Cardiovascular (CV) guidelines stress the need for global intervention to manage risk factors and reduce the risk of major vascular events. Growing evidence supports the use of polypill as a strategy to prevent cerebral and cardiovascular disease, however it is still underused in clinical practice. This paper presents an expert consensus aimed to summarize the data regarding polypill use. The authors consider the benefits of polypill and the significant claims for clinical applicability. Potential advantages and disadvantages, data regarding several populations in primary and secondary prevention, and pharmacoeconomic data are also addressed.
As recomendações cardiovasculares salientam a necessidade de uma intervenção global para controlar os fatores de risco e assim reduzir o risco de eventos vasculares major. Há evidência crescente que apoia o uso da polipílula como estratégia de prevenção de doenças cérebro e cardiovasculares, porém esta ainda é subutilizada na prática clínica. Este artigo apresenta um consenso de especialistas com o objetivo de resumir os dados sobre a utilização de polipílula. Os autores consideram os benefícios da polipílula e a sua possível aplicabilidade clínica. Vantagens e desvantagens potenciais, dados referentes a populações em prevenção primária e secundária e dados fármaco-económicos também são abordados.
Cardiovascular diseases are the leading cause of death worldwide, accounting for 18 million deaths each year, a third of all-cause annual deaths. Ischemic heart disease and stroke represent the majority.1 Despite the differences between coronary heart disease and stroke, these conditions share most risk factors.
The INTERHEART case–control study showed that 90% of the risk of myocardial infarction could potentially be explained by the combination of six major risk factors (dyslipidemia, hypertension, smoking, diabetes, obesity, psychosocial stress) with the absence of three protective factors (exercise, daily diet rich in fruit and vegetables, moderate alcohol intake).2 According to the prevalence of these risk factors and the magnitude of the estimated risk, dyslipidemia showed the highest population attributable risk (49.2%).2
Similarly, the risk factors for stroke were studied in the INTERSTROKE case-control study.3 Major risk factors identified were hypertension, dyslipidemia, smoking, central obesity, diabetes, alcohol intake, cardiac disease, stress, diet, and physical inactivity. Hypertension had the highest population attributable risk for stroke (47.8%), followed by dyslipidemia.3
Overall, hypertension and dyslipidemia, both modifiable vascular risk factors, are the major contributors to the annual incidence of myocardial infarction and ischemic stroke. Furthermore, it is acknowledged that these are mediated by arterial disease and thrombosis.2,3
Hypertension, dyslipidemia and atherothrombosis in PortugalIt is widely known that hypertension and dyslipidemia are two prevalent risk factors in the Portuguese population.
The first landmark study regarding arterial hypertension prevalence, knowledge, treatment and control was the PAP study.4 Among 5023 adult individuals, a representative sample of the Portuguese population in 2003, the prevalence of hypertension was 42.1%. Of these, 46.1% had a previous diagnosis of hypertension, 39.0% were treated with anti-hypertensive drugs and only 11.2% had controlled hypertension. More recently, the Portuguese HYpertension and SAlt (PHYSA) study revealed a similar prevalence of hypertension (43.1%), but a higher proportion of knowledge (62.8%), treatment (69.9%) and control (32.1%), albeit still far from desirable goals.6Table 1 summarizes the major Portuguese hypertension studies.
Key studies on the prevalence of hypertension and dyslipidemia in Portugal.
| Key studies on hypertension prevalence in Portugal | |||
|---|---|---|---|
| Study | Study period | Prevalence of hypertension | Control of hypertension |
| PAP4 | 2003 | 42.1% | 11.2% |
| PHYSA5 | 2011–2012 | 42.2% | 42.6% |
| e_COR6 | 2012–2014 | 43.1% | 32.1% |
| Key studies of dyslipidemia prevalence in Portugal | |||
|---|---|---|---|
| Study | Study period | Prevalence of elevated total cholesterol | Prevalence of elevated LDL cholesterol |
| BECEL7 | 2001 | ≥190 mg/dl: 68.5% | ≥115 mg/dl: 71% |
| VALSIM9 | 2006–2007 | ≥200 mg/dl: 47% | ≥130 mg/dl: 38.4% |
| HIPOCRATES8 | 2008 | ≥190 mg/dl: 56% | ≥115 mg/dl: 41% |
| e_COR6 | 2012–2014 | ≥200 mg/dl: 56.3% | ≥130 mg/dl: 51.5% |
Regarding dyslipidemia, several cross-sectional epidemiological studies have been performed in the last two decades, however prevalence is more heterogeneous due to the use of different criteria, time points and cholesterol thresholds.
In the BECEL study comprising 1500 individuals, the prevalence of dyslipidemia defined as total cholesterol ≥190 mg/dl, was 68.5% with 71% of the individuals having LDL cholesterol ≥115 mg/dl.7 The HIPOCRATES study, including 1585 individuals representative of the Portuguese population aged 18–75 years old, revealed a prevalence of total cholesterol ≥190 mg/dl or the use of lipid lowering drugs of 56% and a prevalence of LDL cholesterol ≥115 mg/dl of 41%.8 The largest epidemiological study published to date with information concerning risk factors, especially dyslipidemia, in a primary care setting in Portugal, was the VALSIM study.9 It assessed 16856 patients followed in primary care and concluded that 47% had total cholesterol ≥200 mg/dl and 38.4% had a LDL cholesterol ≥130 mg/dl.9 More recently, the eCOR study showed that 31.5% of the participants had LDL cholesterol ≥160 mg/dl and 51.5% ≥130 mg/dl.6 These studies share a factor in common: cholesterol (more specifically LDL cholesterol thresholds) was not adjusted for the baseline cardiovascular risk, possibly underestimating the prevalence of dyslipidemia burden and control. Table 1 summarizes the major Portuguese studies on dyslipidemia.
Regarding cardiovascular events, the Atlas of the European Society of Cardiology (ESC) showed that Portugal had an age-standardized incidence (435 per 100000 inhabitants) and prevalence (4871 per 100000 inhabitants) of cardiovascular disease. This included atherosclerotic and non-atherosclerotic cardiovascular disease.10 From these, the incidence (83 per 100000 inhabitants) and prevalence (1325 per 100000 inhabitants) of ischemic heart disease were relatively low among all ESC member countries,10 despite the average of 362 primary percutaneous coronary interventions per million of inhabitants.10 Stroke estimates (incidence 76 and prevalence 804 per 100000 inhabitants) were lower than ischemic heart disease, but Portugal's ranking in comparison with other ESC member countries was not so favorable.10
One of the most comprehensive studies evaluating the epidemiological data of atherosclerotic cardiovascular disease in Portugal11 estimated that in 2016 about 674000 individuals had atherothrombotic disease and that 15000 deaths were attributable to this condition (approximately 14% of all-cause mortality), illustrating the importance of atherosclerosis in Portugal.11
Scope and methodology of consensusAn expert panel was established to gather consensus on areas of uncertainty in polypill applicability and supported by scientific evidence. It involved a multidisciplinary panel of clinicians, with expertise in cardiovascular prevention, representing several specialties: Primary Care, Cardiology, Neurology, Internal Medicine and Clinical Pharmacology. The authors conducted a literature review on Pubmed with the key words “polypill”, “cardiovascular disease”, “cardiovascular prevention” and “CNIC”, for Centro Nacional de Investigaciones Cardiovasculares (CNIC), and selected all the review articles, randomized controlled trials, systematic reviews and meta-analysis. Bibliography was shared for individual reflection. All the experts contributed to the most relevant topics in their areas of expertise, and a discussion meeting was held. A draft of the document was produced and reviewed by all authors for final consensus. Ferrer Laboratories supported the meeting and the medical writing but had no participation in the discussion or drafting of the document.
The polypill conceptThe acknowledgment of the importance of cardiovascular risk factors in global morbidity and mortality, and the evidence of cardiovascular event risk reduction with drug therapy, led to a meeting between representatives of the Welcome Trust and the World Health Organization in 2001 to establish the principles and requirements of a single pill combination for cardiovascular prevention. The potential benefits of this cardiovascular polypill, at that time combining a statin, a blood pressure drug, aspirin, and folic acid, were raised by Yusuf in 2002,12 and proposed by Wald and Law in 2003. They claimed that it could theoretically reduce the population risk of cardiovascular events by at least 80%. These measures were the most important in the development of the concept of polypill for cardiovascular risk reduction in the population.13
The cardiovascular polypill is a fixed dose combination of drugs with proven benefits in the prevention of cardiovascular disease, usually a statin and a blood pressure drug(s), with or without aspirin. The concept of the polypill was first approved in 2011 with a single pill containing aspirin, ramipril, and simvastatin.14 The candidate drugs had to warrant stability testing for all components together, preserve their pharmacokinetics (bioavailability), pharmacodynamics (the effects on blood pressure, LDL cholesterol, and platelet function) and ideally influence “hard” cardiovascular outcomes.
Currently there are various polypills available or being investigated. In Portugal, only the combination of aspirin–ramipril–atorvastatin (also termed CNIC-polypill) and perindopril–amlodipine–atorvastatin are available (Table 2).
Polypills under investigation or with market approval based on Webster et al., 2017.14
| Cardiovascular polypills currently available in Portugal | Studied polypills currently not available in Portugal |
|---|---|
| CNIC polypill: aspirin, ramipril and atorvastatin | Aspirin, simvastatin, atenolol, thiazide and ramipril |
| Perindopril, amlodipine, and atorvastatin | Aspirin, losartan, atenolol, and atorvastatin |
| Clopidogrel, Atorvastatin, and ramipril | |
| Aspirin, Ramipril, metoprolol, and atorvastatin | |
| Aspirin, atorvastatin, hydrochlorothiazide, and, valsartan | |
| Aspirin, atorvastatin, hydrochlorothiazide, and enalapril | |
| Amlodipine, rosuvastatin | |
| Pitavastatin*, valsartan14 | |
| Ramipril, hydrochlorothiazide, atenolol, simvastatin |
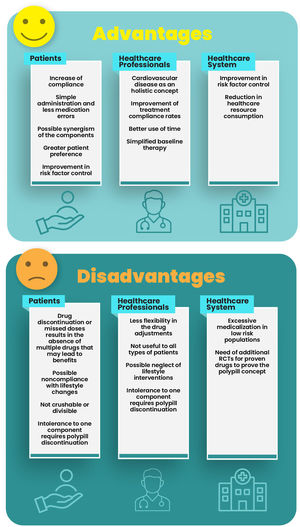
Polypills have both advantages and disadvantages for patients, health care professionals and healthcare systems (Table 3). The main advantage is the simplification of the drug treatment regimen, which can lead to increase in patient compliance, enhanced patient preferences, fewer medication errors, and thus an increase in the likelihood of reaching the target values for blood pressure and LDL cholesterol.15
Main characteristics of randomized controlled trials that evaluated the compliance with the polypill.
| Trials | Population | Polypill | Follow-up | Results |
|---|---|---|---|---|
| UMPIRE24 | 2004 patients with established CVD or at risk of CVD | Aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg, and atenolol 50 mg or hydrochlorothiazide 12.5 mg | 15 months | Polypill significantly improved medication adherence by 33% |
| IMPACT25 | 513 patients at high CV risk | Aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg, and atenolol 50 mg or hydrochlorothiazide 12.5 mg | 12 months | Polypill significantly improved medication adherence by 75% |
| Kanyini-GAP26 | 623 patients with established CVD or at risk of CVD | Aspirin 75 mg, simvastatin 40 mg, lisinopril 10 mg, and atenolol 50 mg or hydrochlorothiazide 12.5 mg | 18 months | Polypill significantly improved medication adherence by 49% |
| FOCUS Phase 222 | 695 at secondary prevention after MI | Aspirin 100 mg, simvastatin 40 mg, and ramipril 2.5, 5, or 10 mg | 9 months | Polypill significantly improved medication adherence |
Another possible advantage is the potential combination of the polypill components. In a pharmacodynamic open-label study, Juanatey et al. found that the CNIC-polypill was associated with an additional 7% decrease in LDL cholesterol in comparison with the equivalent dose of isolated atorvastatin alone in a per-protocol analysis of the study.16 The absolute reduction in LDL cholesterol was small (4.7 mg/dl), but the relative reduction (7%) was similar to doubling the statin dose. Despite reservations regarding the methodological limitations and the clinical relevance of this LDL cholesterol decrease, a synergistic effect cannot be ruled out, as this phenomenon has even been described in prior studies evaluating metabolic and endothelial parameters.17–19 Furthermore the use of both ACE inhibitors and statins have demonstrated benefits in clinical outcomes.19
The main disadvantage of the polypill is related to a less flexible dose adjustment of individual drug components. If necessary, this can be overcome by adding the additional medication needed. On the other hand when patients start to use drugs that increase the concentration of one or more of the compounds of the polypill, the potential dose adjustment requires a new prescription due to being a single compound. Similarly, the use of these drugs in elderly patients and those with conditions with decreased excretion of any of the compounds should be tightly monitored. Other disadvantages are related to missing doses and treatment discontinuation, implying missing or discontinuation of multiple drugs at the same time, or even neglecting other non-pharmacological risk reduction intervention. However, results of clinical trials do not confirm this hypothetical disadvantage.20 Other potential advantages and disadvantages are shown in Figure 1.
The difficulty in selecting polypill components makes it unsuitable for some types of patients. Certain drugs might not be indicated or tolerated by specific patients. This may be perceived as a disadvantage of the polypill. Currently, the most controversial components are antiplatelets agents for patients in primary prevention and beta-blockers.
Aspirin (or clopidogrel in the event of intolerance) is recommended by ESC guidelines for the secondary prevention of cardiovascular disease.21 The same guidelines recommend against antiplatelet therapy in individuals with low or moderate cardiovascular risk due to the potentially hazardous risk-benefit balance, namely due to increased risk of major bleeding. Nevertheless, the use of aspirin may be considered in patients with diabetes in primary prevention in those with high or very high cardiovascular risk, in the absence of contraindications.21,22
In Portugal, among the two available cardiovascular polypills, neither of them includes a beta blocker and one contains aspirin, which implies that prescribing should be tailored according to the previously mentioned criteria.
Consensus position: the simplification of the treatment regimen with a polypill, can lead to an increase in patient compliance, and better control of cardiovascular risk factors, namely LDL-C and blood pressure levels. The main disadvantage of the polypill may be the interruption of multiple drugs in the event of polypill discontinuation, and the inclusion of an antithrombotic in the polypill when used in a low risk primary prevention setting.
Polypill and treatment compliance, patient satisfaction, and quality of lifeAs previously mentioned, one of the main advantages of the polypill is the simplification of drug therapy, which can increase compliance.23 The problem of non-is estimated to affect about 40–50% of the patients,23–25 and is estimated to be responsible for 13 of every 100000 CVD deaths per year, and approximately 9% of all cardiovascular disease cases in Europe.24 It is also worth noting that in secondary prevention there is an increased risk of recurrence of major adverse cardiovascular events according to the level of adherence, with those who do not adhere having the highest event rates.26
Four major studies were published supporting the hypothesis that polypill increases adherence to drug therapy. Three of them, UMPIRE,27 IMPACT28 and Kanyini-GAP,29 evaluated the use of the polypill vs. standard of care (Table 3), and were pooled into the SPACE individual patient data meta-analysis.15 The other study was the FOCUS project, which had a first phase to determine the prevalence of non-compliance and its main determinants, and a second phase which was a randomized controlled trial that allocated patients to polypill or to the separate components of the polypill (Table 4).25
Summary characteristics of randomized controlled trials that evaluated the polypill in primary prevention of atherosclerotic cardiovascular disease.
| Characteristic/trial | TIPS-332 | HOPE-333 | PolyIran34 |
|---|---|---|---|
| Antiplatelet component | Aspirin 75 mg | No antiplatelet component | Aspirin 81 mg |
| Lipid lowering component | Simvastatin 40 mg | Rosuvastatin 10 mg | atorvastatin 20 mg |
| Blood pressure lowering component | Ramipril 10 mgAtenolol 100 mgHydrochlorothiazide 25 mg | Candesartan 16 mg Hydrochlorothiazide 12.5 mg | Enalapril 5 mg or valsartan 40 mgHydrochlorothiazide 12.5 mg |
| Number of patients included in the trial | 5713 | 6348 | 6101 |
| Inclusion criteria | Men≥50 years and women≥55 years with an INTERHEART risk score≥10, or men and women≥65 years with an INTERHEART risk score of ≥5 | Men≥55 years or women≥65 with at least one of the following cardiovascular risk factors: elevated waist-to-hip ratio, history of a low level of high-density lipoprotein cholesterol, current or recent tobacco use, hyperglycemia, family history of premature coronary disease, and mild renal dysfunction | Age 50≥years, living in rural areas |
| Trial design | Randomized double-blinded placebo-controlled trial | Randomized double-blinded placebo-controlled trial | Cluster randomized controlled trial, with stratification by districts and using villages as the unit of clustering for randomization (236 clusters) |
| Comparator | Matching placebos | Matching placebos | Minimal care (blood pressure measurement and risk factor counselling) |
| Main conclusions | Polypill with aspirin reduced the incidence of cardiovascular events in persons at intermediate cardiovascular risk | Rosuvastatin, candesartan and hydrochlorothiazide reduced the risk of cardiovascular events in persons at intermediate risk | Polypill reduced major cardiovascular events with high medication adherence and low incidence of adverse events |
These studies were randomized controlled trials with an open-label design, possibly increasing the risk of bias for efficacy and safety analysis, but their design was adequate for compliance assessment.
The SPACE meta-analysis included 3140 patients and showed that the polypill patients had a compliance (defined as taking the drugs ≥4 days in the last week at 12 months of follow-up) of 80% compared to 50% in standard of care, corresponding to a significant increase of 58% in compliance (RR 1.58, 95% confidence interval (CI) 1.32–1.90). Patients undertreated at the baseline showed an early decrease in compliance rates but overall, the improvement in compliance was greater at long-term in this subgroup.
The FOCUS project further strengthened the concept of polypill as a compliance enhancer against the prescription/administration of each drug component separately.25 Furthermore, using the validated Morisky-Green questionnaire, FOCUS concluded that younger patients, those with depression, or with complex medication regimens, and those with a lower level of social support were less compliant, making these subgroups the most likely to benefit from polypill when indicated.
The main drawback of the completed randomized controlled trials evaluating polypill compliance, was in trying to establish a direct relationship between increased compliance and effective event reduction in the same trial. This might be related to the low number of events and short follow-up period, making it difficult to detect such differences.
The AURORA was a Spanish multicentric, cross-sectional study that provided information about treatment satisfaction.30 Using the Treatment Satisfaction Questionnaire for Medication 9 items (TSQM9), investigators found that polypill was associated with higher satisfaction and consistently with higher compliance, compared to patients receiving each drug component individually.30
The pooled data analysis of UMPIRE,27 IMPACT28 and Kanyini-GAP29 in the Cochrane Systematic Review published in 2017 showed no difference of polypill versus standard of care regarding quality of life measured by the EQ-5D scale.31
Consensus position: Polypill improves medication adherence in high risk patients both in primary as well as secondary prevention. Although plausible, a direct relationship between adherence due to fixed combinations and better cardiovascular outcomes has yet to be established in randomized controlled trials. Higher compliance and patient satisfaction have been reported, but improvement in quality of life remains to be clarified in future trials.
The main evidence on the polypill in primary prevention derives from three trials – TIPS-3, HOPE-3, and PolyIran (subgroup of patients without cardiovascular disease) – which are summarized in Table 4.32–34 The TIPS-3 and HOPE-3 trials compared the polypill with matching placebos and in the Polyran study the control was “minimal care”, which did not include pharmacologic therapy.
These data were aggregated in a systematic review with individual patient data meta-analysis,35 including 18 182 primary prevention patients, with a mean age of 63.0 years, a proportion of men of 50.2%, 63% with arterial hypertension, 19% with diabetes, and 23% of smokers. The mean baseline LDL was 121.7 mg/dl, the mean blood pressure was 137.7/81.5 mmHg and mean fasting plasma glucose was 105.7 mg/dl. The median follow-up was five years.35
The estimates of this meta-analysis are depicted in Table 5, with significant risk reductions of major adverse cardiovascular events (MACE) and each of its individual components comprising cardiovascular death, myocardial infarction, stroke, or arterial revascularization. The number needed to treat (NNT) to prevent one MACE with the polypill was 52 in five years, being lower in the polypill with aspirin with a NNT of 37 in five years. The polypill showed increased risk of muscle pain and dizziness, but the proportion of gastrointestinal bleeding was not significantly increased (calculated number need to harm of 554).35
Estimates from the meta-analysis evaluating the effects of a polypill with or without aspirin on cardiovascular outcomes and all-cause mortality in primary prevention.
| HR (95%CI) for polypill (with or without aspirin) vs control | HR (95%CI) for polypill (with aspirin) vs control | HR (95%CI) for polypill (without aspirin) vs control | |
|---|---|---|---|
| MACE | 0.62 (0.53–0.73) | 0.53 (0.41–0.67) | 0.68 (0.57–0.81) |
| Cardiovascular mortality | 0.65 (0.52–0.81) | 0.51 (0.37–0.72) | 0.73 (0.57–0.93) |
| Myocardial infarction | 0.52 (0.38–0.70) | 0.47 (0.32–0.69) | 0.59 (0.39–0.88) |
| Stroke | 0.59 (0.45–0.78) | 0.49 (0.32–0.73) | 0.62 (0.44–0.86) |
| All-cause mortality | 0.90 (0.79–1.03) | 0.85 (0.70–1.03) | 0.90 (0.78–1.05) |
CI: confidence interval; HR: hazard ratio; MACE: major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, revascularization).32
Also in primary prevention, the open-label VULCANO trial randomized 492 patients at high or very high cardiovascular risk for usual care or CNIC-polypill and found that the latter significantly decreased the LDL level by 8.48 mg/dl and provided more 8.8% patients with LDL levels on target compared with usual care.36 VULCANO did not show non-inferiority of CNIC-polypill for blood pressure reduction or blood pressure control.36
Consensus statement: polypill, with or without aspirin, improves risk factors control and cardiovascular outcomes in primary prevention, reducing cardiovascular mortality and major cardiovascular events in comparison with placebo or no pharmacologic treatment. The use of aspirin should be assessed in an individual basis, weighting the risk of thrombosis versus the risk of bleeding.
For secondary prevention of cardiovascular disease (known and established atherosclerotic disease), in addition to lifestyle changes, drug therapy with antithrombotics and comprehensive cardiovascular risk factor control are the mainstay to decrease the recurrence of atherosclerotic cardiovascular events. This is applicable for patients with coronary, cerebrovascular, or peripheral artery disease.
The effectiveness of polypill treatment in secondary prevention in Spain was recently evaluated in the Neptuno Study.37 This was a real world retrospective non-interventional study using a propensity match scoring with 23 prespecified variables, establishing four cohorts with comparable baseline characteristics. A total of 6546 patients were distributed in cohort 1, CNIC-polypill; cohort 2, with the same drugs in separated monocomponents; cohort 3, equipotent monocomponents; and cohort 4, other monocomponents. The main outcome was the cumulative incidence of MACE (acute myocardial infarction, angina, ischemic stroke, transient ischemic attack, peripheral artery disease, claudication, ischemia, amputation and cardiovascular mortality) at 2 years follow-up. The CNIC-polypill cohort was associated with a reduction of LDL cholesterol and blood pressure compared with other cohorts. There was also an association with decreased MACE risk and time to event risk in the polypill cohort compared with cohorts 2 (HR 0.76; IC95% 0.66–0.88), 3 (HR 0.82; IC95% 0.71–0.94) and 4 (HR 0.83; IC95% 0.72–0.95).
The recently published Secure triale was a phase 3, controlled clinical trial that assigned 2499 patients with myocardial infarction within the previous six months in seven European countries. Patients were randomized to a polypill-based strategy or usual care. Follow-up had a median of 36 months. in seven European countries. Mean age was 76, with almost 60% over 75 years old. The majority were men, 57% had diabetes and 51% smokers. The primary composite outcome (cardiovascular death, nonfatal type 1 myocardial infarction, nonfatal ischemic stroke, or urgent revascularization) was reduced 24% (HR ratio, 0.76; 95% confidence interval [CI], 0.60–0.96; p=0.02). The key secondary end point, a composite of cardiovascular death, nonfatal type 1 myocardial infarction, or nonfatal ischemic stroke had also a 30% risk reduction (hazard ratio, 0.70; 95% CI, 0.54–0.90; p=0.005). Adherence to medication was higher in the polypill group than in the usual care group at two years follow-up. Adverse events were similar between groups and the management of dyslipidemia and hypertension was similar in both groups. The reduction in events could be in part due to the pleiotropic effect of statins and angiotensin-converting-enzyme (ACE) inhibitors related with greater adhesion or also due to greater adhesion to aspirin in the polypill arm.37
Together with to the Neptuno (an observational) study, the evidence to supporting the polypill as an option for reducing events in secondary prevention is now growing. To our knowledge, no randomized evidence exists on the use of the polypill for secondary prevention in cerebrovascular or peripheral artery disease.32–34
Consensus statement: A cardiovascular polypill is a valid option after an atherosclerotic cardiovascular event, arising from coronary, cerebrovascular, or peripheral arterial disease, in patients with indication for the composite of the monocomponents, assuming that this strategy can improve treatment adherence. This is in accordance with the first stepwise approach in patients with established atherosclerotic cardiovascular disease as endorsed by the ESC 2021 Prevention Guidelines,38 which proposes antithrombotic therapy, lipid and hypertension control and smoking cessation as the baseline strategy in the prevention of recurrent events.38 The SECURE trial proved the efficacy of a polypill in reducing MACE in an elderly population with a previous myocardial infarction.
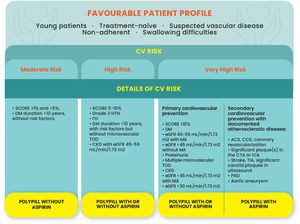
There are some phenotypes of patients that could derive greater benefit from the use of the polypill. The identification of these subpopulations has to crossmatch patients’ characteristics with their estimated cardiovascular risk.
The type of patients that are more likely to benefit from the polypill are:
- -
Younger patients that may be less compliant with complex regimens (according to the FOCUS study)25;
- -
Patients that are treatment-naïve and are simultaneously recognized as eligible for these drugs;
- -
Asymptomatic patients with cardiovascular risk factors and suspected vascular disease (for example: patients with positive treadmill stress test for myocardial ischemia, in addition to risk factors that increase the likelihood of the presence of coronary artery disease);
- -
Non-adherent patients, regardless of age;
- -
Patients with difficulty in swallowing multiple pills (e.g., some post-stroke patients with mild dysphagia);
- -
Primary prevention patients with diabetes at high cardiovascular risk due to additional risk factors, especially those with body mass index over 30 kg/m2 in whom aspirin was deemed to be more beneficial in the ASCEND trial.39
The type of polypill suitable for these patients will depend on cardiovascular risk, as per the Cardiovascular Prevention Guidelines21:
- -
Moderate Risk – it is reasonable to prescribe a polypill without aspirin to control the cardiovascular risk factors, and HOPE-3 trial supports this recommendation.33
- -
High or very high risk – patients might be eligible for the polypill with or without aspirin.
Figure 2 summarizes the polypill options based on patient characteristics and CV risk.
Polypill eligibility according to the patients’ profile and cardiovascular risk. ACS: acute coronary syndrome; CCS: chronic coronary syndrome; CTA: computerized tomographic angiography; CKD: chronic kidney disease; CV: cardiovascular; DM: diabetes; eGFR: estimated glomerular filtration rate; FH: familial hypercholesterolemia; HTN: hypertension; ICA: invasive coronary angiography; MA: microalbuminuria; PAD: peripheral arterial disease SCORE: Systematic Coronary Risk Estimation for cardiovascular mortality at 10 years; TIA: transient ischemic attack; TOD: target organ damage.
It is also important to determine if polypill prescription leads to the reaching of risk factor control targets, with or without additional drugs.21
The prescription of the polypill needs to weigh up the benefits and risks and, whenever possible, the patient should be involved in the decision process, stressing the importance of compliance.
Consensus statement: Patients eligible for an aspirin-containing polypill are those in secondary cardiovascular prevention and, on an individual basis, those at very high risk such as patients with diabetes with target organ disease and low bleeding risk. Non-adherent patients, younger patients, and the medication-naïve are also suitable candidates for polypill use.
Cardiovascular disease (including ischemic heart disease and stroke) is the foremost cause of disease burden worldwide according to the GBD 2019 Disease and Injuries report.40 The importance of atherosclerotic disease in Portugal was further stressed in the reports on the national cost and burden of atherosclerosis.11,41 In 2016, atherosclerosis was responsible for 14.3% of overall mortality and for 260943 cisability-adjusted life years, of which 75% of them were due to premature death and 25% to disability.41 Atherosclerosis was estimated to yield costs equivalent of 1% of the Portuguese gross domestic product and 11% of the health expenditures in the same year.41 This emphasizes that potential beneficial interventions may be valuable not only for patients but for all stakeholders.
Regarding polypill cost-effectiveness, the available reports support that the cardiovascular polypill is cost-effective using different models and data from different countries.42–47 Being cost-effective translates into gains in health for the population with an acceptable cost for the healthcare system, usually expressed in incremental cost-effectiveness ratio (ICER) per quality adjusted life years (QALY). There are some published models with different polypills. Wald and Jowett evaluated a polypill with statin and three low-dose anti-hypertensive drugs (ACE inhibitor/angiotensin receptor blocker, hydrochlorothiazide and amlodipine) as a primary prevention strategy in the United Kingdom in two different pharmacoeconomic studies.43,47 Both included the potential benefits of cardiovascular events due to control of risk factors but only Wald considered the potential benefits of increased compliance.43,47 The other cost-effective studies (with scenarios for United Kingdom, United States of America, and other countries such as China, India, Mexico, Nigeria, and South Africa, as well as models for low-income and middle-income countries) evaluated aspirin-containing polypills with or without beta-blockers, mostly in secondary prevention and used estimates for risk reduction of cardiovascular events due to risk factors improvement and increased treatment compliance.42,44–46 In both primary and secondary prevention scenarios these models supported the polypill strategy as ‘cost-effective’.
For the Portuguese scenario, the aspirin-containing polypill cost-effectiveness was evaluated for patients with established coronary disease and for post-stroke patients (the Mercury Study).48 Using a Markov model, the authors compared the polypill with monocomponents over a lifetime horizon and from the perspective of the Portuguese national healthcare system.48 Patient characteristics were derived from the Portuguese Registry of Acute Coronary Syndromes subgroup that had previous history of coronary disease.49 The evaluation for post-ischemic stroke patients, followed the same methodology, but the patients characteristics were derived from the Central Administration of the Portuguese Health System, and the polypill was cost-effective with an ICER of €2353/QALY.50 The treatment effects of the polypill and monocomponents were extrapolated from the Spanish retrospective real world NEPTUNO study, which found differences in the lipid and blood pressure profiles of polypill vs. separate monocomponents.51 In the Mercury study, the incremental cost-utility ratio was €1557/QALY gained. Assuming a willingness-to-pay threshold of €30000/QALY gained, there is a 79.7% probability of the CNIC-Polypill being cost-effective compared with the combination of monocomponents.48 In spite of these results, it is important to highlight the limitations inherent to this analysis, such as the use of clinical registries and observational data to extrapolate the pharmacoeconomic impact of the polypill.
Consensus statement: Currently both polypills marketed in Portugal (aspirin, atorvastatin, ramipril; and perindopril, amlodipine, atorvastatin) are reimbursed, after a cost-minimization analysis that determined that the price of the fixed combinations is inferior to the sum of all individual components.52,53
The polypill may be useful in the prevention of cardiovascular disease by improving cardiovascular risk factors and increasing compliance with drug therapy. A possible synergistic effect resulting from the combination of its components in risk factors’ control cannot be excluded. There are subgroups of patients that can benefit more from fixed dose combinations of drugs that aim to prevent cardiovascular events (e.g., younger patients, treatment-naïve patients, or patients with diabetes). The polypill has proved to improve risk factor control both in primary and secondary prevention settings and there are data supporting the use of the polypill for the reduction in cardiovascular events.
Conflicts of interestFerrer laboratories supported the expert panel meeting and the medical writing but did not have any participation in the discussion or drafting of the document.
FA has received honoraria for lecturing, investigation, and consulting activities from Akcea, Astra Zeneca, Bayer, Bial, Boehringer Ingelheim, Daiichi Sankyo, Ferrer, Jaba, Lilly, Menarini, Medinfar, MSD, Novartis, Novo Nordisk, Servier, Tecnifar, Tecnimede.
DC has participated in educational meetings and/or attended a conferences or symposia (including travel, accommodation and/or hospitality in the last 5 years) with Bristol-Myers Squibb, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Menarini, Merck Serono, Ferrer, Pfizer, Novartis and Roche.
CA has received honoraria for consultancy from Ferrer.
JPA nothing to declare.
NC has received honoraria for consultancy from Ferrer.
VC participated in educational meetings and received honoraria for lecturing and consulting activities from Bial, Ferrer, Jaba, Medicamenta, Medinfar and Servier.
LF has received honoraria for lecturing, investigation, and consulting activities from Boehringer Ingelheim and Novo Nordisk; has participated in educational meetings and/or attended a conferences or symposia (including travel, accommodation and/or hospitality) with Bayer, Boehringer Ingelheim, Daiichi Sankyo, Ferrer.
JPM has received honoraria for lecturing, investigation, and consulting activities from Amgen, Astrazeneca, Bayer, Bial, Boehringer Ingelheim, Ferrer, MSD, Takeda, Tecnimede.
VPD has received honoraria for lecturing, and consulting activities from Delta, JABA, Servier, Tecnimede.
HR has received honoraria for consultancy from Ferrer.
VTC has received honoraria for lecturing, investigation, and consulting activities from Bayer; Boehringer Ingelheim; Biogen Idec; Bioportugal; Bristol Meyers Squibb/Pfizer; Roche; Novartis; Abbott; Ferrer; Astra Zeneca; PHRI.
CG has received honoraria for lecturing, investigation, and consulting activities from AstraZeneca, Bayer, Bial, Boehringer Ingelheim, Daiichi Sankyo, Ferrer, Lilly, MSD, Novartis, Novo Nordisk and Servier.