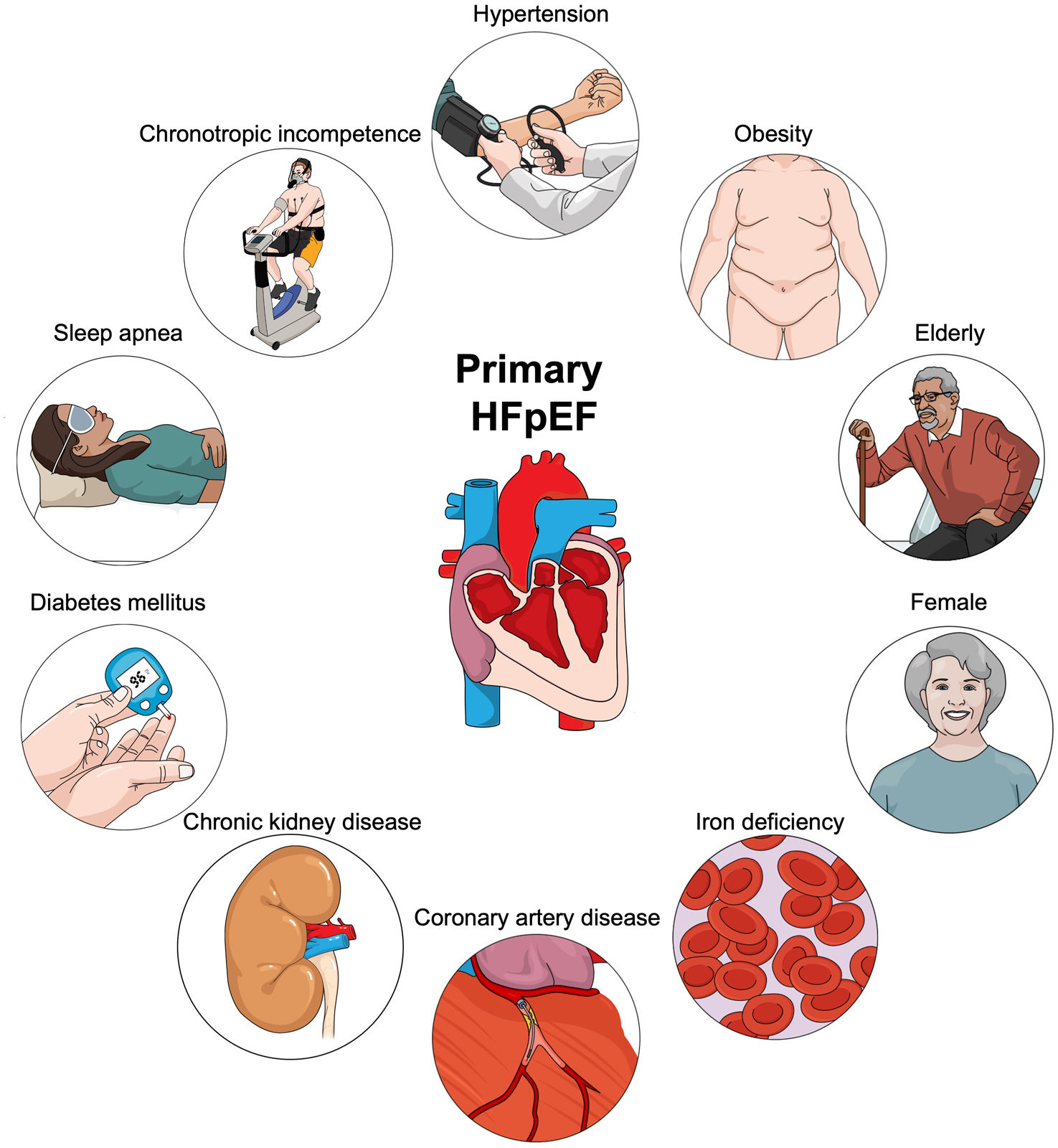
Heart failure with preserved ejection fraction (HFpEF) is characterized by diverse underlying pathophysiological mechanisms and can be divided into two subgroups based on the identification of the specific cause: primary and secondary HFpEF. Primary HFpEF is caused by primary impairments in myocardial relaxation or compliance with the contribution of several risk factors. Therefore, we have reviewed current literature on pathophysiology and treatment in patients with primary HFpEF. Patients with primary HFpEF represent specific “phenotypes” and are usually elderly, more commonly women, and often with a history of arterial hypertension, obesity, iron deficiency (ID), coronary artery disease (CAD), sleep apnea, diabetes, chronic kidney disease (CKD), and chronotropic incompetence. Some of the main pathophysiological mechanisms for each phenotype of primary HFpEF are as follows: arterial hypertension, which promotes left ventricular hypertrophy and fibrosis; obesity, which contributes through systemic inflammation and metabolic dysregulation; aging, which leads to ventricular-vascular stiffening; gender differences, with women experiencing changes due to smaller heart size and hormonal shifts; ID, which affects mitochondrial function; CAD, which impairs myocardial blood flow; diabetes, which is associated with hyperglycemia, lipotoxicity, insulin resistance, and microvascular rarefaction; CKD, which leads to hypertension, metabolic disturbance, systemic inflammation, and endothelial dysfunction; sleep apnea, which induces cardiac changes through pressure swings and hypoxia; and chronotropic incompetence, which is due to reduced cardiac β-receptor responsiveness. In conclusion, each factor intricately contributes to the complex pathophysiology of HFpEF. Understanding these interrelated mechanisms is critical for tailoring management strategies to improve outcomes in HFpEF patients.
A insuficiência cardíaca com fração de ejeção preservada (ICpFE) é consequência de diversos mecanismos fisiopatológicos e pode ser dividida em dois grupos tendo por base a identificação da causa específica: primária ou secundária. A ICpFE primária é causada pela perturbação primária do relaxamento ou da distensibilidade miocárdica com a contribuição de vários fatores de risco. Neste artigo, é feita uma revisão da fisiopatologia e do tratamento de algumas das formas de apresentação mais relevantes de ICpFE primária. Os doentes com ICpFE primária representam «fenotipos» específicos e são habitualmente idosos, do sexo feminino e mais frequentemente têm uma história de hipertensão arterial, obesidade, deficiência de ferro, doença coronária, apneia do sono, diabetes mellitus, doença renal crónica e de incompetência cronotrópica. Alguns dos principais mecanismos fisiopatológicos de cada fenótipo da ICpFE primária são os seguintes: hipertensão arterial, que promove hipertrofia ventricular esquerda e fibrose; obesidade que ocasiona inflamação sistémica e desregulação metabólica; envelhecimento que contribui para a rigidez ventriculo-vascular; diferenças entre sexos com o sexo feminino expressando-se com coração de menores dimensões e oscilações hormonais; deficiência de ferro contribuindo para a disfunção mitrocondial; doença coronária ocasionando redução do fluxo sanguíneo miocárdico; diabetes mellitus, que está associada com hiperglicémia, lipotoxicidade, resistência à insulina e rarefação microvascular; doença renal crónica, que ocasiona hipertensão arterial, perturbação metabólica, inflamação sistémica e disfunção endotelial;apneia do sono, que induz variações tensionais e hipóxia; e incompetência cronotrópica que se deve a redução da resposta dos recetores beta adrenérgicos. Em conclusão, cada fator interage com outros fatores contribuindo para o complexo fisiopatológico da ICpFE. A compreensão destes mecanismos interrelacionados é crítica para estabelecer estratégias terapêuticas adequadas para melhorar o prognóstico da ICpFE.
Heart failure (HF) is defined as a clinical syndrome consisting of symptoms that may be accompanied by signs. It arises from structural or functional heart anomalies and leads to elevated intracardiac filling pressures and/or inadequate cardiac output at rest and/or during exercise. In daily practice, HF is often categorized by left ventricular (LV) ejection fraction (EF). The proposed classifications of EF are an important feature of the universal definition; classification is defined as follows: (1) HF with reduced EF (HFrEF): HF with an LVEF of ≤40%; (2) HF with mildly reduced EF (HFmrEF): HF with an LVEF of 41–49%; (3) HF with preserved EF (HFpEF): HF with an LVEF of ≥50%; and (4) HF with improved EF: HF with a baseline LVEF of ≤40%, a ≥10-point increase from baseline LVEF, and a second measurement of LVEF of >40%.1,2 HF with supra-normal EF is still a matter of debate. Approximately 50% of patients with HF are reported to have HFpEF, which is characterized by diverse underlying pathophysiological mechanisms.3 Patients with HFpEF can be divided into two subgroups based on the identification of a specific cause. Secondary HFpEF is caused by conditions such as valvular heart disease, hypertrophic cardiomyopathy, restrictive/infiltrative cardiomyopathies, and constrictive pericarditis. Primary HFpEF, on the contrary, is characterized by primary impairments in myocardial relaxation or compliance, with the contribution of several risk factors.4 The diagnosis of primary HFpEF, as a clinical entity, should be reserved for those cases in which there is primary diastolic dysfunction leading to HF, caused by the interaction of multiple risk factors such as hypertension, aging, systemic inflammatory status, or metabolic disorders, but without any specific underlying cardiac cause and in which there are currently no specific medical or surgical therapies.
Patients with primary HFpEF represent specific “phenotypes” and are usually elderly, more commonly women, and often with a history of arterial hypertension, obesity, iron deficiency (ID), coronary artery disease (CAD), sleep apnea, diabetes, CKD, or chronotropic incompetence (Figure 1).5 In addition, “preserved EF” does not always mean normal LV systolic performance when assessed by preload recruitable stroke work, mitral annular systolic excursion and velocity, midwall fractional shortening, and longitudinal and circumferential strain.6–8 For example, global longitudinal strain offers a sensitive assessment of LV function, and decreased global longitudinal strain in HFpEF is independently predictive of future clinical events and deterioration in LVEF.9,10 Numerous research findings indicate that minor deficits in systolic function at rest are significantly exacerbated during physical activity in patients with HFpEF; this results in reduced exercise tolerance, impaired early diastolic recoil/LV suction, decreased cardiac output, and increased LV filling pressures.7,10–12 In this article, we have reviewed the intertwined pathophysiology of some of the most relevant phenotypes in primary HFpEF (Table 1).
Central illustration. Phenotypes of primary heart failure with preserved ejection fraction. Patients with primary HFpEF represent specific “phenotypes” and are usually elderly, more commonly women, and often with a history of arterial hypertension, obesity, iron deficiency, coronary artery disease, diabetes mellitus, chronic kidney disease, sleep apnea, and chronotropic incompetence.
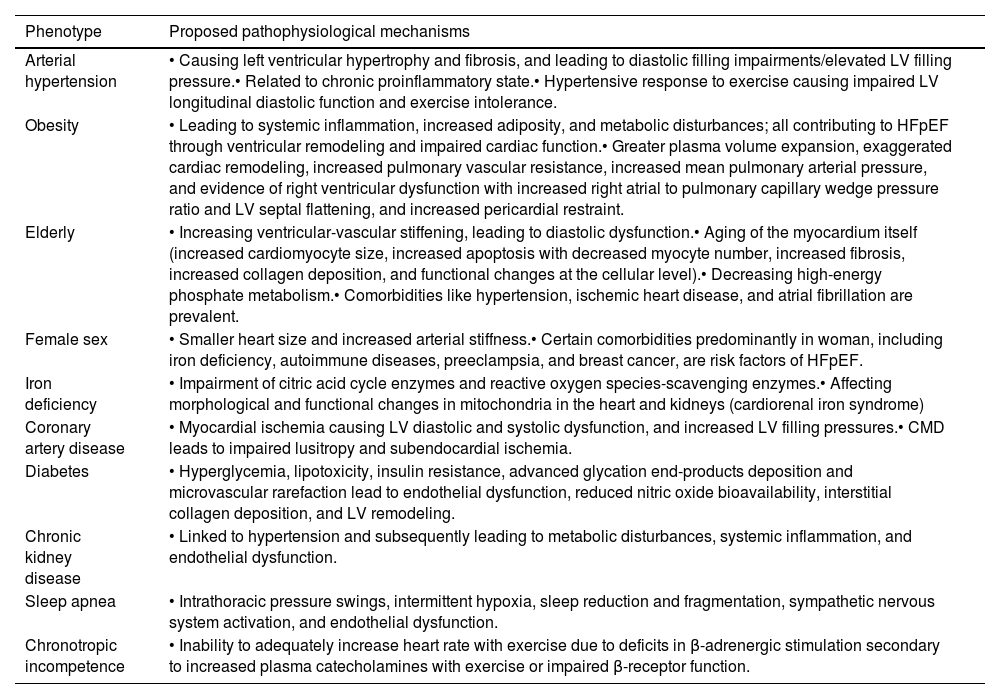
Main pathophysiological mechanisms by different phenotypes of primary HFpEF.
| Phenotype | Proposed pathophysiological mechanisms |
|---|---|
| Arterial hypertension | • Causing left ventricular hypertrophy and fibrosis, and leading to diastolic filling impairments/elevated LV filling pressure.• Related to chronic proinflammatory state.• Hypertensive response to exercise causing impaired LV longitudinal diastolic function and exercise intolerance. |
| Obesity | • Leading to systemic inflammation, increased adiposity, and metabolic disturbances; all contributing to HFpEF through ventricular remodeling and impaired cardiac function.• Greater plasma volume expansion, exaggerated cardiac remodeling, increased pulmonary vascular resistance, increased mean pulmonary arterial pressure, and evidence of right ventricular dysfunction with increased right atrial to pulmonary capillary wedge pressure ratio and LV septal flattening, and increased pericardial restraint. |
| Elderly | • Increasing ventricular-vascular stiffening, leading to diastolic dysfunction.• Aging of the myocardium itself (increased cardiomyocyte size, increased apoptosis with decreased myocyte number, increased fibrosis, increased collagen deposition, and functional changes at the cellular level).• Decreasing high-energy phosphate metabolism.• Comorbidities like hypertension, ischemic heart disease, and atrial fibrillation are prevalent. |
| Female sex | • Smaller heart size and increased arterial stiffness.• Certain comorbidities predominantly in woman, including iron deficiency, autoimmune diseases, preeclampsia, and breast cancer, are risk factors of HFpEF. |
| Iron deficiency | • Impairment of citric acid cycle enzymes and reactive oxygen species-scavenging enzymes.• Affecting morphological and functional changes in mitochondria in the heart and kidneys (cardiorenal iron syndrome) |
| Coronary artery disease | • Myocardial ischemia causing LV diastolic and systolic dysfunction, and increased LV filling pressures.• CMD leads to impaired lusitropy and subendocardial ischemia. |
| Diabetes | • Hyperglycemia, lipotoxicity, insulin resistance, advanced glycation end-products deposition and microvascular rarefaction lead to endothelial dysfunction, reduced nitric oxide bioavailability, interstitial collagen deposition, and LV remodeling. |
| Chronic kidney disease | • Linked to hypertension and subsequently leading to metabolic disturbances, systemic inflammation, and endothelial dysfunction. |
| Sleep apnea | • Intrathoracic pressure swings, intermittent hypoxia, sleep reduction and fragmentation, sympathetic nervous system activation, and endothelial dysfunction. |
| Chronotropic incompetence | • Inability to adequately increase heart rate with exercise due to deficits in β-adrenergic stimulation secondary to increased plasma catecholamines with exercise or impaired β-receptor function. |
CMD: coronary microvascular dysfunction; HFpEF: heart failure with preserved ejection fraction; LV: left ventricular.
Arterial hypertension is the leading cause of HFpEF development, with prevalence ranging from 60 to 90%.5,13 In patients with hypertension, pressure overload leads to left ventricular hypertrophy (LVH), myocardial fibrosis, and impaired diastolic filling, which could trigger HFpEF.14 Also, previous studies have demonstrated that patients with hypertension have impaired global longitudinal strain and an accompanying exaggerated circumferential strain.10,15 Laplace's Law is one of the primary mechanisms of the progression from hypertension to HFpEF. According to Laplace's Law, wall stress in a sphere increases in direct proportion to the internal pressure and radius, and decreases in inverse proportion to wall thickness, resulting in concentric geometry.16,17 Arterial hypertension leads to increased LV pressure, which induces wall thickening to reduce wall stress, and the concentric geometry leads to a reduction in chamber size and shifts end-diastolic property of the pressure–volume relationship leftward, leading to LV stiffening. Consequently, LVH leads to further myocardial dysfunction, including myocardial ischemia caused by a lack of myocardial blood supply, particularly in the midmyocardial layer.17,18 In addition, hypertension has also been found to be correlated with a chronic proinflammatory state.19 These result in fibrotic remodeling and ventricular stiffening, promoting elevated LV filling pressures and consequent LV diastolic dysfunction.13
In addition to the relationship between arterial hypertension and HFpEF, hypertensive response to exercise may also be associated with HFpEF. Although there is no universal definition of hypertensive response to exercise, a systolic blood pressure elevation during exercise testing ≥210 mmHg for men and ≥190 mmHg for women, or diastolic blood pressure elevation >110 mmHg in both genders, are used in some previous studies.17 Irrespective of the presence of resting hypertension, E/e′ values were significantly higher in patients with hypertensive response to exercise as a surrogate parameter for elevated LV filling pressure and impaired LV diastolic function, and these patients had impaired LV longitudinal diastolic function and exercise intolerance.20 Another systematic review showed that patients with hypertensive response to exercise have a 36% higher risk of developing adverse cardiovascular events including CAD, which could be another factor related to HFpEF development.21
ObesityObesity is a major risk factor for HFpEF.22 Increasing body mass index (BMI) above the normal range (≥25 kg/m2) was found to be associated with being at an increased risk of HFpEF; a dose-dependent relationship was observed.23 Obese HFpEF was found to be associated with decreased quality of life, worse symptoms of HF, greater systemic inflammation, worse exercise capacity, and higher metabolic cost of exertion in comparison with nonobese HFpEF.24 In obese patients with HFpEF, dysregulation of energy storage (adipose tissue) plays an important role. Increased adiposity promotes inflammation, hypertension, insulin resistance, and dyslipidemia and also impairs diastolic, systolic, arterial, skeletal muscle, and physical function.14,25 There is a significant inverse relationship between adiposity or BMI and brain natriuretic peptide.26 On the other hand, increased metabolic demand seems to lead to high-output HF in obese patients with HFpEF, but most obese HFpEF patients do not have a high cardiac index.27 The pathophysiology of obese patients with HFpEF includes greater plasma volume expansion, exaggerated cardiac remodeling, increased pulmonary vascular resistance, increased mean pulmonary arterial pressure, as well as evidence of right ventricular dysfunction with increased right atrial to pulmonary capillary wedge pressure ratio, LV septal flattening, and increased pericardial restraint.5,22
The elderlyThe prevalence of heart failure with preserved ejection fraction is expected to rise owing to the aging population.28,29 Like hypertension, aging is linked to elevated ventricular-vascular stiffening, which leads to higher prevalence of LV diastolic dysfunction with aging.30 Aging of the myocardium itself is a specific pathophysiological process. Normal cardiac aging is characterized by structural and functional changes. Increased cardiomyocyte size, increased apoptosis with decreased myocyte number, increased fibrosis, increased collagen deposition, as well as functional changes at the cellular level may all contribute to abnormal diastolic function with normal aging.31 Another mechanism is the decline in high-energy phosphate metabolism and peak cardiac power with age. Aging itself is associated with widespread comorbidities including arterial hypertension, macro/microvascular ischemic heart, atrial fibrillation, restrictive/infiltrative/dilated cardiomyopathies, metabolic syndrome, diabetes, CKD, thyroid disease, chronic lung disease, anemia, and chronic inflammatory disease; therefore, HFpEF can be caused by aging and its related diseases.32–34
Female genderEpidemiological and registry studies show that women have an incidence of HFpEF similar to that in men.35,36 Interactions between estrogen, gene expression, inflammation, anthropometry, and comorbidities drive the higher relative prevalence of HFpEF in women.37 The following phenotypic characteristics of the myocardium and cardiovascular (CV) system predispose women to HFpEF more than men: smaller ventricular chambers and vasculature; increased ventricular wall thickness and concentric myocardial remodeling; decreased diastolic compliance and diastolic reserve; increased myocardial blood flow and oxygen consumption with prominent myocardial lipid metabolism; greater arterial stiffness and pulse pressure leading to greater pulsatile afterload; and impaired pulmonary vascular reactivity with pulmonary vascular dysfunction and remodeling.5,37 The highest prevalence of anatomically small hearts, which have an abnormally low LV end-systolic volume, is reported in older women and is associated with an increased LVEF; women with smaller hearts may live under constant hyperdynamic conditions, compensating for the disadvantage of smaller ventricular volumes.2 Aging of the heart increases the susceptibility of elderly women to HFpEF because LV concentric remodeling and diastolic dysfunction are characteristic features of HFpEF, and these conditions are more pronounced in women than in men with HFpEF. Furthermore, during physical exertion, women exhibit a more significant increase in LV diastolic elastance compared to men, coupled with diminished chronotropic and contractile reserve, leading to exercise intolerance, a key clinical symptom of HFpEF.38
Certain comorbidities and clinical conditions, particularly prevalent or exclusive in women, increase the likelihood of developing HFpEF. These include metabolic risk factors such as obesity, hypertension, diabetes, and dyslipidemia. Additional contributing factors include ID and autoimmune diseases, which lead to increased inflammation and a higher risk of pulmonary hypertension. Furthermore, preeclampsia and breast cancer, along with its treatments, also elevate the risk. Many of these factors become more pronounced due to a deficiency in sex hormones following the menopause.37
Iron deficiencyIron deficiency is observed in 50–75% of patients with HFpEF.39,40 ID is thought to be due to an increase in the regulatory protein hepcidin, which prevents iron release from enterocytes and the reticuloendothelial system and can be elevated in the setting of systemic inflammation.39 Iron plays an important role beyond oxygen transport and storage, as it is also essential for the normal activity of key citric acid cycle enzymes and reactive oxygen species-scavenging enzymes.41,42 Furthermore, iron is crucial in mitochondria for the final step in producing adenosine triphosphate from glucose, fatty acids, or ketones; therefore, ID leads to several morphological and functional changes in mitochondria in the heart and kidneys, in which mitochondria are abundant with high-energy metabolism.43 The interaction of ID, HF, and CKD, is called cardiorenal iron syndrome.44 In patients with HFpEF and ID, diastolic function E/e′ has been negatively correlated with iron and transferrin saturation, whereas peak VO2 was positively correlated with these iron parameters.45 Furthermore, a meta-analysis showed that patients with HFpEF and ID had lower maximal oxygen consumption, more dyspnea, reduced six-minute walking distance, and reduced health-related quality of life.37
Coronary artery diseaseBoth obstructive epicardial CAD and coronary microvascular dysfunction (CMD) are frequent in patients with HFpEF, although co-existence of HFpEF and obstructive coronary artery disease is less common than in the HFrEF population.5,46 Myocardial ischemia due to CAD can cause LV diastolic and systolic dysfunction, and patients with obstructive CAD have higher estimated LV filling pressures on echocardiography.46 CAD is also associated with more advanced impairment of right ventricular function, right ventricular filling, and diastolic or systolic dysfunction in HFpEF.47 On the other hand, CMD may result from altered endothelial cell function leading to decreased nitric oxide (NO) production or from the inability of vascular smooth muscle cells to vasodilate in response to NO.48 A strong association between CMD and HFpEF has been observed, and the PROMIS-HFpEF study reported a 75% prevalence of CMD in patients with HFpEF, with impaired coronary flow velocity reserve associated with systemic endothelial dysfunction and elevated natriuretic peptide levels.49 Two potential mechanisms, linking CMD and HFpEF, have been identified: impaired lusitropy and subendocardial ischemia. Limited endothelium-dependent NO bioavailability has been shown to promote fibroblast and myofibroblast proliferation and impair energy-dependent cardiomyocyte relaxation through hypophosphorylation of the cytoskeletal protein titin, resulting in impaired lusitropy and LV diastolic reserve.50 In addition, reduced adenosine triphosphate production due to inadequate myocardial perfusion is likely to lead to incomplete diastolic relaxation due to diastolic cross-bridge cycling, and repeated cardiomyocyte injury over time may lead to subendocardial ischemia and myocardial fibrosis.48
Sleep apneaPathophysiological abnormalities related to sleep apnea include intrathoracic pressure swings, intermittent hypoxia, sleep reduction and fragmentation, sympathetic nervous system activation, and endothelial dysfunction.51–53 Exaggerated intrathoracic pressure swings can contribute to cardiac remodeling.17 Obstructive sleep apneas (OSA) increase LV transmural pressure through the generation of negative intrathoracic pressure and elevations in systemic blood pressure secondary to hypoxia, arousals from sleep, and increased sympathetic nervous system activity. Apnea also suppresses the sympathetic inhibitory effects of lung stretch receptors, further enhancing sympathetic nervous system activity. The combination of increased LV afterload and increased heart rate secondary to increased sympathetic nervous system activity increases myocardial oxygen.54,55
Intermittent hypoxia induced by OSA results in widespread stimulations of the sympathetic nervous system, the renin–angiotensin–aldosterone system and, importantly, a systemic inflammatory state associated with oxidative stress.56,57 Regarding biomarkers, circulating interleukin-10 levels were found to be positively associated with major adverse cardiovascular events in men with HFpEF and OSA.58
DiabetesDiabetes is one of the common comorbidities in patients with HFpEF.5 Diabetic cardiomyopathy is the clinical entity in which patients who had been diagnosed with diabetes for a long period exhibited clinical symptoms, such as cardiomegaly and pulmonary congestion, and pathological examinations revealed myocardial hypertrophy, fibrosis, and thickening of the microvascular walls due to the buildup of acid mucopolysaccharides.59 However, recent clinical reports have noted a shift in the presentation of diabetic cardiomyopathy, showing a phenotype that diverges from the dilated form to the restrictive form.59,60 Currently, a typical patient with diabetic cardiomyopathy is described as an older woman with obesity and type 2 diabetes, characterized by a small LV cavity, normal LVEF, thickened LV walls, increased LV filling pressures, and an enlarged left atrium. This profile is indicative of a restrictive rather than a dilated cardiomyopathy and is characterized as HFpEF. Pathophysiological mechanisms include hyperglycemia, lipotoxicity, insulin resistance, advanced glycation end-products deposition, and microvascular rarefaction.61–63 In diabetic cardiomyopathy, the endothelial dysfunction is attributed to hyperglycemia, lipotoxicity, and the accumulation of advanced glycation end-products. Additionally, microvascular rarefaction contributes to reduced nitric oxide bioavailability, while hyperinsulinemia promotes cardiomyocyte hypertrophy. Hyperglycemia and lipotoxicity also elevate protein kinase C activity in fibroblasts, enhancing interstitial collagen deposition. Coronary microvascular endothelial dysfunction induces LV remodeling and dysfunction by decreasing myocardial nitric oxide bioavailability and protein kinase G activity.59
Chronic kidney diseaseThe prevalence of CKD in HFpEF has been reported to be 26–49%.64,65 CKD is pathophysiologically linked to hypertension and subsequently leads to metabolic disturbances, systemic inflammation, and endothelial dysfunction. These factors are considered crucial in the development of HFpEF, mediated by interactions between the endothelium and cardiomyocytes, resulting in myocardial stiffening, hypertrophy, and interstitial fibrosis.66 First, elevated fibroblast growth factor 23 in patients with CKD causes endothelial dysfunction due to increased superoxide and decreased nitric oxide bioavailability.67 Second, vitamin D deficiency in CKD is associated with systemic inflammation, endothelial dysfunction, and LV remodeling.68 Third, high levels of phosphorus and parathyroid hormone are associated with ventricular hypertrophy and fibrosis.68,69 In addition, erythropoietin deficiency is associated with endothelial dysfunction, nitric oxide availability, and inflammation.66 Finally, proteinuria and uremic toxins affect inflammation and endothelial dysfunction by inhibiting nitric oxide.70,71 Therefore, CKD induces inflammation and endothelial dysfunction, all of which could lead to HFpEF.
Chronotropic incompetenceChronotropic incompetence, broadly defined as the inability of the heart to increase its rate commensurate with increased activity or demand, is common in patients with HFpEF, and more than 50% of the patients have chronotropic incompetence.72–74 The exaggerated decrease in chronotropic reserve in HFpEF may be due to deficits in β-adrenergic stimulation secondary to increased plasma catecholamines with exercise.75 Another study demonstrated that patients with HFpEF had reduced cardiac β-receptor responsiveness, suggesting that impaired β-receptor function may contribute to chronotropic incompetence.76 Therefore, exercise intolerance is the cardinal symptomatic manifestation of HFpEF. Administration of beta-blockers blunts the chronotropic response to exercise; however, their effect on outcomes in HFpEF is not well established.5,77,78 Conversely, beta-blocker withdrawal increased the functional capacity and oxygen uptake significantly, and this cessation may be particularly beneficial in patients with smaller end-systolic LV volume.79,80 Furthermore, selective heart rate reduction of HFpEF using ivabradine, an If-inhibitor, does not show any benefit on exercise tolerance or echocardiographic parameters in HFpEF.81,82 In patients with HFpEF and chronotropic incompetence, the implantation of a pacemaker to enhance exercise heart rate did not improve exercise capacity, symptoms, or exercise cardiac output.74
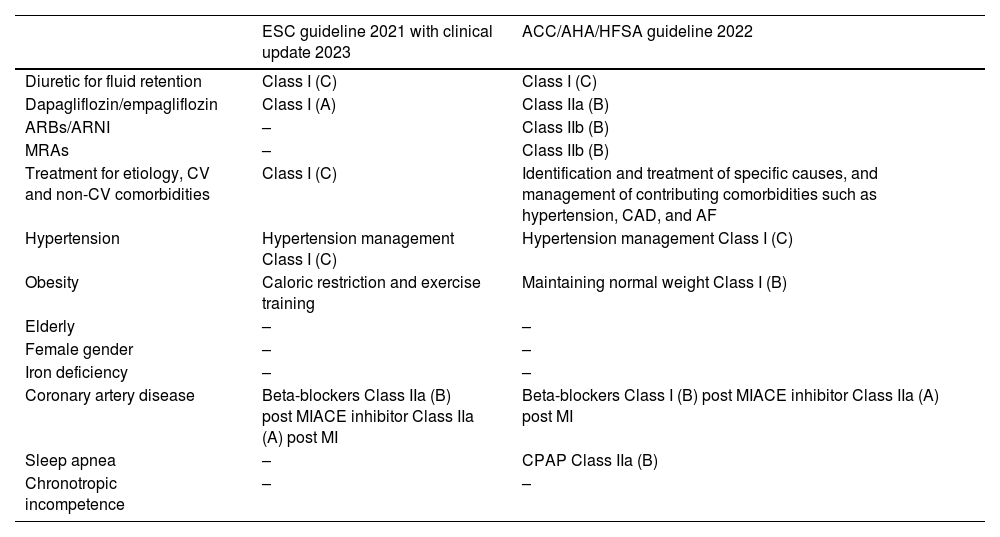
From pathophysiology to treatment of heart failure with preserved ejection fractionTreatment of HFpEF requires a comprehensive approach that identifies potentially treatable comorbidities as discussed above, such as pathophysiology. The European Society of Cardiology (ESC) 2021 Guidelines with clinical update 2023 and ACC/AHA/HFSA guideline 2022 recommend identification and treatment for etiology and comorbidities.13,83,84 Therefore, identifying the causes of HFpEF pathophysiology and treating them is very important. A comparison of major differences in the ESC and American HF Guidelines regarding HFpEF treatment is shown in Table 2. Both guidelines list diuretics for fluid retention as a Class I recommendation. The current evidence for treating HFpEF in general and according to pathophysiology is reviewed in the aforementioned table.
Comparison of major differences in the European Society of Cardiology and American Heart Failure Guidelines for HFpEF treatment.
| ESC guideline 2021 with clinical update 2023 | ACC/AHA/HFSA guideline 2022 | |
|---|---|---|
| Diuretic for fluid retention | Class I (C) | Class I (C) |
| Dapagliflozin/empagliflozin | Class I (A) | Class IIa (B) |
| ARBs/ARNI | – | Class IIb (B) |
| MRAs | – | Class IIb (B) |
| Treatment for etiology, CV and non-CV comorbidities | Class I (C) | Identification and treatment of specific causes, and management of contributing comorbidities such as hypertension, CAD, and AF |
| Hypertension | Hypertension management Class I (C) | Hypertension management Class I (C) |
| Obesity | Caloric restriction and exercise training | Maintaining normal weight Class I (B) |
| Elderly | – | – |
| Female gender | – | – |
| Iron deficiency | – | – |
| Coronary artery disease | Beta-blockers Class IIa (B) post MIACE inhibitor Class IIa (A) post MI | Beta-blockers Class I (B) post MIACE inhibitor Class IIa (A) post MI |
| Sleep apnea | – | CPAP Class IIa (B) |
| Chronotropic incompetence | – | – |
Parentheses indicate levels of evidence, as defined in the guidelines.
ACC: American College of Cardiology; ACE: angiotensin-converting enzyme; AF: atrial fibrillation; AHA: American Heart Association; ARB: angiotensin-receptor blocker; ARNI: angiotensin-receptor neprilysin inhibitor; CPAP: continuous positive airway pressure; CV: cardiovascular; ESC: European Society of Cardiology; HF: heart failure; HFSA: Heart Failure Society of America; MI: myocardial infarction.
Based on the results of the EMPEROR-Preserved and DELIVER trials, both dapagliflozin and empagliflozin have shown beneficial effects on CV mortality and HF hospitalization.85,86 A meta-analysis revealed that dapagliflozin and empagliflozin reduced composite cardiovascular death or first hospitalization for HF (hazard ratio 0.80 [95% confidence interval (CI) 0.73–0.87]) with consistent reductions in both components: cardiovascular death (0.88 [0.77–1.00]) and first hospitalization for HF (0.74 [0.67–0.83]) among 12251 participants from DELIVER and EMPEROR-Preserved trials. These drugs are recommended for patients with HFpEF as Class I in the 2023 Focus Update of 2021 ESC HF guidelines and Class IIa in the ACC/AHA/HFSA guideline 2022.
Angiotensin-receptor blocker/angiotensin-receptor neprilysin inhibitorNone of the HFpEF trials with the angiotensin-converting enzyme inhibitor (perindopril) or the angiotensin-receptor blockers (ARBs (candesartan and irbesartan) achieved their primary endpoints.87–89 Regarding the angiotensin-receptor neprilysin inhibitor (ARNI), sacubitril/valsartan did not significantly reduce the primary endpoint of CV death and total HF hospitalizations in the PARAGON trial in patients with LVEF >45%.90 In addition, one subanalysis of the PARAGON-HF trial suggested that women with HFpEF are more likely to benefit from ARNI treatment than men. Another subanalysis indicated that patients with a LVEF of 57% or lower showed a significant benefit from sacubitril/valsartan compared to valsartan alone. Therefore, this analysis suggested that the treatment was more effective particularly in patients with an LVEF in the range of 45–57% or among female patients. Additionally, in a prespecified participant-level pooled analysis of PARAGLIDE-HF and PARAGON-HF, ARNI significantly reduced total HF events and CV death compared with valsartan [rate ratio of 0.86, 95% CI 0.75–0.98, p=0.027].91 American guidelines assign a Class IIb recommendation, while no recommendation is listed in the ESC guidelines.
Mineralocorticoid receptor antagonistThe TOPCAT trial using spironolactone concluded that while spironolactone did not significantly reduce the primary composite outcome in the overall population of patients with HFpEF, it did reduce the rate of hospitalization for HF and showed potential regional differences in effectiveness. In American patients, spironolactone reduced the primary endpoint,92 therefore, American guidelines assign a Class IIb recommendation, while no recommendation is listed in the ESC guidelines.
Finerenone is a nonsteroidal MRA that is more selective for the mineralocorticoid receptor than spironolactone or eplerenone. FINEARTS-HF, a global, randomized, double-blind, parallel-group, event-driven trial comparing the efficacy and safety of finerenone with placebo in patients with chronic HFmrEF or HFpEF, showed that finerenone resulted in a significantly lower rate of a composite total worsening HF events and death from cardiovascular causes than placebo.93
HypertensionHypertension is the most important cause of HFpEF, and patients with HFpEF also frequently have an exaggerated hypertensive response to exercise and may present with hypertensive acute pulmonary edema. Therefore, hypertension management is recommended for patients with HFpEF as Class I in both the ESC and American HF Guidelines. Although blood pressure targets are uncertain in HFpEF, a treatment goal of <130/80 mmHg is recommended for those with a cerebrovascular disease risk of ≥10%.94 However, recent findings from the OPTIMIZE-HF registry suggest that a systolic blood pressure of <120 mmHg may be linked to worse outcomes in older patients with HFpEF.95 In patients with HFpEF, a therapeutic strategy with all major antihypertensive drug classes (angiotensin-converting enzyme (ACE) inhibitor or ARBs, beta-blockers, calcium channel blockers, and thiazide/thiazide-like diuretics) is recommended. The substitution of ACE inhibitor/ARBs with ARNI and treatment with an MRA independent of resistant hypertension can be considered, particularly in the lower HFpEF spectrum.96 Although there is no specific indication to prefer one antihypertensive class over another, a network meta-analysis of 223313 patients showed that diuretics, ACE inhibitors, and ARBs were the most effective classes of drugs for reducing the incidence of HF compared with placebo (odds ratios of 0.59, 0.71, and 0.76, respectively) and were more effective than calcium channel blockers in preventing the development of HF.97
ObesityObesity is a risk factor for hypertension and CAD and is also associated with an increased risk of HF. Caloric restriction and exercise training were found to have additive beneficial effects on exercise capacity and quality of life of patients with obesity and HFpEF.98 In the STEP-HFpEF trial, the glucagon-like peptide 1 (GLP-1) agonist, semaglutide, administered once weekly at a dose of 2.4 mg for one year significantly decreased body weight (13.3% loss vs. 2.6% in the placebo group) and improved the KCCQ clinical summary score and six-minute walking distance among obese HFpEF patients (body mass index ≥30 kg/m2).99,100 In the SURMOUNT-1 trial, the combined GLP-1/glucose-dependent insulinotropic polypeptide agonist tirzepatide (administered subcutaneously at 5–15 mg once weekly) showed notable efficacy in inducing weight loss among patients with obesity.101 Regarding the effect of bariatric surgery for obesity on the incidence of HF, a meta-analysis showed that the pooled hazard ratio for incident HF after bariatric surgery versus control was 0.50 (95% CI 0.38–0.66, p<0.001). This analysis also found that bariatric surgery in patients with obesity was associated with reduced all-cause and CV mortality as well as lowered incidence of several CV diseases.102
Female genderAs described earlier, female patients with HFpEF may benefit from taking ARNIs. The reduction in the composite outcome of total HF hospitalizations and cardiovascular death was more pronounced in women. The interaction p-value for sex was 0.017, suggesting a statistically significant difference in treatment effect between men and women.103 In the I-PRESERVE trial, which included patients with an LVEF of 45% or higher, subgroup analysis indicated that women using irbesartan experienced a lower incidence of all-cause mortality or first cardiovascular hospitalization compared to men.104 Conversely, in the TOPCAT trial (LVEF ≥45%), spironolactone did not demonstrate a reduction in the occurrence of cardiovascular death or HF hospitalization. However, the subgroup analysis revealed that women had a greater reduction in the risk of cardiovascular and all-cause mortality than men.105 A recent meta-analysis found no significant trend toward reducing events with other drugs in HFpEF patients across both sexes.106
Iron deficiencyIn a meta-analysis study, ID in HFpEF was found to be associated with decreased peak oxygen consumption, increased dyspnea, decreased six-minute walk oxygen uptake, and decreased health-related quality of life.39 Some studies suggest that ID in HFpEF may be associated with worse clinical outcomes, although others have found no association with hospitalization or death.5,39 The use of ferric carboxymaltose has been associated with a significant improvement in functional status in patients with HFpEF according to the NYHA scale.107 The potential benefits of intravenous iron supplementation in HFpEF are being investigated in ongoing trials such as FAIR-HFpEF (NCT03074591) and PREFER-HF (NCT03833336).
Coronary artery diseaseIn patients with an LVEF of >40% following myocardial infarction, ACE inhibitors and beta-blockers should be considered. In patients with HF post-myocardial infarction, beta-blockers are recommended for patients with preserved EF as Class IIa in ESC and Class I in AHA/ACC guidelines.13,84 ACE inhibitors are recommended for patients with preserved EF as Class IIa in both guidelines.13,84
In the absence of positive clinical trials, ARNI or SGLT-2 inhibitors should not be used routinely in high-risk patients immediately after myocardial infarction, although if a patient develops chronic symptomatic HF in the weeks and months following acute myocardial infarction, then ARNI and SGLT-2 inhibitors should be initiated at the earliest opportunity according to HF guidelines.108
Treatments for patients with HFpEF and CMD are limited and not well established. The 2019 ESC guidelines suggest using β-blockers, ACE inhibitors, and statins, in addition to lifestyle changes and weight loss, for patients with a coronary flow reserve below 2.0 or an index of microcirculatory resistance >25 who have a negative acetylcholine provocation test.109 However, these recommendations are not specifically targeted at HFpEF patients.
Diabetes and chronic kidney diseaseSee “Sodium-glucose cotransporter-2 inhibitor, Angiotensin-receptor blocker/angiotensin-receptor neprilysin inhibitor, Mineralocorticoid receptor antagonist” sections for information on individual types of therapeutic agents. SGLT-2 inhibitors and finerenone, a nonsteroidal and selective MRA, are now recommended as Class IA for the prevention of HF in patients with type 2 diabetes and CKD.83,110 DAPA-CKD and EMPA-KIDNEY trials demonstrated reduced cardiovascular death, HF hospitalization, and kidney disease progression in CKD patients with or without diabetes.111–113 Furthermore, FIDELIO-DKD and FIGARO-DKD trials showed the benefits of finerenone on cardiovascular and kidney outcomes compared to placebo in patients with CKD and type 2 diabetes.
Sleep apneaSince sleep apnea is associated with obesity, the treatment might be similar to that for obesity itself. Therapeutic strategies for patients with HFpEF and sleep apnea are limited, especially with respect to pharmacological interventions. When focusing on the treatment of positive airway pressure therapy for sleep apnea, observational studies have indicated that older, obese HFpEF patients with sleep-disordered breathing might benefit the most from adaptive servo-ventilation (ASV) therapy, as evidenced by significant reductions in cardiovascular mortality and morbidity, along with high adherence to the therapy.114,115
Another small trial involving HFpEF patients (EF >50%, apnea–hypopnea index >15/hour, n=36) demonstrated that ASV therapy could improve diastolic function, reduce brain natriuretic peptide levels, and lower the combined risk of cardiac death and worsening HF.116 In addition, treating obstructive sleep apnea may help prevent the progression of HFpEF by reducing risk factors like arterial hypertension and cardiac workload, thereby preventing cardiac remodeling.117 Despite these findings, there is a lack of data from large-scale clinical trials for HFpEF and sleep-disordered breathing; thus, further studies are warranted.
Chronotropic incompetenceBeta-blockers are widely prescribed in patients with HFpEF, although the evidence for beneficial effects in these patients is poor. Many patients with HFpEF experience chronotropic incompetence, which may deteriorate further when treated with beta-blockers.118 A recent study investigated the effects of discontinuing beta-blocker treatment on the percentage change in predicted peak oxygen consumption, indexed left ventricular diastolic volume, indexed left ventricular systolic volume (iLVESV), and LVEF in patients with HFpEF and chronotropic incompetence.79 Participants in the PRESERVE-HR trial were randomized to either stop or continue their beta-blocker therapy and then switched to the alternate intervention after two weeks. The findings indicated that HFpEF patients with chronotropic incompetence who had lower iLVESV experienced a greater short-term enhancement in maximal functional capacity following the cessation of β-blocker treatment.
ConclusionsIn conclusion, we have provided a contemporary overview of the intertwined pathophysiology of primary HFpEF, which includes arterial hypertension, obesity, aging, gender differences, ID, CAD, diabetes, CKD, sleep apnea, and chronotropic incompetence, as well as its treatments. HFpEF, characterized by diverse etiological factors and clinical phenotypes, poses significant challenges in both diagnosis and management. Understanding these interrelated mechanisms is critical for tailoring management strategies to improve outcomes in HFpEF patients.
Human and animal rights and consentThis article does not contain any studies with human and animal subjects conducted by any of the authors.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interestsThe authors declare no competing interests.