Heart failure (HF) secondary to acute myocardial infarction (AMI) is still a worldwide problem with a high mortality rate. The current study aimed to explore early and reliable predictive biomarkers of HF following AMI.
MethodsThe gene expression profile GSE59867 was downloaded from GEO. Array data from peripheral blood mononuclear cells (PBMCs) was used from 46 control patients and 111 patients with AMI at four time points: (i) first day of AMI; (ii) 4-6 days after AMI; (iii) one month after AMI; and (iv) six months after AMI. Among the 111 AMI patients, nine with HF and eight without HF were studied. CIBERSORT was used to analyze the relative proportions of immune cells in PBMCs. The proportions of immune cells in different groups were compared. Differentially expressed genes (DEGs) were analyzed with R language packages.
ResultsThe percentages of monocytes and neutrophils increased significantly on the first day of AMI, and then decreased gradually. The percentage of regulatory T cells increased significantly 4-6 days after AMI, while the percentage of resting memory CD4 cells, CD8 T cells, and resting natural killer cells decreased significantly on the first day of AMI, and then increased gradually. Patients who developed HF had a significantly higher proportion of neutrophils in PBMCs on the first day of AMI, but had a significantly lower proportion of naive CD4 T cells. Two shared genes, interleukin-1 receptor 2 (IL1R2) and leucine-rich repeat neuronal protein 3 (LRRN3), were found to have potentially important roles in predicting the development of HF following AMI.
ConclusionA higher proportion of neutrophils and a lower proportion of naive CD4 T cells in PBMCs on the first day of AMI may be correlated with the development of HF following AMI. IL1R2 and LRRN3 may exert functions in the development of HF following AMI.
A insuficiência cardíaca (IC) secundária ao enfarte agudo do miocárdio (EAM) constitui um problema mundial devido à elevada taxa de mortalidade. Este estudo tem o objetivo de identificar biomarcadores preditores de IC pós o EAM.
MétodosO perfil da expressão do gene GSE59867 foi descarregado do GEO. Foram utilizados os dados da matriz das células sanguíneas mononucleares periféricas (CSMP), incluindo 46 doentes controlo e 111 doentes com EAM em quatro momentos: i) 1.° dia após EAM; II) 4–6 dias após EAM; iii) um mês após EAM; e iv) seis meses após EAM. Dos 111 doentes com EAM, foram estudados nove doentes com insuficiência cardíaca (IC) e oito sem IC. O CIBERSORT foi utilizado para analisar as proporções relativas das células imunes nas CSMP. As proporções das células imunes foram comparadas nos diferentes grupos. Os genes expressos de forma diferente (GEDs) foram analisados com pacotes de linguagem R.
ResultadosA percentagem de monócitos e de neutrófilos aumentou significativamente no primeiro dia após o EAM e foi diminuindo gradualmente. A percentagem de Treg aumentou significativamente 4–6 dias após o EAM. A percentagem de repouso da memória CD4, as células CD8 T e as células de repouso NK diminuíram significativamente no primeiro dia após o EAM e seguidamente aumentaram gradualmente. Os doentes com prognóstico de IC apresentaram uma proporção mais elevada de neutrófilos nas CSMP no primeiro dia após o EAM, tendo, no entanto, apresentado uma proporção significativa menos elevada de células CD4 T. Dois genes partilhados, a interleucina 1 recetor 2 (IL1R2) e a proteína neuronal de repetição rica em leucina 3 (LRRN3), foram consideradas como tendo um papel potencialmente importante no prognóstico de IC após o EAM.
ConclusãoUma proporção mais elevada de neutrófilos e uma proporção mais baixa de células CD4 T naïve nas CSMP no primeiro dia após o EAM podem correlacionar-se com o prognóstico de EAM. A IL1R2 e a LRRN3) podem exercer funções potenciais no desenvolvimento da IC pós-EAM.
Heart failure (HF) is a major health problem worldwide. Although the treatment of HF has improved dramatically, the five-year mortality rate of HF patients is still 50%.1–4 Acute myocardial infarction (AMI), which may lead to cardiac dysfunction, is a common cause of HF. Studies have demonstrated that 14-36% of AMI patients will eventually develop HF.5,6 Early and reliable prediction of HF and appropriate intervention following AMI are considered an effective strategy to prevent the development of HF. Brain-type natriuretic peptide and N-terminal pro-brain natriuretic peptide have been proved to be associated with the development of HF and are widely applied in its clinical diagnosis and assessment of prognosis.7,8 However, these biomarkers are not specific, since they can be elevated in congestive HF, renal failure, primary aldosteronism and thyroid disease.9 Recently, the relationship between neutrophilia and lymphopenia has been shown to be an independent marker to predict mortality in patients with acute HF.10,11 The neutrophil-to-lymphocyte ratio has been shown to predict long-term mortality in patients with ST-segment elevation myocardial infarction (STEMI).9,12,13 But the relationship between neutrophil and lymphocyte counts and HF following AMI is still unclear. Using genome-wide gene expression profiling, researchers have shown that inflammatory response and immune response pathways are associated with the development of HF following AMI, and C-X-C motif chemokine ligand 8 (CXCL8) and interleukin 1β (IL1B) are hub genes.14 However, they used gene expression profiles from peripheral blood mononuclear cells (PBMCs), and neglected changes in different cell types and numbers following AMI.
In this study, we used the microarray data of the GSE59867 from the Gene Expression Omnibus (GEO) database and the CIBERSORT analytical tool to identify relations between AMI and proportions of different PBMCs, then explored the relations between the development of HF following AMI and proportions of different PBMCs.
MethodsData preparationData of the gene expression profile GSE59867 were downloaded from GEO (www.ncbi.nlm.nih.gov/geo). The dataset was a gene array of 436 PBMCs from 46 control patients with stable coronary artery disease and 111 patients with STEMI at four time points: (i) first day of AMI, 111 samples; (ii) 4-6 days after AMI, 101 samples; (iii) one month after AMI, 95 samples; and (iv) six months after AMI, 83 samples. Among the 111 patients with STEMI, nine diagnosed with HF and eight not considered to have HF at six months after AMI were studied. From the nine HF patients, 34 samples in total were used in this study (nine samples on the first day of AMI, nine at 4-6 days after AMI, eight at one month after AMI, and eight at six months after AMI); and from the eight patients without HF, 30 samples were used (eight samples on the first day of AMI, six at 4-6 days after AMI, eight at one month after AMI, and eight at six months after AMI). Gene expression data were normalized with the limma R language package for further analysis.
Assessment of immune cellsThe CIBERSORT analytical tool (https://cibersort.stanford.edu/), developed by Newman et al,15 was used to analyze the relative proportions of 22 types of immune cells in PBMCs based on normalized gene expression data. The proportions of different immune cell groups were depicted in heatmaps and barplots. Using Spearman correlation analysis, we explored the relationship between different immune cells. We further applied the Wilcox test to compare the differences in immune cells between HF and non-HF groups.
Identification of differentially expressed genesSamples were divided into high or low neutrophils and naive CD4 groups according to the median value. Data analysis was performed using the limma R language package.16 Log fold change >0.5 or <-0.5 and adjusted p<0.05 were set as the cutoffs to screen for differentially expressed genes (DEGs).
Statistical analysisOnly samples with CIBERSORT p<0.05 were selected for subsequent analysis. Changes in immune cell fractions in different samples were compared using analysis of variance and Tukey's multiple test. Pearson correlation analysis was used to assess the correlations between different immune cells. Statistical analyses were performed using R version 3.6.0 and GraphPad Prism 6 (GraphPad). A p-value <0.05 was considered significant.
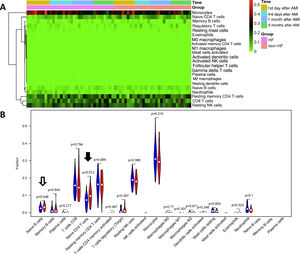
ResultsComposition of peripheral blood mononuclear cells in acute myocardial infarction patientsWe first investigated the landscape of 22 immune cell types in PBMCs of AMI patients using the CIBERSORT algorithm. All 436 samples were eligible based on CIBERSORT p<0.05. The most common cell types in PBMCs were monocytes, T lymphocytes and natural killer (NK) cells (Figure 1A). This fitted with the normal distribution of PBMCs, which confirmed CIBERSORT as a reliable method for analyzing proportions of immune cells based on gene expression data. To further explore the underlying relationships between different cells in PBMCs of AMI patients, we assessed the correlations between every two types of immune cell. In this analysis, the cell type was removed if its percentage was lower than 2%, and nine different cell types were kept. As shown in Figure 1B, the percentages of monocytes and neutrophils were positively related (r=0.45) and that of monocytes was negatively related with CD8 T cells (r=-0.52), resting NK cells (r=-0.5), resting memory CD4 T cells (r=-0.27), and naive CD4 T cells (r=-0.29). Neutrophil percentages were negatively related with CD8 T cells (r=-0.36), resting NK cells (r=-0.36), and naive CD4 T cells (r=-0.26).
Analysis of peripheral blood mononuclear cell (PBMC) sequencing data with CIBERSORT. (A) Heatmap of 22 different cell types in 436 PBMC samples. Samples were divided into five groups: (i) control; (ii) first day of AMI; (iii) 4-6 days after AMI; (iv) one month after AMI; and (v) six months after AMI; (B) correlation of nine different cell types in 436 PBMC samples. A cell type was deleted if its percentage was lower than 2%, and nine different cell types were kept. Red represents positive correlation, blue represents negative correlation. Numbers in the figure are correlation coefficients.
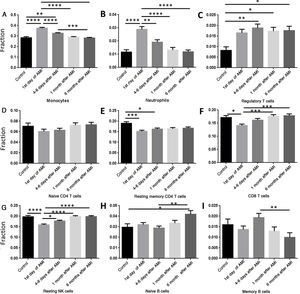
We then analyzed the relation between AMI and percentage changes of PBMCs. Data were classified into five groups, the control group and four other groups according to different time points after AMI: (i) controls; (ii) first day of AMI; (iii) 4-6 days after AMI; (iv) one month after AMI; and (v) six months after AMI. The percentages of monocytes (Figure 2A) and neutrophils (Figure 2B) increased significantly on the first day of AMI compared with the control group, and then gradually decreased to normal levels by six months after AMI. The percentage of regulatory T cells (Tregs) remained high after AMI, and did not change significantly over time (Figure 2C). The percentage of resting memory CD4 cells (Figure 2E), CD8 T cells (Figure 2F) and resting NK cells (Figure 2G) decreased significantly on the first day of AMI, and then increased gradually to normal levels by six months. No significant change was seen in naive CD4 T cells (Figure 2D), and no regular pattern of change was seen in naive B cells (Figure 2H) or memory B cells (Figure 2I).
Comparison of different cell types at different time points. (A) The percentage of monocytes increased significantly on the first day of AMI, and then decreased gradually; (B) the percentage of neutrophils increased significantly on the first day of AMI, and then decreased gradually; (C) the percentage of regulatory T cells increased significantly 4-6 days after AMI; (D) the percentage of naive CD4 T cells did not change significantly following AMI; (E) the percentage of resting memory CD4 T cells decreased significantly on the first day of AMI, and then increased gradually; (F) the percentage of CD8 T cells decreased significantly on the first day of AMI, and then increased gradually; (G) the percentage of resting NK cells decreased significantly on the first day of AMI, and then increased gradually; (H) the percentage of naive B cells increased significantly at six months after AMI; (I) the percentage of memory B cells did not change significantly following AMI. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001; AMI: acute myocardial infarction.
Since HF is a severe complication following AMI, we then examined whether the percentages of immune cells in PBMCs could be a biomarker for HF using CIBERSORT. We first compared 34 samples from nine HF patients with 30 samples from eight non-HF patients. As shown in Figure 3A and 3B, the proportions of naive CD4 T cells (p=0.012) and naive B cells (p=0.045) were significantly lower in HF patients than in non-HF patients.
Comparison of cell types between heart failure and non-heart failure samples. (A) Heatmap showing 22 different cell types in 34 heart failure samples and 30 non-heart failure samples; (B) violin plot comparing 22 different cell types between heart failure and non-heart failure samples. Naive B cells (p=0.046, hollow arrow) and naive T cells CD4 (p=0.012, black arrow) differed significantly between heart failure and non-heart failure samples. No significant difference was seen in other cell types. AMI: acute myocardial infarction; HF: heart failure.
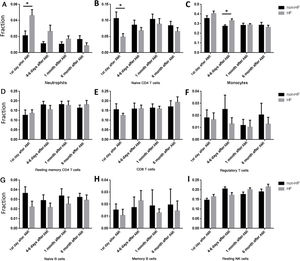
Our results showed that the proportions of cells changed gradually after AMI. So we further analyzed the time effect on HF development. The proportion of neutrophils was significantly higher in HF compared with non-HF patients on the first day of AMI (Figure 4A), while the proportion of naive CD4 T cells was significantly lower in HF than in non-HF patients on the first day of AMI (Figure 4B). However, no significant differences were found at other time points in neutrophils or naive CD4 cells. Interestingly, the proportion of monocytes was significantly higher in HF than in non-HF patients 4-6 days after AMI (Figure 4C), but there was no difference on the first day of AMI.
Comparisons of different cell types at different time point between heart failure and non-heart failure samples. (A) Patients progressing to HF had higher neutrophils on the first day of AMI; (B) patients progressing to HF had lower naive CD4 T cells on the first day of AMI; (C) patients progressing to HF had higher monocytes at 4-6 days after AMI; (D-I) there were no significant changes between patients progressing to HF and non-HF patients in resting memory CD4 cells (D), CD8 T cells (E), regulatory T cells (F), naive B cells (G), memory B cells (H) or resting NK cells (I). *: p<0.05. AMI: acute myocardial infarction; HF: heart failure.
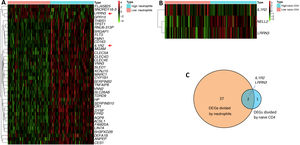
In our study, we confirmed that higher proportions of neutrophils and lower proportions of naive CD4 T cells are biomarkers for HF development. We wondered if there were molecular changes underlying this phenomenon. We therefore further explored DEGs by comparing samples from the first day of AMI (111 samples) according to their proportions of neutrophils and naive CD4 T cells. The median (0.026358017 for neutrophils, 0.054867687 for CD4 cells) was used to classify the proportions as high or low. Log fold change >0.5 or <-0.5 and adjusted p<0.05 were set as the cutoffs to screen for DEGs. A total of 39 DEGs were found when comparing high and low neutrophils, in which four genes were upregulated and 35 genes were downregulated in the high neutrophil group (Figure 5A). Three DEGs were found when comparing high and low naive CD4 T cells, in which two genes were upregulated and one gene was downregulated in the high naive CD4 group (Figure 5B). The Venn diagram showed two shared DEGs in the analysis of neutrophils and naive CD4 cells (Figure 5C, red arrow in Figure 5A). These two genes are interleukin-1 receptor 2 (IL1R2) and leucine-rich repeat neuronal protein 3 (LRRN3).
(A) Heatmap of differentially expressed gene sets between high and low neutrophils. Samples were divided into high and low neutrophil groups according to percentage of neutrophils on the first day of AMI. Log fold change >0.5 or <-0.5 and adjusted p<0.05 were used; (B) heatmap of differentially expressed gene set between high and low naive CD4 cells. Samples were divided into high and low naive CD4 group according to percentage of naive CD4 cells on the first day of AMI. Log fold change >0.5 or <-0.5 and adjusted p<0.05 were used; (C) Venn diagram showing two shared differentially expressed genes in neutrophils and naive CD4 cells. AMI: acute myocardial infarction.
Neutrophilia and lymphocytopenia have been shown to be associated with AMI and mortality in patients with AMI.17,18 In our study, using the CIBERSORT algorithm, we confirmed that increased peripheral neutrophils and decreased peripheral CD4 T cells are associated with AMI and the development of HF. Moreover, it is the first study to correlate AMI with resting memory CD4 T cells and resting NK cells. It is also the first study to correlate the development of HF following AMI with naive CD4 T cells. We also identified genes that potentially have important roles in the development of HF following AMI.
CIBERSORT15 is software for cell proportion enumeration based on gene expression data. Its performance had been validated by flow cytometry, and in particular, a study has shown that CIBERSORT is reliable when analyzing sequence data from PBMCs or blood.19 Therefore, the cell proportions in PBMCs calculated in the current study using CIBERSORT should be reliable. Our finding that AMI correlated with changes in neutrophils and CD4 T cells is consistent with previous studies which confirmed that CIBERSORT is reliable.
Previous studies showed that counts of white blood cells and their subtypes are associated with AMI.20 In the acute period, leukocyctosis usually accompanies AMI in proportion to the magnitude of the necrotic process, inflammation in the coronary arteries and systemic inflammation.20,21 Specifically, research has revealed that activation of neutrophils is observed immediately after AMI, and neutrophils are the first leukocytes to be found in the infarcted myocardial area.22 Moreover, previous studies also identified an association between increased neutrophil count and poor outcomes in STEMI.23–26 Previous studies also confirmed that lymphocytopenia is a common finding in the acute phase of AMI.27,28 In particular, a decreased CD4 T cell count is closely associated with AMI.29 In this study, we found the percentages of monocytes and neutrophils were positively correlated, and the proportions of monocytes and lymphocytes are negatively correlated with lymphocytes (Figure 1B), which is consistent with a previous study showing that AMI causes rapid increases in production of neutrophils and monocytes in the peripheral blood,30 and high neutrophil counts and low lymphocyte counts are predictors of AMI.31 In this study, we also found that the percentage of neutrophils in PBMCs increased significantly on the first day of AMI (Figure 2B), which is in accordance with previous studies. Furthermore, we found that resting memory CD4 T cells, but not other subtypes of CD4 T cells (Figure 2E), decreased significantly on the first day of AMI. In addition, we found significantly increased monocytes, decreased CD8 T cells and resting NK cells on the first day of AMI (Figure 2). These results suggest that AMI may have more effects on peripheral immune cells than previously thought, and more research needs to be done to clarify the potential roles of these immune cells in AMI.
HF is a severe complication of AMI, and so it is important to be able to predict HF following AMI in a reliable and timely manner. Previous studies demonstrated that increased neutrophil count is independently and positively associated with large infarct size, mechanical complications, and mortality in patients with AMI.32,33 Additionally, lymphocytopenia and specifically decreased CD4 counts have been correlated with low ejection fraction, high degree of myocardial necrosis, and mortality in patients with AMI.29,34 In the present study, we also found patients who developed HF had higher proportions of neutrophils in PBMCs on the first day of AMI (Figure 4A), which is in accordance with previous studies. Interestingly, we noted that naive CD4 T cells, but not other subtypes of CD4 T cells, decreased significantly in patients who developed HF on the first day of AMI (Figures 3B and 4B). This result confirmed the correlation between lower CD4 T cell levels and worse prognosis following AMI. More importantly, it provides a deeper insight into a subtype of CD4 T cells. However, only 17 patients were included in this study; further research including more patients is needed to confirm our results and to clarify the potential roles of naive CD4 T cells in the development of HF following AMI.
After classifying samples according to the proportions of neutrophils and naive CD4 T cells on the first day of AMI, we carried out a further analysis on key genes that may affect the development of HF following AMI. Two shared genes, IL1R2 and LRRN3, were shown to have potentially important roles in the development of HF following AMI (Figure 5). Previous studies also demonstrated that IL1R2 may be independently associated with an elevated neutrophil-to-lymphocyte ratio in HF patients as a potentially decisive factor and IL1R2 is independently associated with parameters of adverse left ventricular remodeling following AMI.35,36 Furthermore, LRRN3 may mediate the development of HF following AMI via the MAPK signaling pathway and its downstream effectors.37
ConclusionsIn the present study, firstly, we demonstrated that the proportions of neutrophils and monocytes in PBMCs increased significantly, and the proportions of resting memory CD4 T cells, CD8 T cells and resting NK cells decreased significantly, on the first day of AMI. Secondly, we found that patients who developed HF following AMI had a higher proportion of neutrophils and a lower proportion of naive CD4 T cells in PBMCs on the first day of AMI. Finally, we identified two genes, IL1R2 and LRRN3, as possible target genes for the development of HF following AMI.
FundingNo funding was received.
Conflicts of interestThe authors have no conflicts of interest to declare.