Pediatric obesity is increasingly prevalent in the Portuguese population. Adipocyte dysfunction results in the expression of pro-inflammatory mediators that are responsible for the low-grade inflammatory process that characterizes obesity.
ObjectivesThe aim of this study was to investigate the relationship between markers of adiposity, inflammation and adipokines in a Portuguese obese pediatric population.
MethodsOne hundred and twenty children of both sexes, aged 6–17 years, were included in this study. The control group consisted of 41 healthy normal-weight children. The variables analyzed were age, gender, body mass index, waist circumference, fat mass percentage, high-sensitivity C-reactive protein (hs-CRP), leptin and adiponectin.
ResultsThere were significant differences between controls and obese children for all parameters analyzed. In the obese group, after controlling for age and gender, hs-CRP (p=0.041), adiponectin (p=0.019) and leptin (p<0.001) still showed significant statistical differences. A direct correlation was found between hs-CRP, leptin, body mass index and waist circumference, the strongest being with leptin (r=0.568; p<0.001). This trend remained statistically significant, regardless of gender or pubertal age.
ConclusionsConsidering the role of leptin, adiponectin and hs-CRP in the genesis of endothelial dysfunction, they may be used in clinical practice for risk stratification, as well as in the assessment of weight control programs.
A obesidade pediátrica é prevalente na nossa população. A adiposidade resulta na expressão de marcadores pró-inflamatórios que são responsáveis pelo processo de inflamação de baixo grau que caracteriza a obesidade.
ObjetivosTivemos como objetivo avaliar a relação entre marcadores de adiposidade, inflamação e adipocinas num grupo de crianças portuguesas obesas.
MétodosForam incluídas no estudo 120 crianças obesas, entre os 6 e 17 anos de idade. O grupo controlo continha 41 crianças saudáveis, sem excesso de peso, dentro da mesma faixa etária. As variáveis analisadas foram: idade, género, índice de massa corporal, circunferência abdominal, percentagem de massa gorda, proteína C-reativa ultra-sensível, leptina e adiponectina.
ResultadosTodos os parâmetros analisados encontravam-se significativamente mais elevados no grupo de crianças obesas. No grupo obeso, após avaliação por regressão logística, ajustando à idade e género, a proteína C-reativa ultra-sensível (p=0,041), adiponectina (p=0,019) e leptina (p<0,001), mantiveram significado estatístico. Os marcadores de adiposidade correlacionaram-se diretamente com a leptina (p=0,001) e inversamente com a adiponectina (p=0,029). Encontrámos também uma correlação direta entre a proteína C-reactiva ultra-sensível, leptina, índice de massa corporal e circunferência abdominal, sendo a correlação mais robusta com a leptina (ρ=0,568; p<0,001). Esta tendência manteve-se independentemente do género ou idade puberal.
ConclusõesConsiderando a relação da leptina, adiponectina e proteína C-reativa na génese da disfunção endotelial, estes poderão ser úteis na prática diária para estratificação de risco, assim como, para avaliar medidas implementadas nos programas para redução de peso em crianças obesas.
body mass index
fat mass percentage
high-sensitivity C-reactive protein
tumor necrosis factor alpha
waist circumference
Obesity is considered an independent cardiovascular risk factor,1,2 and in the adult population its relationship with cardiovascular disease is well established. Due to its prevalence, childhood obesity is considered an epidemic by the World Health Organization.3 About 10% of the world's pediatric population is overweight or obese, and it is estimated that 40% of these will be obese as adults.4
In children, obesity is defined by a body mass index (BMI) above the 95th percentile for age and gender.5 However, various studies have shown that body fat distribution, particularly visceral fat, rather than BMI, is linked to cardiovascular events.6,7 About 85% of adipose tissue is subcutaneous, the remainder being located in the abdominal cavity, both intra- and retroperitoneally, and this relationship is maintained regardless of the individual's weight. Magnetic resonance imaging and computed axial tomography are the gold standard references used to quantify visceral fat. However, in clinical practice, access to these exams is limited, and hence waist circumference (WC) is used to infer visceral fat,8 with which it is strongly correlated, as well as having predictive value regarding cardiovascular events.
Adipose tissue is a metabolically active organ that produces various bioactive substances, known as adipokines, involved in metabolic, endocrine and immunological processes.9,10 Of these substances, leptin and adiponectin are the most specifically related to adipose tissue.
Leptin is a hormone closely linked to adiposity, and its levels are influenced by the amount of fat mass and adipocyte size.11 It is a non-glycosylated protein encoded by the ob gene, located on chromosome 7. Through its central actions it controls food intake and, indirectly, energy balance. Paradoxically, in obesity, high levels do not suppress appetite, possibly due to a state of leptin resistance.12,13 It also has pro-inflammatory properties, stimulating the secretion of other cytokines such as interleukin-6 and tumor necrosis factor alpha (TNF-α), all important in the pathophysiological mechanisms of obesity.
Adiponectin is negatively correlated with obesity. It is the most abundant of the adipokines and is an insulin-sensitizing hormone with anti-inflammatory, anti-atherogenic and anti-diabetic properties.14 Synthesis of this hormone is inhibited in situations of cellular hypoxia and oxidative stress, as well as by interleukin-6 and TNF-α, all present in obesity. Its production also appears to be regulated by insulin and inhibited in insulin-resistant states, also common in obesity. Unlike leptin, adiponectin's levels and anti-inflammatory actions are suppressed in obesity, a consequence of the over-expression of pro-inflammatory molecules,15 particularly in abdominal obesity.
Thus, increased adiposity, especially in the visceral component, results ultimately in an imbalance of adipocyte metabolism that favors the release of pro-inflammatory mediators, contributing to the low-grade inflammatory process that characterizes obesity.16 In turn, these mediators affect the actions of other tissues, further stimulating the synthesis of other inflammatory molecules, such as C-reactive protein (CRP), produced in the liver by the action of interleukin-6.
In obesity, CRP is one of the most important inflammatory markers. In laboratory tests it is quantified by assaying high-sensitivity C-reactive protein (hs-CRP). CRP is considered an independent cardiovascular risk predictor due to its direct involvement in the various stages of atheroma plaque formation.17 In the adult population, relative cardiovascular disease risk can be inferred from its levels, even in apparently healthy individuals, classified as low (<0.1 mg/dl), moderate (0.1-0.3 mg/dl) or high (>0.3 mg/dl). Those with hs-CRP above 0.3 mg/dl have twice the risk of acquiring atherosclerotic disease compared to those with low levels (class IIa recommendation, level of evidence B).18,19 In obese children, raised hs-CRP levels suggest a persistent inflammatory process, and, as with the adult population, also confer cardiovascular risk.20,21
The inflammatory cascade induced by adiposity leads to endothelial dysfunction, which in this patient group raises their cardiovascular risk. Implicitly, nitric oxide bioavailability is compromised, as is vessels’ vasodilatory capacity. Endothelial dysfunction is considered the earliest step in the atherosclerotic process and precedes any morphological vessel wall changes. If the inflammatory milieu is maintained, the arterial wall phenotype undergoes progressive changes that culminate in the formation of the atheromatous plaque.22,23 Various studies have shown that atherosclerotic disease begins in childhood, mostly in children with cardiovascular risk factors,24 and thus obesity is an important predictor of morbidity and mortality in adulthood.25
MethodsWe carried out an observational, cross-sectional analysis in a cohort of obese children recruited from the Cardiovascular Risk Clinic of the Department of Pediatric Cardiology of Coimbra Pediatric Hospital, Portugal, over the course of 2012 and 2013. All parents gave their informed consent for the children to participate in the study, which had been approved by the local Ethics Committee.
The inclusion criteria for the study group were children with primary obesity (BMI above the 95th percentile for gender and age) aged 6-17 years without recent or chronic illness. The control group included healthy children within the same age range with a normal BMI (P5-85). All children had undergone a 12-hour fast prior to clinical assessment and blood sampling.
The study group included 120 obese children and the control group 41 children, of both sexes.
Weight (in kg to the nearest 100 g) was determined using a SECA 220 digital weight scale (Seca Medical Scales and Measuring Systems, Germany) and standing height (in cm to the nearest 0.1 cm) was determined using a stadiometer incorporated in the same apparatus, with the children wearing only underwear. BMI was calculated based on the formula: weight (kg)/height (cm)2.26 We used the World Health Organization BMI percentile charts to define BMI (obesity if BMI >P95; normal if BMI P5-P85). WC, to the nearest 0.1 cm, was measured using a flexible plastic tape placed midway between the last rib and the iliac crest and plotted on published WC charts.27 Body fat mass percentage (FM%) was determined by bioelectric impedance using a BIA 101 Anniversary device (Akern Srl, Italy). FM% was defined according to the charts published by McCarthy et al.28
Blood collection and biochemical analysisFasting venous blood samples (15 ml) were obtained to determine hematological parameters. Blood specimens were collected in vacutainer tubes with or without ethylenediaminetetraacetic acid. Serum and plasma were prepared and then frozen at −80°C for storage until analysis.
hs-CRP was determined by immunonephelometry from serum samples and processed in a BN ProSpec® system (Siemens Healthcare Diagnostics Inc.) (undetected if <0.02 mg/dl).
Quantitative measurements of serum leptin and adiponectin levels were performed using commercially available enzyme-linked immunosorbent assay kits (eBioscience, San Diego, CA, USA and BioVendor, Brno, Czech Republic, respectively), and absorbances were measured at 450 nm (Bio-RAD microplate reader model 680, Hercules, CA, USA).
Statistical analysisThe data were analyzed using SPSS version 20. Descriptive analysis of the parametric variables was performed by calculating the mean and standard error of the mean.
Differences in the demographic and hematological characteristics of the case and control groups were analyzed using the Student's t test and the Mann-Whitney U test, depending on population distribution.
For categorical variables, differences between the two groups were assessed using the chi-square test.
Logistic regression was used when clinical variables were controlled between the two groups.
Pearson's and Spearman's correlation were used to establish correlations between the parameters in the obese group.
Results were considered statistically significant at p<0.05.
ResultsOne hundred and twenty obese children, 61 boys and 59 girls, aged between 6 and 17 years (mean 11.65±2.96 years), were included in the study. The control group was made up of 41 healthy, non-obese children, 29 boys and 12 girls, within the same age group (mean 12.73±2.77 years).
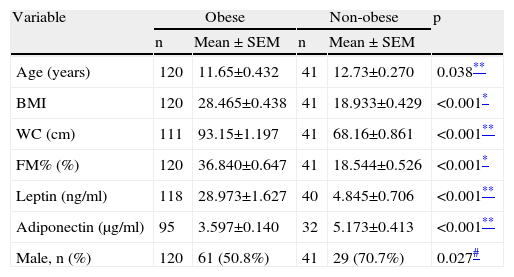
We firstly compared the two groups’ anthropometric, clinical and laboratory parameters, which showed significant differences for all the variables analyzed (Table 1). With the exception of adiponectin, all were higher in the obese group.
Anthropometric and laboratory parameters of obese and non-obese groups.
| Variable | Obese | Non-obese | p | ||
| n | Mean ± SEM | n | Mean ± SEM | ||
| Age (years) | 120 | 11.65±0.432 | 41 | 12.73±0.270 | 0.038** |
| BMI | 120 | 28.465±0.438 | 41 | 18.933±0.429 | <0.001* |
| WC (cm) | 111 | 93.15±1.197 | 41 | 68.16±0.861 | <0.001** |
| FM% (%) | 120 | 36.840±0.647 | 41 | 18.544±0.526 | <0.001* |
| Leptin (ng/ml) | 118 | 28.973±1.627 | 40 | 4.845±0.706 | <0.001** |
| Adiponectin (μg/ml) | 95 | 3.597±0.140 | 32 | 5.173±0.413 | <0.001** |
| Male, n (%) | 120 | 61 (50.8%) | 41 | 29 (70.7%) | 0.027# |
BMI: body mass index; FM%: fat mass percentage; WC: waist circumference.
Data are expressed as means ± standard error of the mean (SEM).
As both age (p=0.038) and gender (p=0.027) were also significantly different between the groups, we used logistic regression to adjust for these parameters and repeated the analysis, which demonstrated that adiponectin (p<0.001), leptin (p<0.001) and hs-CRP (p<0.001) still showed statistically significant differences.
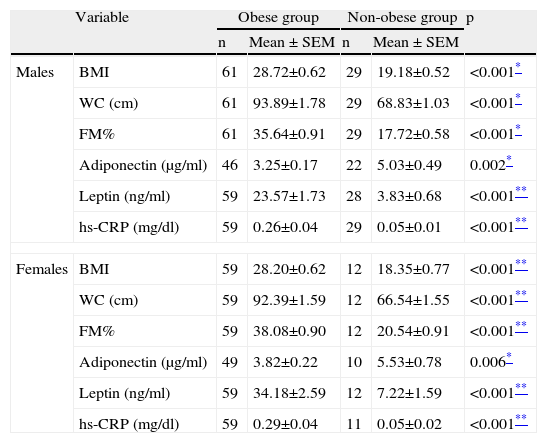
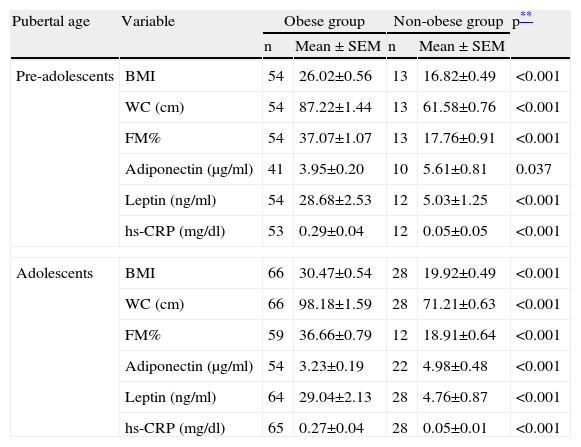
We then analyzed these parameters as a function of gender and pubertal age, and the statistical differences remained, as can be seen in Tables 2 and 3.
Anthropometric and laboratory parameters of obese and non-obese groups by gender.
| Variable | Obese group | Non-obese group | p | |||
| n | Mean ± SEM | n | Mean ± SEM | |||
| Males | BMI | 61 | 28.72±0.62 | 29 | 19.18±0.52 | <0.001* |
| WC (cm) | 61 | 93.89±1.78 | 29 | 68.83±1.03 | <0.001* | |
| FM% | 61 | 35.64±0.91 | 29 | 17.72±0.58 | <0.001* | |
| Adiponectin (μg/ml) | 46 | 3.25±0.17 | 22 | 5.03±0.49 | 0.002* | |
| Leptin (ng/ml) | 59 | 23.57±1.73 | 28 | 3.83±0.68 | <0.001** | |
| hs-CRP (mg/dl) | 59 | 0.26±0.04 | 29 | 0.05±0.01 | <0.001** | |
| Females | BMI | 59 | 28.20±0.62 | 12 | 18.35±0.77 | <0.001** |
| WC (cm) | 59 | 92.39±1.59 | 12 | 66.54±1.55 | <0.001** | |
| FM% | 59 | 38.08±0.90 | 12 | 20.54±0.91 | <0.001** | |
| Adiponectin (μg/ml) | 49 | 3.82±0.22 | 10 | 5.53±0.78 | 0.006* | |
| Leptin (ng/ml) | 59 | 34.18±2.59 | 12 | 7.22±1.59 | <0.001** | |
| hs-CRP (mg/dl) | 59 | 0.29±0.04 | 11 | 0.05±0.02 | <0.001** | |
BMI, body mass index; FM%, fat mass percentage; hs-CRP, high-sensitivity C-reactive protein; WC, waist circumference.
Data are expressed as means ± standard error of the mean (SEM).
Anthropometric and laboratory parameters of the obese and non-obese groups by pubertal age.
| Pubertal age | Variable | Obese group | Non-obese group | p** | ||
| n | Mean ± SEM | n | Mean ± SEM | |||
| Pre-adolescents | BMI | 54 | 26.02±0.56 | 13 | 16.82±0.49 | <0.001 |
| WC (cm) | 54 | 87.22±1.44 | 13 | 61.58±0.76 | <0.001 | |
| FM% | 54 | 37.07±1.07 | 13 | 17.76±0.91 | <0.001 | |
| Adiponectin (μg/ml) | 41 | 3.95±0.20 | 10 | 5.61±0.81 | 0.037 | |
| Leptin (ng/ml) | 54 | 28.68±2.53 | 12 | 5.03±1.25 | <0.001 | |
| hs-CRP (mg/dl) | 53 | 0.29±0.04 | 12 | 0.05±0.05 | <0.001 | |
| Adolescents | BMI | 66 | 30.47±0.54 | 28 | 19.92±0.49 | <0.001 |
| WC (cm) | 66 | 98.18±1.59 | 28 | 71.21±0.63 | <0.001 | |
| FM% | 59 | 36.66±0.79 | 12 | 18.91±0.64 | <0.001 | |
| Adiponectin (μg/ml) | 54 | 3.23±0.19 | 22 | 4.98±0.48 | <0.001 | |
| Leptin (ng/ml) | 64 | 29.04±2.13 | 28 | 4.76±0.87 | <0.001 | |
| hs-CRP (mg/dl) | 65 | 0.27±0.04 | 28 | 0.05±0.01 | <0.001 | |
BMI, body mass index; FM%, fat mass percentage; hs-CRP, high-sensitivity C-reactive protein; WC, waist circumference.
Data are expressed as means ± standard error of the mean (SEM).
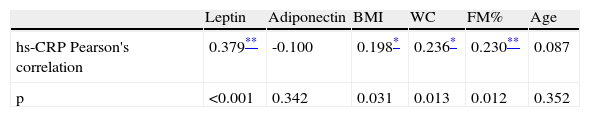
We further aimed to determine correlations between hs-CRP and the other variables.
hs-CRP was directly and moderately correlated with BMI (r=0.507; p<0.001), WC (r=0.496; p<0.001), FM% (r=0.535; p<0.001) and leptin (r=0.568; p<0.001), and inversely but weakly correlated with adiponectin (r=0.240; p=0.007).
The same analysis within the obese group showed that hs-CRP maintained a direct but weak correlation with BMI, WC, FM% and leptin, as demonstrated in Table 4. In boys within the obese group, hs-CRP was directly related to WC (r=0.274; p<0.001), FM% (r=0.285; p=0.029) and leptin (r=0.469; p<0.001). In girls, only leptin was related to hs-CRP (r=0.353; p=0.006). In obese pre-adolescents hs-CRP was related to leptin (r=0.462; <0.001), whereas in the adolescent obese group it was related to BMI (r=0.386; p=0.002), WC (r=0.351; p=0.006), FM% (r=0.261; p=0.036) and leptin (r=0.462; p<0.001).
Correlation between high-sensitivity C-reactive protein, adipokines and markers of adiposity in the obese group.
| Leptin | Adiponectin | BMI | WC | FM% | Age | |
| hs-CRP Pearson's correlation | 0.379** | -0.100 | 0.198* | 0.236* | 0.230** | 0.087 |
| p | <0.001 | 0.342 | 0.031 | 0.013 | 0.012 | 0.352 |
BMI: body mass index; FM%: fat mass percentage; hs-CRP: high-sensitivity C-reactive protein; WC: waist circumference.
In the obese group, apart from hs-CRP, leptin was directly related to BMI (r=0.395; p<0.001), WC (r=0.390; p<0.001) and FM% (r=0.378; p<0.001). Adiponectin was inversely but weakly correlated with BMI (r=-0.276; p=0.002) and WC (r=0.291; p=0.005).
DiscussionWe have demonstrated that in our young obese population there is a correlation between markers of adiposity and leptin, adiponectin and hs-CRP, all important risk factors in the pathogenesis of cardiovascular disease and insulin resistance.
Obesity is a chronic inflammatory disorder, in which leptin, adiponectin and CRP play an important role.29 Besides its relationship with energy balance, leptin is also an important immune modulator. Leptin is a member of the cytokine family and shares structural similarities with molecules such as interleukin-6, and is thus considered a pro-inflammatory cytokine. A leptin receptor is expressed in immune cells, and leptin thereby actively influences the functions of T-lymphocytes, natural killer cells, macrophages and monocytes. It also influences the release of inflammatory markers,30 particularly CRP, in turn a major player in cardiovascular disease through its pro-inflammatory actions.31
Based on these facts, we sought to establish the relationship between these parameters in a population of Portuguese children with primary obesity. To the best of our knowledge this is the first report relating these parameters in our pediatric obese population.
We firstly compared the obese group with a control group made up of healthy non-obese children, and found significant differences in leptin (p<0.001), adiponectin (p<0.001) and hs-CRP (p<0.001) levels between these two groups: in the obese group, leptin and hs-CRP levels were higher, whereas adiponectin levels were lower. These differences are expected, but especially in the obese group, highlight the pathophysiological processes triggered by adiposity present in children as young as six years of age, as clearly shown in our analysis. Age (p<0.038) and gender (p<0.027) were also significantly different, and as such could also account for these differences, but when our sample was adjusted by logistic regression for gender and age, all three variables still remained significantly different, retaining the same pattern.
Leptin reflects fat mass; its levels are influenced by gender and age, particularly at puberty when girls acquire more fat mass.32 Previous studies have shown that in non-obese children leptin levels increase in both boys and girls until puberty, at which point they increase in girls and decrease in boys, most likely due to the effects of testosterone.33 Not surprisingly, in the obese group, we found leptin to be significantly correlated with BMI, WC, and fat mass percentage, particularly in girls, showing strong gender dimorphism, as previously described.34 More importantly, it was also equally and moderately related to hs-CRP. We further aimed to clarify the correlations between hs-CRP, leptin, BMI, WC, fat mass percentage and adiponectin. With the exception of adiponectin, which was inversely but directly related to hs-CRP, all others were directly and moderately correlated with hs-CRP. These trends highlight the role of adiposity and leptin as pro-inflammatory triggers in obesity, and these were evident from our study, regardless of age or pubertal status.
Evidence in animal studies suggests that leptin, like interleukin-6, is capable of directly stimulating the production of hepatic CRP, thus maintaining a low-grade inflammatory state.35 It also appears to compromise nitric oxide bioavailability, and hence contribute towards endothelial dysfunction.36 In our study, leptin was the best predictor of hs-CRP levels. This observation may provide a clue to the link between adipocyte dysfunction, inflammation and ultimately endothelial dysfunction. In contrast to our findings, some authors have found that the best predictor of CRP is BMI, while for others it was adiposity,37–39 but in these studies leptin was not included as a variable.
As expected, adiponectin showed gender dimorphism and was age-related, being higher in girls than boys, and decreased with age. Our findings showed that, in the obese group, it was inversely correlated with BMI (r=-0.212; p=0.39) and WC (r=-0.278; p=0.06), but not correlated with hs-CRP or leptin. In a study by Caprio et al., an association was demonstrated between adiponectin levels and CRP, whereas in ours, as shown, this was not the case. In healthy individuals adiponectin has anti-atherogenic properties and studies have shown that, in adults, hypoadiponectinemia correlates with coronary lesions and is an independent risk factor for the progression of type 2 diabetes.40,41 In the light of our results, we can infer that the presumptive loss of adiponectin's protective properties is yet another cardiovascular risk factor present in our population, and if not abated may contribute to early onset cardiovascular disease and type 2 diabetes in this population.
This study's main limitation relates to the sample size, particularly the control group. It also only includes a Caucasian population, race thus being a possible source of bias in this study.
Based on our findings, these markers may be considered for use in clinical practice for cardiovascular risk stratification in obese children.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank all those who contributed towards this study, particularly those at the Department of Pediatric Cardiology of Coimbra Pediatric Hospital, the Biochemical, Hematological and Immunological Laboratories of Coimbra Pediatric Hospital and the Department of Physiology of Coimbra University Faculty of Medicine.