We performed a collective analysis of a dedicated national post-myocardial infarction ventricular septal defect (VSD) registry to further elucidate controversial areas of this clinical entity's surgical treatment.
MethodsA descriptive statistical analysis was carried out and cumulative survival using the Kaplan-Meier method and multivariate logistic regression of risk factors for 30-day mortality are presented.
ResultsMedian survival of the cohort (n=76) was 72 months (95% CI 4–144 months). Better cumulative survival was observed in patients who underwent VSD closure more than 10 days after myocardial infarction (log-rank p=0.036). Concomitant coronary artery bypass grafting (CABG), different closure techniques, location of the VSD, extracorporeal membrane oxygenation as bridge to closure, or intra-aortic balloon pump as bridge to closure showed no statistically significant differences at Kaplan-Meier analysis. Multivariate binary logistic regression for independent factors affecting status at 30 days showed a statistically significant effect of age (OR 1.08; 95% CI 1.01–1.15) and concomitant CABG (OR 0.23; 95% CI 0.06–0.90).
ConclusionsOur results are comparable with previous reports regarding mortality, risk factors and concomitant procedures. Timing of surgery remains a controversial issue. Later closure seems to be advantageous, however, there is significant observational bias.
Análise coletiva de um registo nacional dedicado a comunicação interventricular (CIV) pós-enfarte (EAM) de forma a elucidar áreas controversas do tratamento cirúrgico desta entidade clínica.
MétodosAnalise descritiva; sobrevida cumulativa utilizando o método de Kaplan-Meier; análise multivariada utilizando regressão logística para fatores de risco para mortalidade a 30 dias.
ResultadosA sobrevida mediana da coorte (n=76) foi de 72 meses (6 anos, 95% CI 4-144 meses). Melhor sobrevida cumulativa foi observada nos doentes submetidos a encerramento cirúrgico da CIV após 10 dias do EAM (log-rank p=0,036). CABG concomitante, diferentes técnicas cirúrgicas de encerramento, localização da CIV, ECMO como ponte para encerramento ou BIA como ponte para encerramento não relevaram diferença estatisticamente significativa na análise de Kaplan-Meier. Análise multivariada para fatores independentemente associados a mortalidade a 30 dias revelou uma diferença estatisticamente significativa da idade (OR 1,08; 95% CI 1-011-15) e CABG concomitante (OR 0,23; 95% CI 0-06-0,90).
ConclusãoOs nossos resultados são comparáveis com os resultados previamente reportados por outros autores. O timing do encerramento cirúrgico em relação ao EAM permanece um assunto controverso. No entanto, um encerramento mais tardio parece ser vantajoso, apesar de existir um importante viés observacional.
Ventricular septal defect (VSD) is a rare complication of myocardial infarction (MI), with an estimated incidence of 0.2% of all MI patients in the current era of widely available reperfusion techniques.
Despite the low reported incidence, post-MI VSD carries a high mortality risk for patients, whether treated medically (exceeding 90%) or surgically (ranging from 19% to 60%). Although there has been considerable improvement in the treatment of acute coronary disease in the past two decades, VSD patients remain a challenging niche.1,2 In this regard, several issues involving the surgical treatment of VSD patients remain controversial, including the timing of surgery, the surgical technique, extracorporeal membrane oxygenation (ECMO) as a bridge to closure, concomitant procedures, and the role of percutaneous closure.
Most of the evidence regarding post-MI VSD is derived from small single-center studies with small sample sizes. Three national registries have been reported in the literature.3–5 These multicenter studies provide the best available evidence regarding this rare entity.
ObjectivesOur objective was to establish the first dedicated post-MI VSD national registry in Portugal. We hope that the collective analysis of data on VSD patients on a national scale will contribute to the evidence available regarding risk factors, surgical treatment, perioperative management and long-term follow-up of post-MI VSD patients.
MethodsData collectionPatient characteristics and procedural and postoperative information were retrospectively retrieved from prospectively collected data from the enrolled centers’ databases; therefore, this information did not alter or influence patient treatment or outcome in any way. In order to achieve maximal completeness of the registry, patients’ individual records were retrospectively consulted to retrieve any missing data.
The data were uniformly collected through an online form available to all the centers involved.
Patient and center enrollmentAll seven public cardiac surgery centers in Portugal were individually contacted and asked to enroll in the database. The response rate was 45–50% of the estimated national representation (calculated by the rate of individual center weight in yearly total reported national cardiac surgery numbers). All patients operated with a diagnosis of post-MI VSD were included. No exclusion criteria were defined. These results correspond to the first 76 patients reported during a pre-specified one-year enrollment timeframe, and therefore consist of a preliminary report with the potential for more patient inclusion.
Statistical analysisDescriptive statistics for outcomes and baseline patient characteristics are presented as percentages for categorical variables and as means with standard deviation (SD) for continuous variables. Cumulative survival was analyzed using the Kaplan-Meier method.
The chi-square test or Fisher's exact test was used to compare categorical variables and the Mann-Whitney U-test and Student's t test were used to compare continuous variables. Binary logistic regression was used to estimate independent risk factors for being alive at 30 days postoperatively. Variables used in the logistic regression were baseline characteristics that were statistically significant at univariate analysis (p<0.1) and timing of intervention, which was significant in the Kaplan-Meier analysis.
A p-value of <0.05 was considered significant.
All analysis was performed using R (R Foundation for Statistical Computing, Vienna, Austria).
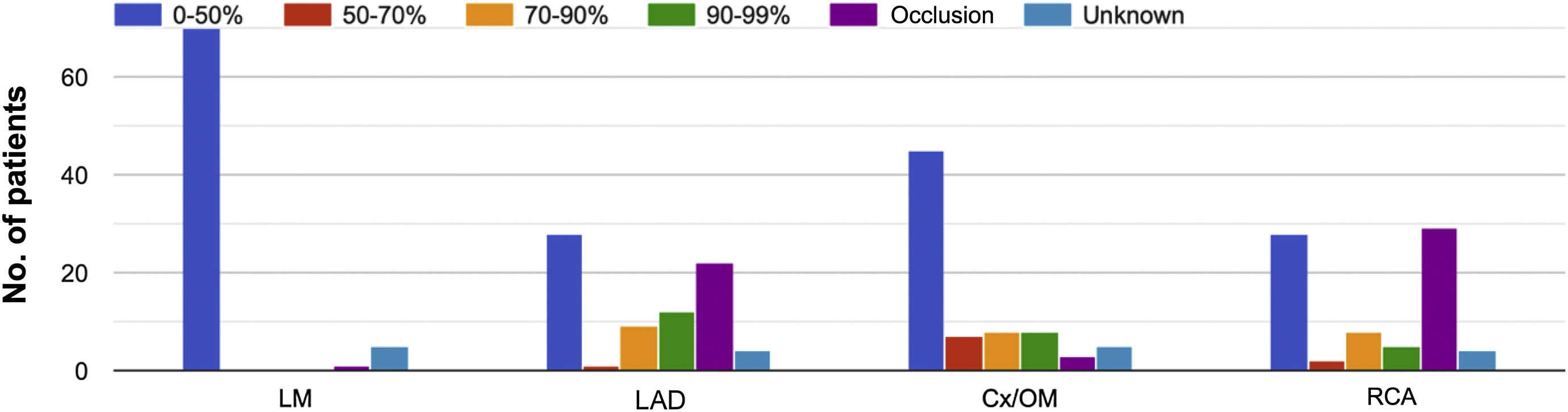
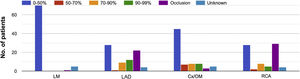
ResultsPatient and procedural characteristics are summarized in Table 1. Fifty-one percent of patients were female. The most common comorbidities in the cohort were hypertension (68%) and diabetes (34.7%). The majority of patients presented no significant hemodynamic lesions in either aortic or mitral valves. Left ventricular ejection fraction was normal in 53.6% of patients. Pulmonary hypertension (pulmonary arterial systolic pressure >60 mmHg) was present in 14.7% of patients. Qp/Qs was 1–1.9 in 10.5% and 2–2.9 in 19.7% of patients, however, Qp/Qs measurement was not available for the majority of patients. Regarding coronary anatomy, the most commonly affected vessels were the left anterior descending and right coronary arteries (Figure 1). Seventy-three percent of patients received preoperative inotropic support. Mechanical support by intra-aortic balloon pump (IABP) as a bridge to closure was used in 30 (39.5%) and ECMO in five (6.6%) patients. The most common closure technique (94.5%) was based on the classic technique described by Daggett.6 Associated percutaneous closure was necessary in four patients (5.3%), all for residual VSD after the index closure. Forty-six percent of patients had a concomitant procedure at the same time as VSD closure, most commonly coronary artery bypass grafting (CABG) (36.8%). The most common postoperative complications were infection (non-specified location) (25%) and renal failure requiring dialysis (18.4%).
Patient, procedural and postoperative characteristics of the cohort (n=76).
| Patient characteristics | |
|---|---|
| Female | 39 (51.3%) |
| Age, years | 69.5±10.7 |
| Location of defect | |
| Apical | 43 (58.9%) |
| Basal | 27 (37%) |
| Mid | 3 (4.1%) |
| Time of surgery after MI | |
| First 24 h | 13 (17.1%) |
| 1–5 days | 29 (38.2%) |
| 6–10 days | 10 (13.2%) |
| >10 days | 24 (31.6%) |
| Hypertension | 51 (68%) |
| Diabetes | 26 (34.7%) |
| Stroke | 5 (6.7%) |
| BMI >40 kg/m2 | 11 (14.7%) |
| eGFR <30 ml/min/1.73 m2 | 6 (8%) |
| COPD | 2 (2.7%) |
| Previous MI | 5 (6.7%) |
| Hemodynamically significant aortic valve lesion | 3 (4.4%) |
| Hemodynamically significant mitral valve lesion | 5 (7.4%) |
| Ejection fraction <50% | 32 (46.4%) |
| PASP >60 mmHg | 10 (14.7%) |
| Qp/Qs | |
| Not calculated | 51 (67.1%) |
| 1–1.9 | 8 (10.5%) |
| >2 | 17 (22.3%) |
| Preoperative support | |
| None | 20 (27.4%) |
| Inotropes | 41 (54.7%) |
| IABP | 30 (39.5%) |
| ECMO | 5 (6.6%) |
| Invasive ventilation | 16 (21.3%) |
| Procedural characteristics (n=76) | |
| Closure technique | |
| Daggett technique | 69 (94.5%) |
| Other | 4 (5.5%) |
| Associated percutaneous closure | 4 (5.3%) |
| Concomitant procedure | 35 (46.1%) |
| CABG | 28 (36.8%) |
| Mitral | 5 (6.6%) |
| Postoperative characteristics (n=76) | |
| Duration of inotropic support, days | 6.5±9.8 |
| Need for IABP | 39 (51.3%) |
| Duration of IABP, days | 5.3±5.8 |
| Need for ECMO | 6 (7.9%) |
| Duration of ECMO, days | 8.7±9.7 |
| Length of hospital stay, days | 18.5±23.1 |
| Alive at discharge | 47 (61.8%) |
| Alive at 30 days | 53 (69.7%) |
| Complications | |
| Stroke | 7 (9.2%) |
| Renal failure requiring dialysis | 14 (18.4%) |
| Bleeding requiring reoperation | 8 (10.5%) |
| Unspecified infection | 19 (25%) |
| Low output/cardiogenic shock | 10 (13.2%) |
| Tracheostomy | 5 (6.6%) |
| Reintervention for residual VSD | 8 (10.5%) |
| Residual VSD at discharge | 7 (9.2%) |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; ECMO: extracorporeal membrane oxygenation; eGFR: estimated glomerular filtration rate; IABP: intra-aortic balloon pump; MI: myocardial infarction; Qp/Qs: pulmonary blood flow to systemic blood flow ratio; PASP: pulmonary arterial systolic pressure; VSD: ventricular septal defect.
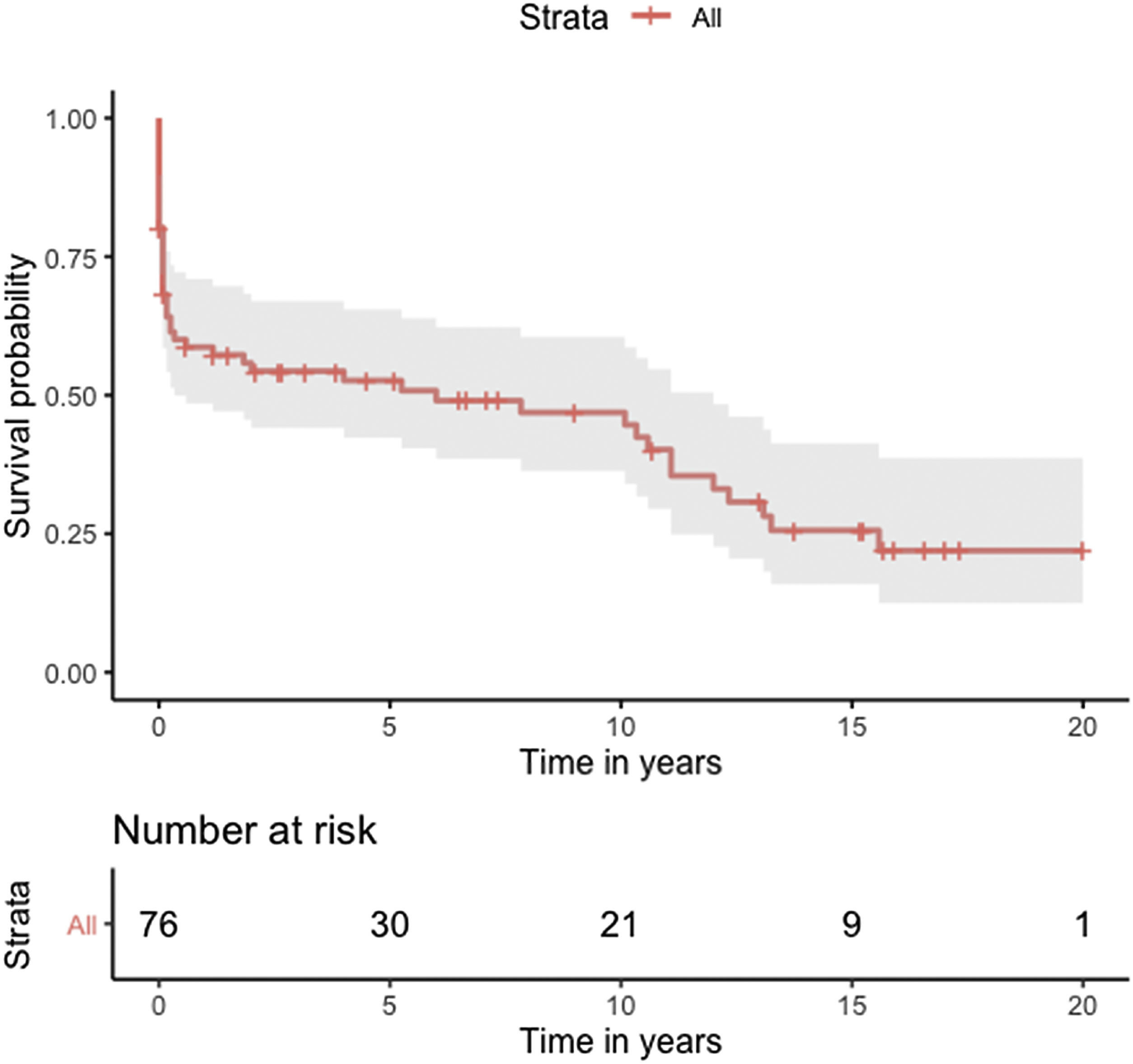
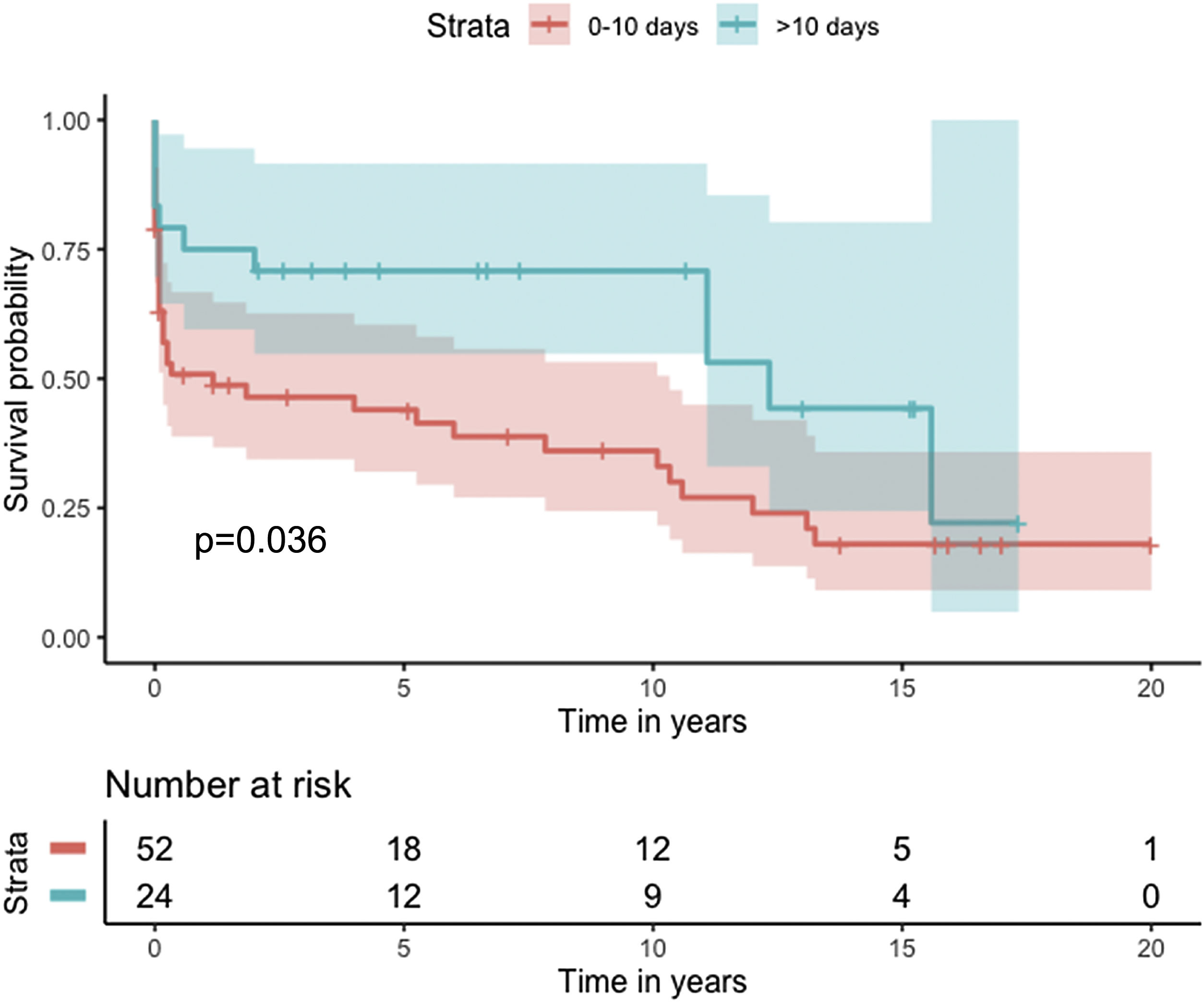
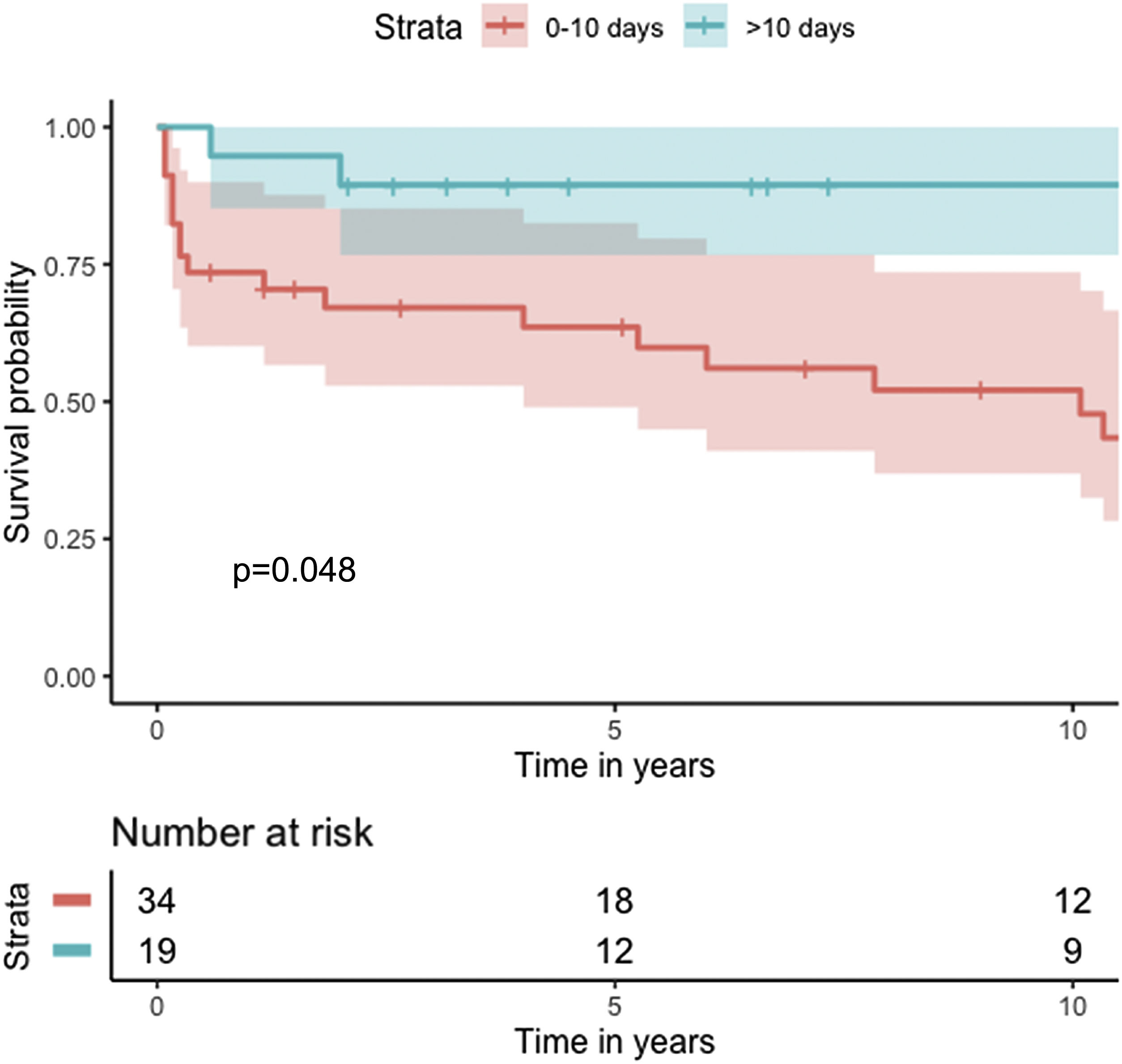
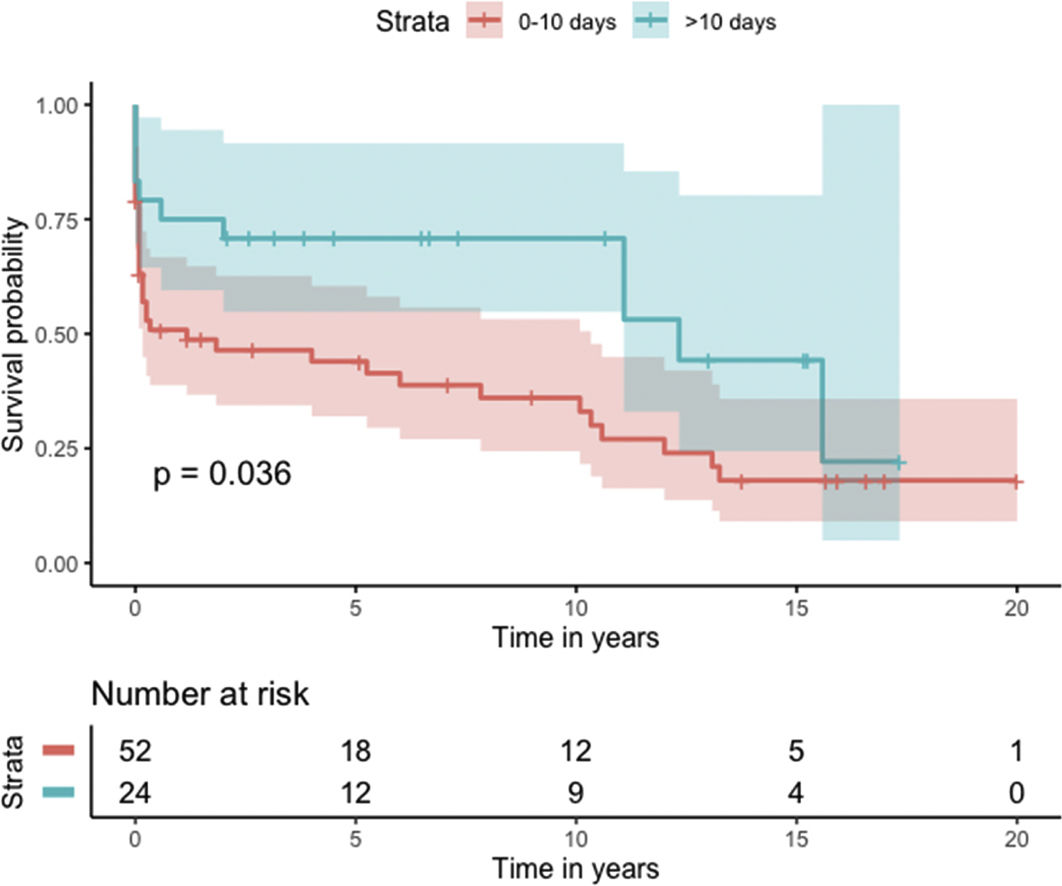
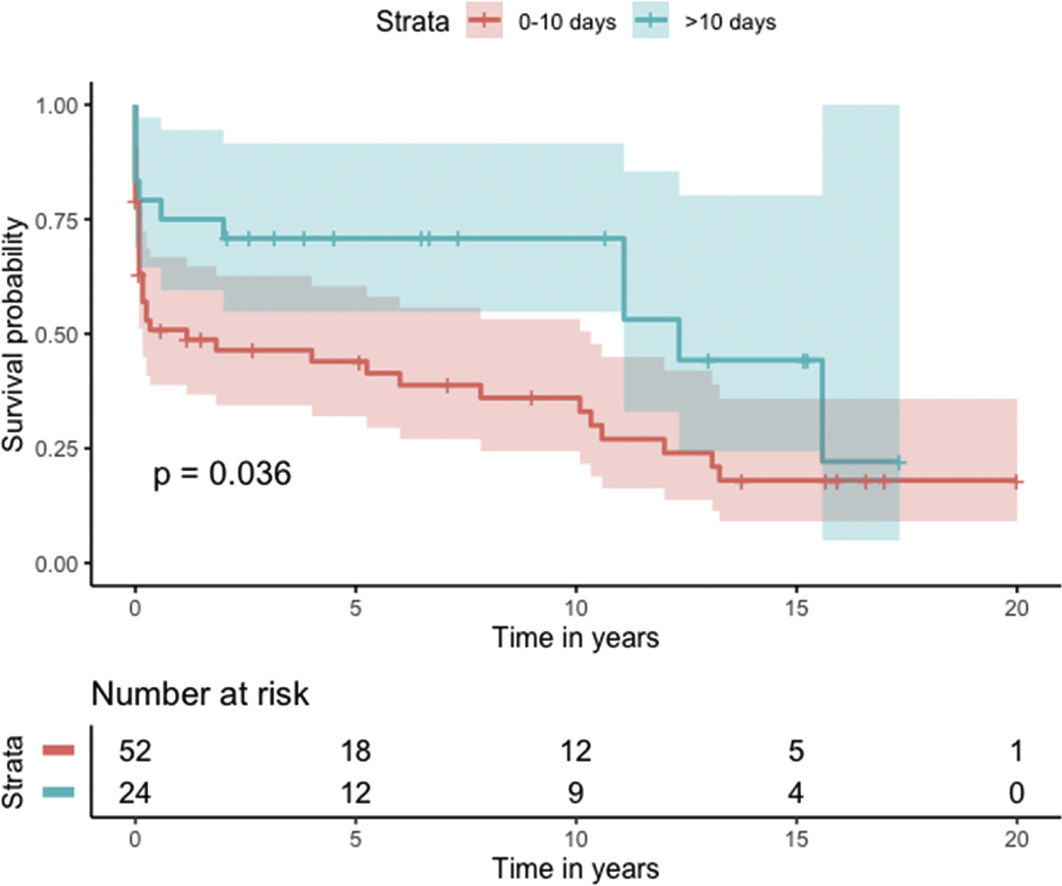
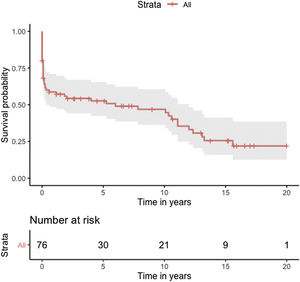
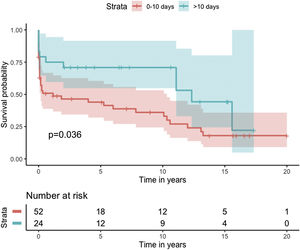
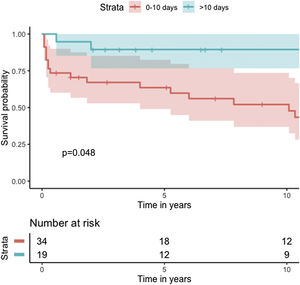
Median survival of the cohort was 72 months (95% confidence interval [CI] 4–144 months) (Figure 2). Two-, six- and 10-year survival rates were 54.3% (95% CI 44.1–67.0), 49.0% (95% CI 38.6–62.3), and 46.9% (95% CI 36.3–60.5), respectively. Survival at discharge was 61.8% and 30-day survival was 69.7%. Patients who underwent VSD closure early rather than later after onset of symptoms seem to have performed worse (first 10 days vs. after 10 days: log-rank p=0.036) (Figure 3). This difference was evident even when only patients alive at the 30-day mark were considered in the analysis (Figure 4).
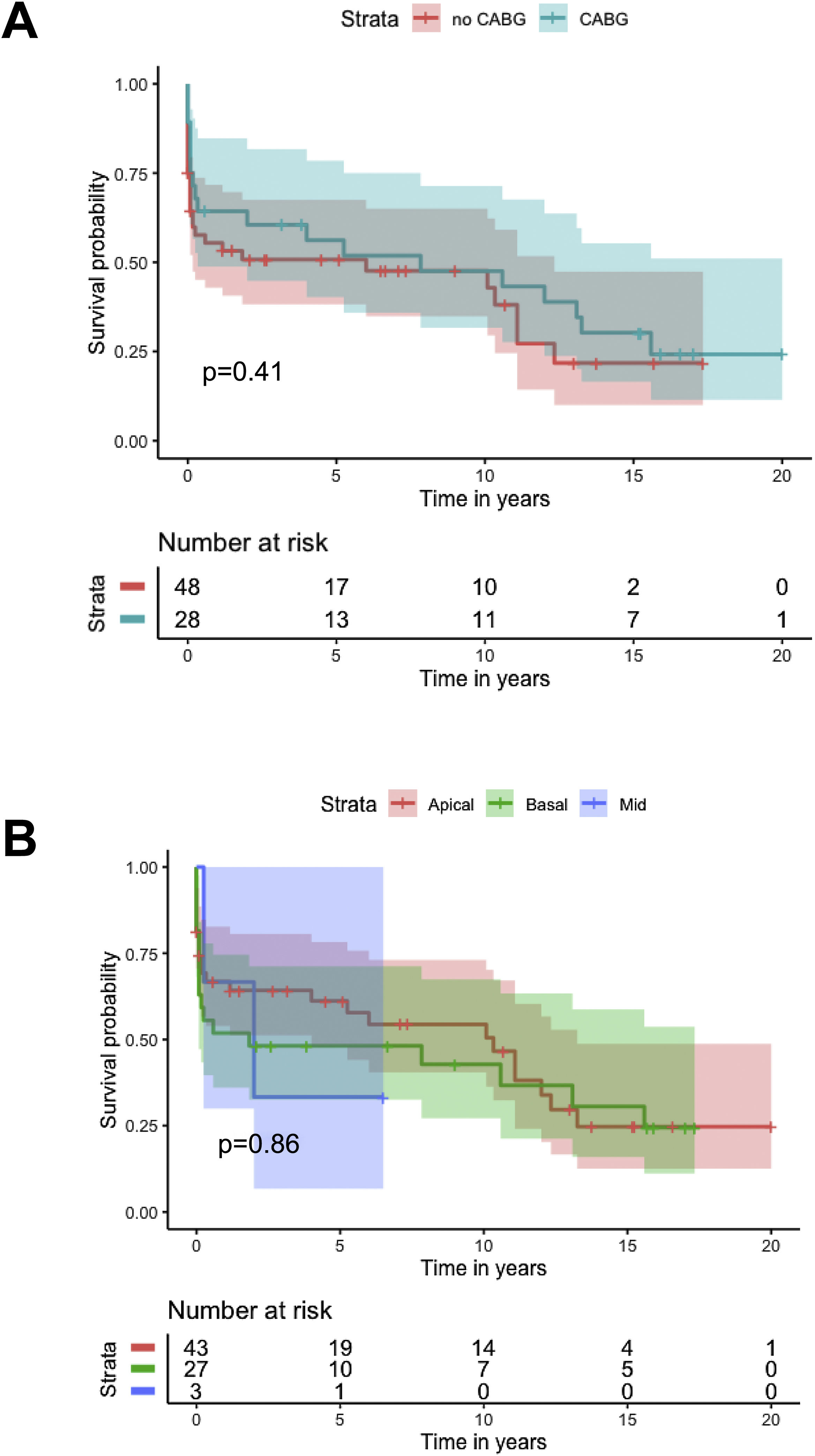
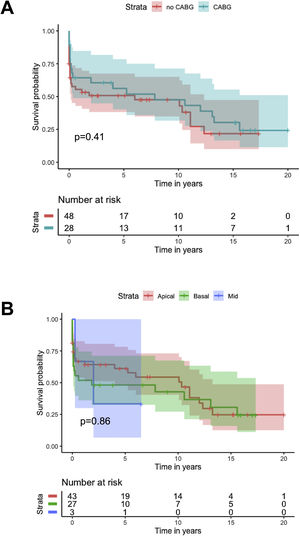
Kaplan-Meier analysis showed no difference in cumulative survival in patients undergoing concomitant CABG (log-rank p=0.4), different closure techniques (log-rank p=0.5), ECMO as a bridge to closure (log-rank p=0.9) or IABP as a bridge to closure (log-rank p=0.6), or with different locations of the VSD (log-rank p=0.9) (Figure 5).
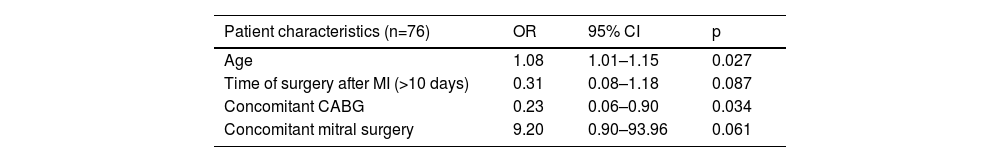
Risk factors for 30-day mortalityStatistically significant differences in preoperative and operative characteristics were seen between 30-day survivors and non-survivors on univariate analysis: age (p=0.024); concomitant mitral surgery (p=0.027); concomitant CABG (p=0.023), as well as in postoperative characteristics: IABP support in the postoperative period (p=0.047); postoperative acute renal failure requiring dialysis (p=0.024); and postoperative cardiogenic shock or low-output syndrome (p<0.001). Multivariate analysis of baseline characteristics with p<0.1 (age, concomitant CABG and mitral surgery) and timing of intervention (which presented a significantly different cumulative survival on Kaplan-Meier analysis as well as clinical interest and plausibility) revealed a statistically significant association between 30-day mortality and age (OR 1.08; 95% CI 1.01–1.15; p=0.27) and concomitant CABG (OR 0.23; 95% CI 0.06–0.90; p=0.034). Concomitant mitral surgery (OR 9.20; 95% CI 0.90–93.96; p=0.06) presented a trend toward worse 30-day outcome (Table 2).
Multivariate binary logistic analysis for independent factors affecting status at 30 days (alive vs. dead).
| Patient characteristics (n=76) | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.08 | 1.01–1.15 | 0.027 |
| Time of surgery after MI (>10 days) | 0.31 | 0.08–1.18 | 0.087 |
| Concomitant CABG | 0.23 | 0.06–0.90 | 0.034 |
| Concomitant mitral surgery | 9.20 | 0.90–93.96 | 0.061 |
Univariate significant factors and timing of closure were considered in the model.
CABG: coronary artery bypass graft; CI: confidence interval; MI: myocardial infarction; OR: odds ratio.
Regarding the timing of post-MI VSD surgical closure, most authors agree that early surgery is indicated, as it avoids prolonged exposure to congestive heart failure and cardiogenic shock leading to multiorgan dysfunction. Advocates of deferred closure argue that a delayed strategy provides time to stabilize the patient, permitting VSD closure in optimal clinical and tissue conditions.7 This is an almost impossible dilemma to resolve in an observational setting, as critically ill patients tend to be operated early and patients in whom surgery is delayed are usually less ill. Adding to this problem is the fact that patients considered inoperable or who died waiting for surgery are not usually included in surgical cohorts. According to the 2013 American College of Cardiology Foundation/American Heart Association guideline for the management of ST-elevation MI,8 surgical treatment should be initiated without delay after diagnosis, whereas the 2017 European Society of Cardiology guidelines on ST-elevation MI9 recommend that delaying intervention may be considered in certain patients.
In our analysis, cumulative survival of delayed intervention was superior both for the overall cohort (Figure 3) and for patients alive at 30 days (Figure 4). This, however, was not apparent in the multivariate model for 30-day mortality. This may be related to underpowering of our model to detect a significant difference. More importantly, however, as previously described, observational studies in the setting of VSD cannot provide a clear picture of the best surgical timing due to the fact that critically ill patients tend to be operated sooner and hemodynamically stable patients tend to be operated later.
One reasonable conclusion is that these patients benefit from being operated as late as their hemodynamic profile permits.
ECMO has been advocated as a bridge to VSD closure. Theoretically ECMO limits the patient's exposure to the detrimental hemodynamic consequences of the VSD, reducing end-organ malperfusion and unloading the heart, while permitting a more opportune timing of closure. However, ECMO is not without its own inherent complications. Morimura et al. reported mechanical circulatory support, including ECMO and IABP, as a bridge to VSD closure in a small cohort, with positive results.10
In our cohort ECMO was used in five patients as a bridge to closure. This strategy was not associated with different cumulative survival (log-rank p=0.9); however, all ECMO patients had a follow-up of less than five years, and three (60%) were alive at discharge.
Model simulations suggest that the Impella device may present a superior hemodynamic benefit in the post-MI VSD setting compared to ECMO, thus permitting a delayed closure strategy with fewer associated complications.11
There is further controversy relating to the benefit of concomitant CABG. Although the majority of studies show no benefit of concomitant CABG regarding early and long-term mortality, results with both improved and reduced survival have been reported. The aforementioned registries3–5 showed no significant difference in survival for patients undergoing concomitant CABG, except for mid-term survival after exclusion of patients who died in the first 30 days. Perrotta et al. reviewed the best available evidence regarding concomitant revascularization in this setting and found that overall, it suggests that revascularization of non-infarcted areas of myocardium and consequent improvement of collateral flow may contribute to better long-term survival.12 Our findings are consistent with the results reported from previous registries, with no difference in cumulative survival (Figure 4), although a protective effect for 30-day mortality was found (OR 0.23; 95% CI 0.06–0.90; p=0.034).
Various techniques of VSD closure have been described in the literature.2,6,13 It is unclear whether any one technique has definite advantages over any other. Furthermore, extent of infarcted area, time of reperfusion and center/surgeon case volume may be more significant factors affecting residual VSD and overall outcome. Our cohort has a clear dominance of the classic technique described by Daggett, with a small number of alternate techniques, which precludes interpretation regarding the relation between closure technique and outcome.
Since the first percutaneous closure of a post-MI VSD in the 1980s, percutaneous intervention has evolved and is viewed with renewed interest. Technical success rates of over 75% with associated in-hospital/30-day mortality of 32% have been reported.14 However, patient selection is an inherent characteristic of such interventional reports, with anatomical feasibility as a limiting factor and most patients not being considered candidates for surgery.
Percutaneous closure in post-MI VSD has been proposed both as a bridge to surgical treatment in patients who are not expected to survive an operation and as a definitive treatment in simple <15 mm defects in the sub-acute or chronic phase.15 Additionally, percutaneous closure is an alternative for reintervention for residual VSD after index closure, which itself consists of a particular subset of high-risk patients. In our cohort we identified four patients in which this approach was used. Three out of these four (75%) patients were discharged alive.
Operative mortality was high in our cohort, albeit in agreement with that described in previous registries: 33.0% in the Japanese registry,5 42.9% in the Society of Thoracic Surgeons Adult Cardiac Surgery Database3 and 41% in the Swedish registry4 (30-day mortality) and 47% in the Global Utilization of Streptokinase and T-PA for Occluded Coronary Arteries (GUSTO) trial.1
Several risk factors for early and late death have been proposed in previous studies.3–5,16,17
Multivariate analysis showed the significant impact of concomitant CABG (OR 0.23; 95% CI 0.06–0.90; p=0.034) and age (OR 1.08; 95% CI 1.01–1.15; p=0.27) on 30-day mortality, as well as a trend regarding concomitant mitral surgery (OR 9.20; 95% CI 0.90–93.96; p=0.061) (Table 2). Other authors have reported similar findings regarding age3,5 and significant mitral regurgitation.3 These findings suggest that surgical complexity and frailty may play a role in early outcomes. A beneficial effect of concomitant CABG has been proposed but remains controversial.15 In our study, due to the outcome being 30-day mortality, long hospitalizations (>30 days) may have skewed the influence of other important baseline characteristics.
As a side note, a uniform approach regarding timing of intervention was not discernible in the three centers included in our cohort, with both epoch- and surgeon-related variation found. A common notion, however, is the need for a pragmatic rather than a dogmatic approach, in the sense that the timing of intervention depends on close monitoring and patient-tailored strategies rather than a predefined timeframe.
Study limitations and meritsDue to its retrospective design and non-randomization of patients this study is at risk of inherent biases in data collection. The large time frame it encompasses (from 2000 to 2021) also presents a risk of bias relating to changes in practices and perioperative care. A ‘VSD repair registry’ rather than a ‘VSD registry’ is presented, in the sense that patients considered inoperable or who died waiting for surgery are not included in this cohort. Nevertheless, it is one of the largest cohorts of surgically treated post-MI VSD patients studied. Its multicenter design provides a reflection of the real-world population and different management strategies.
ConclusionVSD is a dreadful complication of MI. Registries may provide important evidence regarding an entity for which randomized controlled trials are impractical. Percutaneous closure and mechanical support are emerging techniques that may prove useful in selected patients. In our study, age negatively influenced and concomitant CABG positively influenced 30-day survival.
Timing of surgery remains a controversial issue. Later closure seems to be advantageous but there is significant bias. Overall, it seems that these patients benefit from being operated as late as their hemodynamic profile permits.
Learning pointsVSD is a dreadful complication of MI.
Registries may provide important evidence regarding this entity.
CABG positively influenced 30-day survival.
Age negatively influenced 30-day survival.
Later closure seems to be advantageous but an important bias exists.
FundingNo funding was received for this project.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to acknowledge the work of all involved in data collection.