Hyperhomocysteinemia (HHcy) can induce vascular inflammatory and oxidative damage and accelerate intimal hyperplasia. This study investigated the protective effect of pirfenidone (PFD) on the recovery process of injured endothelial arteries during HHcy.
Materials and methodsThirty rabbits were randomly separated into three groups: A control group (n=10, standard rabbit chow), a model group (n=10, control diet plus 30 g methionine/kg food), and a PFD group (n=10, model diet plus oral administration of 90 mg/day of PFD). After 14 weeks of arterial injury, histopathological changes were determined. Plasma homocysteine (Hcy) concentrations, lipid profiles and oxidant and antioxidant status were evaluated. Macrophage infiltration was assessed using immunohistochemical staining.
ResultsPFD supplementation decreased macrophage infiltration of iliac artery significantly without changes in blood lipids and Hcy concentrations. Compared with the model group, PFD restored superoxide dismutase and glutathione peroxidase activities and reduced malondialdehyde and reactive oxygen species levels. A high-methionine diet significantly increased neointimal area and the ratio between neointimal and media area. Systemic administration of PFD inhibited neointimal formation.
ConclusionsPFD can partly alleviate intimal hyperplasia by inhibiting inflammatory and oxidative stress response induced by HHcy during endothelial injury. It may be a potential therapeutic agent for the prevention and treatment of endothelial injury-associated diseases such as atherosclerosis.
A hiperhomocisteinemia pode induzir a inflamação vascular, a lesão oxidativa e acelerar a hiperplasia da íntima. Este estudo analisou o efeito protetor da pirfenidona (PFD) no processo de recuperação de lesões do endotélio arterial durante a hiperhomocisteinemia.
Material e métodosTrinta coelhos foram aleatoriamente incluídos em três grupos: grupo controlo (n = 10, coelho chow padrão), grupo modelo (n = 10, dieta de controlo mais 30g de metionina/kg de comida) e grupo PFD (n = 10, dieta modelo mais administração oral de 90mg/dia de PFD). Após 14 semanas de agressão arterial, foram determinadas as alterações histopatológicas. Foram igualmente avaliadas as concentrações de homocisteína plasmática, os perfis lipídicos e o estado oxidante e antioxidante. A infiltração de macrófagos foi estudada utilizando a coloração imuno-histoquímica.
ResultadosA suplementação de PFD diminuiu significativamente a infiltração de macrófagos na artéria ilíaca sem alterações nas concentrações de lípidos no sangue e de homocisteinemia. Em comparação com o grupo modelo, a PFD restaurou as atividades da superóxido dismutase e da glutationa peroxidase e reduziu os níveis de malonaldadeído e os níveis de espécies reativas de oxigénio. A dieta rica em metionina aumentou significativamente a área da neoíntima e a relação entre esta área e a da média. A administração sistémica de PFD inibiu a formação da neoíntima.
ConclusõesA PFD pode aliviar parcialmente a hiperplasia da íntima ao inibir a resposta ao stress inflamatório e oxidativo induzido pela hiperhomocisteinemia, podendo ser um agente terapêutico potencial para a prevenção e tratamento de doenças associadas à lesão endotelial, tais como a aterosclerose.
Atherosclerosis is recognized as a chronic vascular inflammatory disease related to oxidative stress. Hyperhomocysteinemia (HHcy) is a known independent risk factor for atherosclerosis and it can accelerate intimal hyperplasia by activation of inflammatory reaction and oxidative stress.1–5 Many studies have shown that anti-inflammatories combined with antioxidant treatment could be an ideal strategy in atherosclerosis.6–8 And this therapy strategy could effectively reverse the endothelial dysfunction induced by homocysteine.9 Pirfenidone (PFD) is an anti-fibrotic agent investigated clinically for the treatment of various fibrotic diseases, especially idiopathic pulmonary fibrosis.10–12 Besides its observed effect in fibrosis suppression, PFD has also been shown to decrease inflammation,13,14 alleviate oxidative stress,15,16 and regulate apoptosis.17,18 Several animal studies have suggested PFD prevented ballon-induced neointimal lesion through inhibition of local extracellular matrix deposition and expression of matrix metalloproteinases, governing smooth muscle cell proliferation and migration.19–22 However, few studies have focused on investigating the possible effects of PFD on vascular damage promoted by HHcy. Here, we investigated the role of PFD in injured arteries in hyperhomocysteinemic animals.
MethodsAnimalsWe purchased 30 male New Zealand white rabbits (about 3 kg), 12 weeks old, from the Laboratory Animal Research Center of the 2nd Affiliated Hospital of Harbin Medical University. This study was conducted in accordance with the recommendations of the National Institutes of Health Guidelines for the Use of Laboratory Animals. The protocol was approved by the hospital scientific affairs committee on animal research and ethics. The methods used to create the animal models have been previously described in other studies.23–25 After being anesthetized (xylazine 8 mg/kg and ketamine 35 mg/kg), the right iliac artery damage was performed though balloon inflation (3 mm angioplasty balloon, Cordis, three 1 min inflations, 8 atm). Then all rabbits were randomly separated into three groups: A control group (n=10, standard rabbit chow), a model group (n=10, control diet plus 30 g methionine/kg food), and a PFD group (n=10, model diet plus oral administration of 90 mg/day of PFD). Food intake and body weight were observed each week. After 14 weeks of arterial injury, all the rabbits were anesthetized by 100% diethylether and sacrificed. Blood samples and tissues were collected for further analysis. The iliac arteries were isolated and washed briefly in 0°C phosphate-balanced solution. Each tissue sample was separated and cut into five parts. After marking, proximal, distal, and medial parts were fixed in 10% buffered formalin, embedded in paraffin and serial sections made, with some sections being stained with hematoxylin and eosin and Van Gieson elastin stain for morphometric analysis; others were used for an immunohistochemical study. The last two parts of each tissue were frozen in liquid nitrogen separately for detecting oxidative stress factors.
Measurement of plasma hyperhomocysteinemia and lipid profilingAfter 14 weeks of arterial injury, blood samples were withdrawn from the ear veins, then they were centrifuged and stored at -80°C for further analysis. Plasma Hcy levels and lipid profiles, including low-density lipoprotein cholesterol, total cholesterol (TC), and triglycerides (TG) were analyzed using an automatic biochemical analyzer (HITACHI 7600-020, Hitachi, Ltd., Tokyo, Japan).
Pathological evaluationSix sections, 5-μm thick, were cut from three equally spaced locations and stained with Van Gieson elastin stain for morphometric analysis. Histologic sections were examined microscopically by an investigator blinded to treatment. Ten sites from each section (including the thickest and thinnest parts) were analyzed by computerized morphometry (NIH Image), and the results were averaged. The lumen area (LA), internal elastic lamina area (IELA), and external elastic lamina area (EELA) were measured directly. Neointimal area (NA) was calculated using the equation NA=IELA-LA, and media area (MA)=EELA-IELA, and the ratio between neointimal and media area (N/M)=NA/MA.
Immunohistochemistry for macrophageAfter deparaffinization, hydration and fixing, immunohistochemical staining was performed with a rat monoclonal antibody against rabbit macrophage antibody CD11b (working dilution 1:1200, Sigma Company, USA) using the labeled streptavidin-peroxidase staining kit (Histofine Simple Stain MaxPO Multi, Nichirei, Tokyo, Japan).There were three cross-sections of each arterial specimen to analyze. The positively immunostained area of macrophages in each randomly chosen neointimal cross-section, identified by Brown staining in the cytosol, was quantified using the image analysis system.
Indicators related to oxidative stress detectionThe activities of glutathione peroxidase (GSH-Px, Nanjing Jancheng Bioengineering Institute, Nanjing, China) and superoxide dismutase (SOD, Nanjing Jancheng Bioengineering Institute, Nanjing, China), and the levels of malondialdehyde (MDA, Nanjing Jancheng Bioengineering Institute, Nanjing, China) and reactive oxygen species (ROS, Nanjing Jancheng Bioengineering Institute, Nanjing, China) in the iliac artery were measured by commercially available kits using colorimetric assay according to the manufacturer's protocols. For ROS detection, homogenized iliac artery samples were diluted to protein concentrations of 10 mg/mL, to which 100 μg/mL of digitonin was added. After incubation for 30 min, Amplex Red and Horseradish Peroxidase were added. H2O2 levels in iliac artery homogenates were then detected at 37̊ for 30 min on a Varioskan Flash Multimode Reader (Thermo Scientific, Waltham, MA, USA).
StatisticsAll data were presented as mean ± standard deviation (SD). The mean histological, blood and morphological data for each group were compared by one-way ANOVA with post hoc analysis for multiple comparisons. p<0.05 was considered statistically significant. SPSS 18.0 was used for statistical analysis.
ResultsEffects of pirfenidone on blood lipids and hyperhomocysteinemiaHyperhomocysteinemia was induced by a high-methionine diet, compared with control group, plasma level of Hcy in the model group increased about 4.0-fold (p<0.05, Table 1). However, PFD treatment had no effect on plasma level of Hcy after methionine supplement. There were no differences among the three groups in body weight or levels of TG, TC and LDL (Table 1).
Blood lipids, body weight and homocysteine of each group.
| Parameter | Control group | Model group | PFD group |
|---|---|---|---|
| (n=10) | (n=10) | (n=10) | |
| TC, mmol/L | 2.14±0.32 | 2.23±0.41 | 2.18±0.27 |
| LDL-C, mmol/L | 0.65±0.09 | 0.68±0.19 | 0.66±0.11 |
| TG, mmol/L | 1.09±0.11 | 1.18±0.08 | 1.13±0.12 |
| Body weight, kg | 4.66±0.87 | 4.53±0.58 | 4.41±0.79 |
| Hcy, μmol/L | 8.74±0.32 | 34.56±0.53* | 33.12±0.48* |
Abbreviations: LDL-C: low-density lipoprotein C; TC: total cholesterol; TG: triglyceride; Hcy: homocysteine; PFD: pirfenidone. The data are expressed as mean±SD. p<0.05 indicates a significant difference.
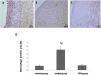
The results of immunohistochemical staining for macrophage of each group are shown in Figure 1A-D. There was no significant difference between control group and PFD group (1.05±0.22% vs. 1.18±0.29%, p>0.05). Compared with the model group, PFD could decrease the expression of macrophage significantly (1.18±0.29% vs. 5.12±0.93%, p<0.05).
Observation of macrophage expression in the vascular intimal with immunohistochemical staining. The scale bar=50 μm. (A), Control group, (B) model group, and (C) Pirfenidone (PFD) group. (D), Bar graph showing increased macrophage expression in arteries of control group, model group and PFD group. Values are represented as mean±SD (n=10). p<0.05 indicates a significant difference. *p<0.05, compared with control group. #p<0.05, compared with model group.
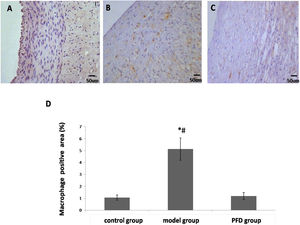
Effect of pirfenidone on oxidative molecule production and antioxidant protein activity. The results are shown in Figure 2A-D. MDA and ROS levels were higher and the activities of GSH-Px and SOD were lower in model group than those of control group (p<0.05). PFD restored SOD and GSH-Px activities (173.21±7.65 vs. 98.76±4.00, p < 0.01; 164.53±12.34 vs. 113.53±16.79, p<0.05) and reduced MDA and ROS levels (5.83±0.81 vs. 16.35±1.07, p<0.01; 546.71±19.36 vs. 1096.21±35.67, p<0.05) in the artery.
Effect of PFD on oxidative damage of iliac artery. (A) MDA level, (B) ROS level, (C) SOD activity and (D) GSH-Px activity were detected according to the manufacturer's instructions. Values are represented as mean±SD (n=10). p<0.05 indicates a significant difference.
*p<0.05, compared with control group.
#p<0.05, compared with model group.
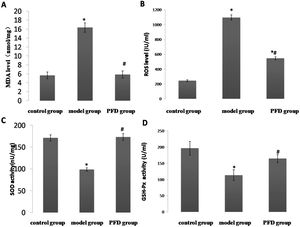
Figure 3A-E showed the morphological analysis results. In the control group, NA was 0.0135±0.0031 mm2, MA was 0.0400±0.0079 mm2, and NA-to-MA ratio (N/M) was 0.3376±0.0449. High-methionine diet significantly increased NA and N/M to 0.0664±0.0092 mm2 and 0.7003±0.0358, respectively. After PFD treatment, intimal hyperplasia was suppressed significantly compared with the model group (NA, 0.0398±0.0067mm2 vs 0.0664±0.0092 mm2, p<0.05; N/M, 0.5845±0.0771 vs 0.7003±0.0358, p<0.05).
Intimal hyperplasia of each group at 14 weeks of arterial injury. Forty magnification photomicrographs (hematoxylin and eosin and Van Gieson elastin stain) of arterial sections from control group (A), model group (B), and PFD group (C). Note thicker intimal in model group. (D), Bar graph shows neointima formation of each group. (E), Bar graph shows the ratio between neointimal and media area of each group. The scale bar=1 mm. Values are represented as mean±SD (n=10). p<0.05 indicates a significant difference. *p<0.05, compared with control group. #p<0.05, compared with model group. N, intimal, M, media.
Through this study, we confirmed firstly that PFD could alleviate vascular damage caused by HHcy. Furthermore, PFD improved intimal hyperplasia and the degree of lumen stenosis in high- methionine-diet rabbits. The specific role of PFD may be associated with inhibition of inflammation and oxidative damage of vascular intima induced by homocysteic acid.
Hyperhomocysteinemia is an independent risk factor for atherosclerosis in addition to traditional factors, especially in patients with early onset and multiple site lesions.26 As we all know, vascular endothelial injury is the initiation of atherosclerosis. Inflammatory response and oxidative stress are two key mechanisms involved in the initiation of vascular endothelial injury. Hcy produces excessive reactive oxygen species during metabolism and causes inflammation, which directly causes damage to the vascular endothelium and enhances the oxidation of low-density lipoprotein, which in turn leads to endothelial dysfunction, and ultimately accelerates the process of atherosclerosis.27–31 At present, vitamin B (including folic acid) is mainly used to reduce homocysteine level and treat HHcy.32,33 However, studies have found that supplements combining folic acid and vitamins B6 and B12 did not reduce the risk of major cardiovascular events in patients with vascular disease,34 and the benefits of simply reducing homocysteine level may be offset by the harmful effects of vitamin B (including folic acid). Therefore, it is of great clinical significance to seek other drugs that can inhibit or alleviate the vascular injury caused by HHcy. Our results showed that PFD can present a potential therapeutic effect during this process. PFD is a fibrosis inhibitor with anti-inflammatory and antioxidant activities. Many experimental studies have found PFD can suppress tumor necrosis factor, interleukin-1, interleukin-6 and other inflammatory cytokines secretion35–37 and inhibit macrophage infiltration in kidney,38 liver,39 lung,40 and so on. As it is known, macrophages play a crucial role during the initiation and development of atherosclerosis and local inflammation, as well as, several diseases associated with dysfunctional endothelium. Therefore, reducing macrophage infiltration is an important therapeutic target of anti-atherosclerotic therapy. In this study, we found that PFD inhibits macrophage infiltration in the vascular wall caused by HHcy. It may be one of the main mechanisms of PFD antagonizing vascular inflammatory injury induced by HHcy. As we all know, ROS and MDA are key indicators of oxidative stress. Consistent with some studies,41,42 our results show that ROS levels and MDA activities are increased significantly in HHcy rabbits, which indicates that Hcy causes oxidative stress damage to vascular intima. SOD is an important antioxidant enzyme, which can remove superoxide anion free radicals and protect cells and tissues from free radicals. GSH-Px can decompose and remove peroxide and block lipid peroxidation. In the present work, we also appraised the antioxidant property of PFD and found it can promote the expression of SOD and GSH-Px and decrease the levels of ROS and MDA. It may be another main mechanisms of PFD antagonizing vascular injury induced by HHcy.
Study limitationsThis study is limited to observations in animal models and it is also unknown whether the results can be reproduced in humans. Therefore, it is necessary to conduct clinical research so that we can evaluate the anti-inflammatory and antioxidant effect of PFD on human atherosclerotic lesions with HHcy.
ConclusionsPirfenidone can partially alleviate intimal hyperplasia by inhibiting inflammatory and oxidative stress response induced by HHcy during endothelial injury. It may be a potential therapeutic agent for the prevention and treatment of endothelial injury-associated diseases such as atherosclerosis.
Source of fundingThe authors did not receive support from any organization for the submitted work.
Conflicts of interestThe authors have no conflicts of interest to declare.