reduced LVEF, left atrial (LA) dilatation, AV block or bundle branch block (p=NS for all).
Mitral valve diseaseMitral disease was more frequent in women than in men (58.9% vs. 41.1%, p=0.002), and was associated directly with rheumatic etiology (p=0.011), ischemic heart disease (p<0.001), HF (p=0.001), AF (p<0.001), LV dilatation (p<0.001) and reduced LVEF (p=0.003). No significant association was found between mitral valve disease and LA dilatation.
Tricuspid valve regurgitationThe presence of moderate-to-severe TR did not differ between genders (p=0.702), and correlated directly with age (p<0.001) and with the presence of previously implanted cardiac devices (p<0.001). The latter association was observed only when TR coexisted with aortic or mitral disease (of any etiology). A significant association was also found between TR and mitral disease (p<0.001), right chamber dilatation (p<0.001), AF (p<0.001) and pulmonary hypertension (p<0.001), but not with chronic pulmonary disease.
Valve intervention proceduresThirty-one patients (10.8%) had previously undergone valve intervention (repair or replacement), most frequently SAVR (58.1%), followed by mitral valve repair (38.7%) and TAVI (12.9%). No patient had been previously submitted to percutaneous mitral valve intervention.
During the index hospitalization, 118 patients (41.1%) underwent valve intervention. Percutaneous procedures were the most frequently performed (74.6%), mainly TAVI (65.3%); surgical replacement was performed in 12.7% of patients (aortic valve in 12 patients, mitral valve in four), and surgical repair in 5.9% of cases (mitral valve in four patients and aortic valve in three). Percutaneous mitral valve interventions were performed in 11 (9.3%) patients.
Regarding the 31 patients who had previously undergone valve intervention, most were admitted with decompensated HF (48.4%), 16.1% were admitted electively for another valve procedure, and 6.5% presented with infectious endocarditis. Furthermore, 35.5% of this population underwent valve intervention again, 25.8% on the same valve. In-hospital mortality was 12.9% in this high-risk population.
Of note, 4 patients underwent CABG during the index hospitalization, and 2 also underwent SAVR.
Discharge and post-discharge dataOverall, mean LOS was 11.98±14.33 days. Most patients were discharged home from hospital (84.3%) and 5.2% were transferred to another hospital (in their area of residence) for continued care. Overall in-hospital mortality was 9.8% and cardiovascular mortality was 8.7%. Most deaths (33.3%) occurred in the context of ACS, followed by advanced refractory HF and cardiogenic shock due to VHD (29.6%), and to severe refractory pulmonary hypertension (related to valve disease and/or pulmonary embolism) in 7.4%. Other causes of death were infectious endocarditis of prosthetic valves (7.4%) or devices (3.7%), acute complications after prosthetic valve implantation (surgical or percutaneous) (7.4%), and pericardial effusion (3.7%).
DiscussionFrom an overall analysis of the data on this population, the first point to highlight is that significant VHD has no gender predominance, a finding consistent with the results of large population studies.2–4 The second point is the median age of the population, almost 10 years older than those included in the Euro Heart Survey.4 According to Eurostat 2015 data,18 the proportion of people aged 65 years or more in Portugal was 20.5%, compared to the overall median percentage of 19% in Europe, but this small difference does not appear to account for the discrepancy. The most likely explanation is that the Euro Heart Survey included both inpatients and outpatients, whereas the cohort presented here included only inpatients, a population with more advanced and severe disease, and probably also an older one compared to VHD patients in an outpatient setting.1,4 This difference would also explain the 56% of patients with AS found in our hospitalized population compared to the 43% reported in the Euro Heart Survey.4 Of note, we found a predominance of females in patients with rheumatic valve disease and consequently in patients with mitral disease (which was significantly associated with the latter), and rheumatic etiology mainly affected younger patients.
Although about 11% of included patients were born in Portuguese-speaking African countries, most of them had been living in Portugal for decades, and it was thus impossible to compare the epidemiology of valve disease in Africa and Portugal. In the VALVAFRIC study, a prospective hospital registry of patients with rheumatic valve disease in west and central sub-Saharan Africa, 40.2% presented with moderate to severe disease, the median age of the population was 29.3±15.6 years, and female gender predominated (60%).14
Decompensated HF was the main reason for admission in our cohort, reflecting the long-term evolution of valve disease, the advanced age of the population, and the severity of the clinical condition of patients with VHD needing hospitalization. Additionally, AF and pulmonary hypertension related to valve disease were both frequent conditions with significant hemodynamic consequences. Underlying the clinical impact of VHD, a significant proportion of patients also presented with echocardiographic evidence of LV hypertrophy and/or dilatation, features linked to an unfavorable prognosis.19 Contrary to expectations, neither aortic nor mitral valve disease was significantly associated with LA dilatation, highlighting the multiplicity of conditions that can contribute to alterations in atrial architecture and function.
Aging is associated with degenerative changes that affect not only the aortic annulus but also the conduction system, and complete AV block was another frequent reason for hospital admission, particularly in patients with degenerative AS, and was a common indication for PM implantation. Advanced AV block was also present in many patients with MR, albeit less frequently, as it may occur as a consequence of CAD and fibrosis.
The majority of patients with VHD had several cardiovascular risk factors and multiple comorbidities, mostly significant CAD, another frequent cause for hospital admission. In a recent retrospective study, Emren et al.20 assessed the prevalence of concurrent CAD in 241 patients (51% female) who underwent surgery due to severe VHD. CAD was detected in 57.7% of patients with AS and in 41.9% of those with MR. CAD and severe AS frequently coexist,20–22 although the significance and severity of CAD in AS may be particularly difficult to assess. Concomitant CAD had a clinical negative impact in our cohort, being responsible for 33% of all-cause in-hospital mortality, highlighting the need for CAD to be managed concurrently during hospitalization for VHD.
Coronary revascularization at the time of aortic valve replacement may be associated with improved long-term survival without affecting operative risk in some patient subsets.23 However, this is an open-ended issue and most studies looking at the outcomes of CAD and PCI in patients undergoing TAVI reveal no benefit in terms of mortality or major cardiovascular events.22–27
A significant proportion of patients (35%) presented with TR associated with other valve disease, mostly mitral disease, thus showing a significant association with rheumatic disease and with secondary MR. Dilated cardiomyopathy was particularly observed, in agreement with the current literature.1 Besides, cardiac devices previously implanted in the right heart were associated with development of TR, as previously described,1 with most patients also having MR. Decision for intervention in moderate-to-severe secondary TR is frequently a matter of debate, being recommended when left-sided valve surgery is indicated, or when right HF is manifest.8
In the index hospitalization 40% of patients underwent valve intervention, primarily TAVI, reflecting recent changes in the paradigm regarding valve disease management. Calcified AS is associated with a higher risk of myocardial infarction, stroke and death, independently of traditional cardiovascular risk factors, and since there is no effective medical approach and five-year survival without intervention ranges from 15% to 50%,9 percutaneous valve interventions are increasingly important in the setting of the old, frail patient with multiple comorbidities, who frequently is not considered suitable for surgery.6
In summary, the findings in the cohort presented herein may be considered a real-world picture of current VHD in developed countries. Globally, VHD is most commonly degenerative, mainly affects the elderly with multiple comorbidities, is severe and has already run a long course. It is frequently associated with CAD, HF, AF and advanced conduction disease, and has an unfavorable impact on prognosis. This includes quality of life (particularly symptoms and long LOS) and in-hospital mortality. Valve intervention is often necessary, and the percutaneous approach is a therapeutic option in many of these high-risk patients.
LimitationsThe data presented are derived from a single center and may not reflect the circumstances of other centers specializing in the management of patients with VHD. Furthermore, the information was not acquired in the setting of a prospective registry, but consists of retrospective data collected from clinical files and echocardiographic reports, which in some cases may not be as detailed as would be desirable. However, the data collected reflect the actual profiles of patients hospitalized with VHD in the cardiology ward of a tertiary hospital, and the thorough manual analysis and multiple revisions performed provide a precise and objective set of real-world information, similar in general respects to previous work published in this field.
The influence of the different variables on mortality was not studied, as this was beyond the scope of this study. Our aim was to characterize the profiles of patients with VHD currently admitted to a tertiary hospital in contemporary Europe, in which the new therapeutic options for patients with VHD are available, and where the European guidelines for the treatment of heart disease are applied.
ConclusionOn the basis of the data presented, two main profiles can be delineated for patients currently hospitalized with VHD: a dominant one, characterized by the elderly patient, male or female, with multiple comorbidities, admitted with decompensated HF in the setting of degenerative AS, presenting with LV hypertrophy and/or dilatation but with preserved LVEF, frequently with AF, who is preferably treated by TAVI; and a second, less prevalent profile, that of a younger patient, predominantly female, also admitted with decompensated HF but in the setting of functional mitral regurgitation (secondary to ischemic heart disease or dilated cardiomyopathy), also presenting with AF and LV dilatation but with reduced LVEF, who less often undergoes valve intervention, but is treated medically for HF. Of note, common denominators of both profiles were HF and AF.
Author contributionsAFE and DB conceived and designed the research, and drafted the first version of the manuscript; JR, IR, MMP, and FV contributed to the research and data analysis; AFE and RP performed the statistical analysis. All authors critically reviewed the manuscript for drafting and key intellectual content, helped revise the paper and gave final approval for the version to be published.
Conflicts of interestsThe authors have no conflicts of interest to declare.
ICD-9 (International Classification of Diseases) diagnosis of valve diseases used:
394.0 (rheumatic mitral stenosis), 394.1 (rheumatic mitral regurgitation), 394.2 (rheumatic mitral stenosis with insufficiency), 394.9 (other and unspecified mitral valve diseases), 395.0 (rheumatic aortic stenosis), 395.1 (rheumatic aortic insufficiency), 395.2 (rheumatic aortic stenosis with insufficiency), 395.9 (other and unspecified rheumatic aortic diseases), 396.0 (mitral valve stenosis and aortic valve stenosis), 396.1 (mitral valve stenosis and aortic valve insufficiency), 396.2 (mitral valve insufficiency and aortic valve stenosis), 396.3 (mitral valve insufficiency and aortic valve insufficiency), 396.8 (multiple involvement of mitral and aortic valves), 396.9 (mitral and aortic valve diseases, unspecified), 397.0 (diseases of tricuspid valve), 397.1 (rheumatic diseases of pulmonary valve), 397.9 (rheumatic diseases of endocardium, valve unspecified), 424.0 (mitral valve disorders), 424.1 (aortic valve disorders), 424.2 (tricuspid valve disorders, specified as nonrheumatic), 424.3 (pulmonary valve disorders) and/or 424.9 (endocarditis, valve unspecified).
Ana Fátima Esteves and Dulce Brito are both first authors in the study.