Patent ductus arteriosus (PDA) in preterm infants has been associated with increased mortality and comorbidities. This study aimed to characterize the population of preterm infants diagnosed with PDA and to identify predictive factors of response to medical treatment of PDA.
MethodsAn eight-year retrospective observational study was carried out, which included all preterm infants with a gestational age (GA) between 23 and 32 weeks diagnosed with PDA, admitted to the Neonatal Unit of the CHUSJ. Univariate comparative analysis was performed, and models for predicting the effectiveness of PDA treatment with ibuprofen were explored by multivariate logistic regression analysis.
Results115 cases were included and 34 were excluded, with a final sample of 81 preterm infants with PDA. The univariate analysis revealed significant differences in the closure efficacy via medical treatment with ibuprofen in several variables, and a multivariate logistic regression model was obtained (discriminative capacity 72.2%, sensitivity 98.1%, specificity 57.1%), taking into account the effect of GA, type of delivery, need for diuretics treatment and platelet transfusion.
ConclusionThis study enabled the population of preterm infants diagnosed with PDA to be characterized and the identification of a predictive model that can help predict the efficacy of medical treatment and thus contribute to optimizing the medical approach to the non-responders.
A patência do canal arterial (PCA) nos recém-nascidos de pré-termo (RNPT) tem vindo a ser associada ao aumento de mortalidade e de comorbilidades. Este estudo objetivou caracterizar a população de RNPT diagnosticados com PCA e identificar fatores preditivos de resposta ao tratamento médico.
MétodosFoi realizado um estudo observacional retrospetivo de oito anos, que incluiu todos os RNPT com idade gestacional entre as 23 e as 32 semanas com diagnóstico de PCA, admitidos no Serviço de Neonatologia do Centro Hospitalar Universitário de São João (CHUSJ). Realizou-se análise comparativa univariada e foram explorados os modelos preditivos da eficácia do tratamento de PCA com ibuprofeno, por análise de regressão logística multivariável.
ResultadosCumpriram critérios de inclusão no estudo 115 casos e foram excluídos 34, obtendo-se a amostra final de 81 RNPT com PCA. A análise univariada revelou diferenças significativas na eficácia de encerramento pelo tratamento médico com ibuprofeno em diversas variáveis e obteve-se um modelo de regressão logístico multivariado (capacidade discriminativa 72,2%, sensibilidade 98,1%, especificidade 57,1%), considerando o efeito das variáveis: idade gestacional, tipo de parto, necessidade de tratamento com diuréticos e de transfusão de plaquetas.
ConclusãoEste estudo permitiu caraterizar a população de RNPT com diagnóstico de PCA e a identificação de um modelo preditivo que poderá auxiliar na previsão da eficácia de resposta ao tratamento médico e deste modo contribuir para aprimorar a estratégia de abordagem aos não respondedores ao tratamento médico.
The transition to extrauterine life is a critical phase in physiological adaptation. It has a major impact on several organs and systems, particularly on the lung and heart. Most neonates complete this transition phase without complications; however, dysregulation of normal postnatal adaptation can lead to cardiopulmonary instability, requiring advanced intensive care, especially in premature newborns.1,2
In the fetus there are at least three shunts: the ductus venosus, the foramen ovale (FO), and the ductus arteriosus (DA); several authors also consider the aortic isthmus to be the fourth shunt. Almost all of the non-oxygenated right cardiac output circulates through the DA to the descending aorta where it is transported by the aorta and umbilical arteries to the placenta. Given the low fetal oxygen tension, pulmonary vasoconstriction is maintained with high pulmonary resistance, which promotes the right-left shunt through the FO and DA.1
In the first 48-72 hours after birth, there is a progressive decrease in pulmonary vascular resistance (PVR) in response to recruitment and increased alveolar oxygen concentration. As PVR decreases, the direction of flow through the DA and FO changes to a bidirectional pattern or exclusive left-right direction, which promotes pulmonary circulation.1 Increased arterial oxygen saturation, endothelial bradykinin production, and decreased levels of circulating placental prostaglandins E2 induce constriction of the DA, followed by its physiological closure.3 Thus, in about 96% of healthy term newborns, functional closure of the DA occurs within the first 48 hours of life, and in the remaining cases, a small canal with restrictive left-right flow may remain.4 On the other hand, patent ductus arteriosus (PDA) is the most frequent cardiovascular alteration in preterm newborns and is inversely proportional to gestational age (GA) and birth weight.5 PDA occurs in about 33% of very low birth weight preterm infants (equal to or less than 1500 grams), and in about 65% of extremely low birth weight preterm infants (equal to or less than 1000 grams).6,7 It is estimated that the frequency of PDA is approximately 20% in preterm infants with a GA of 32 weeks, while in extremely low birth weight preterm infants with a GA of 26 weeks or less, the frequency is around 80-90%.8 The presence of systemic inflammatory mediators leads to an increase in reactive oxygen species, as well as prostaglandins, which may contribute to the failure of spontaneous closure of the DA. Additionally, other aspects that may contribute to PDA in preterm infants are the increased sensitivity of smooth muscle cells to the effects of vasodilator substances,9 adrenal insufficiency,10 thrombocytopenia,11–13 and platelet dysfunction.14 Other factors related to resuscitation and postnatal care in preterm infants, such as exposure to positive pressure ventilation, oxygen concentration, and the use of exogenous surfactant may also contribute to PDA.3
The clinical significance of PDA is related to its size, the magnitude of the shunt, and the consequent cardiovascular and respiratory repercussions resulting from pulmonary hyperflow, cardiac overload, and systemic hypoperfusion.15 The clinical picture of hemodynamically significant PDA includes respiratory signs (increased oxygen requirement, ventilation dependence, and apnea), and hemodynamic signs (systolic or continuous murmur, hyperdynamic precordium, wide pulse pressure, diastolic hypotension, cardiomegaly, hepatomegaly, or metabolic acidosis).16 Hemodynamically significant PDA in preterm infants has been associated with increased mortality and comorbidities.5 Preterm infants with PDA often present with several other complications of prematurity, such as pulmonary hemorrhage, peri/intraventricular hemorrhage, necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), and periventricular leukomalacia. Whether these complications arise in a general setting related to the immaturity of preterm infants or whether there is a relationship with PDA remains to be seen.5
The diagnosis of PDA with hemodynamic significance should be early and based on a set of echographic parameters. This echocardiographic evaluation should be performed in the first 72 hours in newborns with gestational age equal to or less than 28 weeks and/or birth weight equal to or less than 1000 grams; or newborns with gestational age between 28 and 30 weeks with associated risk factors (absence of prenatal corticotherapy, sepsis, invasive ventilation, peripartum asphyxia, and mother under magnesium sulfate therapy). In all other preterm infants, echocardiographic evaluation should be performed if there is a suggestive clinical picture.16
Medical treatment for early closure of PDA is intended to prevent clinical repercussions and should be initiated in the first five days of life with a cycle of ibuprofen that includes three doses at 24-hour intervals (first dose of 10 mg/kg/day, second and third doses of 5 mg/kg/day, at 15-minute intravenous infusions).16 The efficacy of PDA closure after the first cycle of ibuprofen treatment is 70%.17 In case of non-response, a second course of treatment is indicated, and a third course may be considered in exceptional cases.16 Surgical closure of PDA is indicated only in cases of pharmacological treatment failure, or in the presence of other comorbidities that contraindicate pharmacological treatment: renal failure, thrombocytopenia, active bleeding or coagulation alterations, necrotizing enterocolitis, severe sepsis, pulmonary hypertension, hyperbilirubinemia, and intestinal perforation. The surgical approach is quite effective and safe overall, however, there are risks of severe hemodynamic disturbance with some documented cases of potentially serious cardiovascular failure in the immediate postoperative period. However, the complications most commonly associated with surgical PDA ligation include hemorrhage, hypotension, acute cardiac dysfunction, recurrent or phrenic nerve damage, pleural effusion, pneumothorax, and chylothorax.17
This study aimed to characterize the population of preterm infants diagnosed with PDA, and to identify factors predictive of response to medical treatment. In addition, this study sought to contribute to a better understanding of this condition and the most frequently associated comorbidities, thus enabling better stratification of care delivery to preterm infants with hemodynamically significant PDA.
MethodsStudy populationA retrospective observational study was conducted on all preterm infants diagnosed with PDA, with a GA between 23 and 32 weeks (23 weeks and 0 days and 31 weeks and 6 days), determined by ultrasound up to 20 weeks, admitted to the Neonatology Department from the Obstetric Department/Birth Center of the University Hospital Center of São João (CHUSJ) between January 2010 and December 2018.
Preterm infants with a GA of <28 weeks were assessed according to a PDA screening protocol. All others were evaluated on clinical grounds, such as the presence of murmurs and hemodynamic or ventilatory instability.
Exclusion criteria were: (1) Preterm infant born at another institution and underwent neonatal transport to the CHUSJ Neonatology Department; (2) Preterm infant with an additional diagnosis of: a) TORCH group infection; b) major congenital anomaly; c) chromosomopathy: (d) asphyxia (Apgar score at 5 minutes less than or equal to 5 or umbilical cord blood pH<7.0); (e) feto-fetal transfusion syndrome; (f) fetal growth discrepancy (>20% difference compared with the weight of the larger fetus); g) severe anemia on admission (hemoglobin<12 g/dL); h) diagnosis of inborn errors of metabolism at a prenatal appointment or during the neonatal period; i) suspicion or diagnosis of neuromuscular disease.
Clinical and demographic dataData on prenatal history, birth, delivery room resuscitation, comorbidities, and treatments were collected by consulting the clinical database.
Hemodynamically significant PDA was defined as the presence of significant left-right shunt, with echocardiographic criteria of a hemodynamic repercussion (relating to ductal size, pulmonary hyperflow, left heart overload, and systemic hypoperfusion).
To ensure data confidentiality, a code was assigned to each file for patients included in the study and known only to the investigator. This study was approved by the ethics committee and authorized by the Board of Directors and the Access to Information Officer of the Centro Hospitalar Universitário de São João.
Statistical analysisNormality was tested using the Kolmogorov-Smirnov test. Categorical variables were described as total number and percentage, continuous variables with normal distribution were described by mean and standard deviation, and continuous variables with non-normal distribution were described according to the median, minimum and maximum value. Univariate comparative analysis was studied using Chi-squared tests for categorical variables, Student's t-test for continuous variables with normal distribution, and Wilcoxon-Mann-Whitney test for continuous variables with non-normal distribution. Multivariate logistic regression (forward method) was used to explore predictive models of the efficacy of PDC medical treatment of PDA with ibuprofen from the associations found by univariate analysis (p<0.25), a criterion used to choose the variables selected for multivariable regression. An alpha level of 0.05 was considered. Statistical analysis was performed with SPSS 26.0® software (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.).
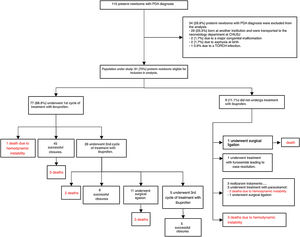
ResultsDescriptive analysis of the study populationBetween January 2010 and December 2018, 115 PDA diagnoses were recorded in preterm infants with GA between 23 and 32 weeks, admitted to the Neonatology Department of the Centro Hospitalar Universitário de São João. Of these, 34 preterm infants were excluded: 29 born at another institution and who underwent neonatal transport to the CHUSJ Neonatology Department, two due to diagnosis of major congenital anomaly, two due to asphyxia (Apgar score at 5 minutes<5), and one due to a TORCH group infection. The characteristics of the population of 81 preterm infants included in the study are shown in Table 1 and the data on diagnosis, approach, and treatment of ductus arteriosus patency are shown in Table 2 and Figure 1.
Characteristics of the population of preterm newborns included in the study.
| Newborn data at birth | |
| Weight (g) (mean, SD) | 995.84 (301.25) |
| Length (cm) (median, min-max) | 34.5 (25,0-52.0) |
| Head circumference (cm) (median, min-max) | 25.5 (15.0-33.0) |
| Gestational age (weeks) (median, min-max) | 27 (23-32) |
| Maternal age (years) (median, min-max) | 33 (19-47) |
| Male gender (no., %) | 38 (46,9) |
| Hemoglobin (g/dl) (mean, SD) | 16.2 (2.6) |
| MCV (%)(mean, SD) | 45.9 (7.1) |
| Leukocytes (×109/liter) (median, mini-max) | 7.3 (2.6-53.2) |
| Neutrophils (%)(mean, SD) | 33.5 (17.4) |
| Lymphocytes (%)(mean, SD) | 52.2 (19.0) |
| Platelet count (mean, SD) | 185 873 (48 635) |
| Prenatal factors | |
| Primigravida (no.,%) | 46 (56.8) |
| Spontaneous pregnancy (no., %) | 70 (86.4) |
| Supervised pregnancy (no., %) | 77 (95.0) |
| Single pregnancy (no., %) | 52 (64.2) |
| Antenatal corticotherapy (no., %) | 70 (86.4) |
| Complete corticotherapy cycle (no., %) | 55 (78.6) |
| Magnesium sulfate (neuroprotective protocol) (no., %) | 11 (13.6) |
| Full cycle (no., %) | 10 (91.0) |
| Delivery by cesarean section (no., %) | 56 (69.1) |
| Premature rupture of membranes (>18 hours) (no., %) | 9 (11.1) |
| Clinical chorioamnionitis (no., %) | 6 (7.4) |
| Cervical incompetence (no. %) | 10 (12.4) |
| Premature detachment of normally inserted placenta (no., %) | 12 (14.8) |
| Pregnant women with chronic hypertension (no., %) | 3 (3.7) |
| Preeclampsia (no., %) | 19 (23.5) |
| Eclampsia (no., %) | 1 (1.2) |
| HELLP Syndrome (no., %) | 4 (5.0) |
| Gestational hypertension (no., %) | 1 (1.2) |
| Type I diabetes (no., %) | 1 (1.2) |
| Gestational diabetes (no., %) | 5 (6.2) |
| Need for insulin during pregnancy (no., %) | 0 (0.0) |
| Smoking during pregnancy (no., %) | 6 (7.4) |
| Fetal growth restriction (no., %) | 6 (7.4) |
| Action in the delivery room | |
| MV (no., %) | 67 (82.7) |
| Invasive mechanical ventilation with endotracheal tube (no., %) | 35 (43.2) |
| Nasal CPAP ventilation (no., %) | 47 (58.0) |
| Supplemental oxygen (no., %) | 76 (93.8) |
| Fraction of inspired oxygen (FiO2) maximum (median, min-max) | 0.3 (0.0-1.0) |
| 1 min Apgar score (median, min-max) | 6 (2-9) |
| 5 min Apgar score (median, min-max) | 8 (4-10) |
| Postnatal period | |
| Bronchopulmonary dysplasia (no., %) | 28 (34.6) |
| Hyaline membrane disease (no., %) | 73 (90.1) |
| Mild (no., %) | 31 (42.5) |
| Moderate (no., %) | 31 (42.5) |
| Severe (no., %) | 11 (15.1) |
| Surfactant use (no., %) | 68 (84.0) |
| 1 dose (no., %) | 28 (41.2) |
| 2 doses (no., %) | 25 (36.8) |
| 3 doses (no., %) | 13 (19.1) |
| 4 doses (no., %) | 2 (2.9) |
| Pneumothorax (no., %) | 11 (13.6) |
| Pulmonary atelectasis (no., %) | 6 (7.4) |
| Use of MV (no., %) | 78 (96l.3) |
| Duration of mechanical ventilation use (days) (mean, SD) | 38.5 (23.3) |
| Use of nasal continuous positive airway pressure (CPAP) (no., %) | 69 (85.2) |
| Duration of nasal CPAP use (days) (mean, SD) | 25.8 (18.6) |
| Use of invasive MV (no., %) | 65 (80.2) |
| Duration of invasive MV use(median, min-max) | 8 (1-73) |
| Use of HFOV (no., %) | 9 (11.1) |
| Duration of use of HFOV (median, min-max). | 8 (3-23) |
| Maximum inspired oxygen fraction at admission (median, min-max) | 0.4 (0.21-1.0) |
| Number of days with supplemental oxygen (median, min-max) | 35 (0-157) |
| Need for supplemental oxygen at discharge (no., %) | 28 (34.6) |
| Treatment with systemic corticoid (no., %) | 10 (12.3) |
| Treatment with inhaled corticosteroids (no., %) | 18 (22.2) |
| Inhaled bronchodilator treatment (no., %) | 19 (23.5) |
| Treatment with diuretics (no., %) | 9 (11.1) |
| Days of total parenteral nutrition (median, min-max) | 3 (0-206) |
| Need for red blood cell transfusion (no., %) | 58 (71.6) |
| Need for platelet transfusion (no., %) | 20 (24.7) |
| Necrotizing enterocolitis (no., %) | 5 (6.2) |
| Early sepsis (<72h of life) (no., %) | 2 (2.5) |
| In-hospital sepsis (>72h of life) (no., %) | 33 (40.7) |
| Pneumonia (no., %) | 12 (14.8) |
| Meningitis (no., %) | 2 (25) |
| Intraventricular hemorrhage (no., %) | 39 (48.1) |
| Periventricular venous infarction associated with intraventricular hemorrhage (no., %) | 10 (25.6) |
| Periventricular leukomalacia (no., %) | 51 (63.0) |
| Grade 1 (no., %) | 44 (86.2) |
| Grade 2 (no., %) | 6 (11.8) |
| Grade 3 (no., %) | 1 (2.0) |
| ROP (no., %)a | 43 (53.1) |
| Grade I (no., %) | 27 (62.8) |
| Grade II (no., %) | 9 (21.0) |
| Grade III (no., %) | 7 (16.3) |
| Surgery for retinopathy of prematurity (no., %) | 13 (30.2) |
| Death (no., %) | 15 (18.5) |
| Deaths under 36 weeks gestational age (no., %) | 14 (93.3) |
| Deaths at GA>36 weeks (no., %) | 1 (6.7) |
| Age at death (median, min-max) | 11.5 (6-96) |
| Data at discharge | |
| Length of stay (days) (mean, SD) | 58.44 (37.8) |
| Weight (g) (median, min-max) | 2175 (485-3420) |
| Length (cm) (median, min-max) | 43 (30-50) |
| Head circumference (cm) (median, min-max) | 32 (22.5-38) |
a ROP assessment is unknown in 7 of the cases included in the study.
GA: gestational age; CPAP: continuous positive airway pressure; HFOV: high frequency oscillatory ventilation; MCV: mean corpuscular volume; MV: mechanical ventilation; ROP: retinopathy of prematurity; SD: standard deviation.
Diagnosis, approach and treatment of patent ductus arteriosus.
| Diagnosis and treatment of patent ductus arteriosus | |
|---|---|
| Preterm newborns diagnosed with PDA | 81 |
| Day of PDA diagnosis (median, min-max) | 3 (1-50) |
| Treatment of PDA with ibuprofen (no., %) | 72 (88.8) |
| One cycle (no., %) | 46 (63.0) |
| Two cycles (no., %) | 21 (25.9) |
| Three cycles (no., %) | 5 (6.8) |
| Day of ibuprofen treatment initiation (median, min-max) | 3 (1-31) |
| Successful closure after ibuprofen treatment (no., %) | 58 (80.6) |
| Treatment of PDA with paracetamol (no., %) | 3 (3.7) |
| Surgical closure (no., %) | 13 (16.0) |
| Day of life of surgical closure (median, min-max) | 14 (7-50) |
| Post-surgery complications (no., %) | 11 (84.6) |
PDA: patent ductus ateriosus.
Therapeutic approach and results obtained in preterm newborns with patent ductus arteriosus included in the study.
Key: PTNB: preterm newborns; PDA: patent ductus arteriosus; CHUSJ: Centro Hospitalar Universitário de São João; TORCH acronym for: Toxoplasmosis, Other (syphilis, varicella-zoster virus (VVZ), parvovirus B19), Rubella, Cytomegalovirus (CMV), and herpes simplex virus (HSV).
Univariate analysis of the efficacy of closure via medical treatment with ibuprofen and the various variables studied revealed significant differences according to the type of delivery (eutocic or cesarean); gestational age, mean weight, length, head circumference, mean hemoglobin, and globular volume at birth; the number of days of use of nasal continuous positive airway pressure and total parenteral nutrition, the need for and the number of red blood cell (RBC) and platelet transfusions; the need for invasive mechanical ventilation during hospitalization; the need for treatment with corticosteroids and inhaled bronchodilators and diuretics; the need for supplemental oxygen at discharge; and the diagnosis of retinopathy of prematurity (ROP), human immunodeficiency virus (HIV) and HIV-associated periventricular venous infarction (Table 3).
Univariate analysis of the efficacy of closure with ibuprofen medical treatment and the various variables studied.
| Treatment effective (n=58) | Treatment ineffective (n=14) | p-value | |
|---|---|---|---|
| GA (mean, SD) | 28.0 (1.9) | 26.1 (2.4) | 0.000 |
| GA<28 weeks (no., %) | 24 (42.1) | 13 (86.7) | 0.002 |
| Gestational ≥28 weeks (no., %) | 33 (57.9) | 2 (13.3) | |
| Type of delivery - Eutocic (no.,%) | 13 (22.8) | 8 (53.3) | 0.021 |
| -Cesarean section (no., %) | 44 (77.2) | 7 (46.7) | |
| Birth weight (g) (mean, SD) | 1069.3 (290.5) | 752.4 (216.6) | 0.000 |
| Length (cm) (mean, SD) | 35.8 (4.2) | 32.3 (4.5) | 0.000 |
| HC (cm) (mean, SD) | 26.1 (2,3) | 23.9 (3.6) | 0.000 |
| Hemoglobin (g/dl) (mean, SD) | 16.8 (2,7) | 14.3 (1.6) | 0.000 |
| Globular volume (%) (mean, SD) | 47.3 (7,5) | 41,5 (3,9) | 0.003 |
| RBC transfusion (no) (no., %) | 20 (35.1) | 0 (0,0) | 0.007 |
| (yes) | 37 (649) | 15 (100,0) | |
| Platelet transfusion NO (no., %) | 48 (84.2) | 6 (40) | 0.000 |
| YES | 9 (15.8) | 9 (60) | |
| Number of RBC transfusions (mean, SD) | 2.4 (3.6) | 8.6 (8.0) | 0.000 |
| Number of platelet transfusions (mean, SD) | 0.35 (1.2) | 3.4 (7.4) | 0.002 |
| Mechanical ventilation (NO) (no., %) | 12 (21.1) | 0 (0,0) | 0.046 |
| (YES) | 45 (78.9) | 15 (100) | |
| Duration of nasal CPAP use (mean, SD) | 29.5 (16.0) | 21.6 (23.7) | 0.034 |
| Need for oxygen at discharge (NO) (no., %) | 43 (75.4) | 7 (46.7) | 0.031 |
| (YES) | 14 (24.6) | 8 (53.3) | |
| Days of parenteral nutrition (mean, SD) | 3.1 (3.5) | 24.4 (49.9) | 0.001 |
| Inhaled corticosteroid treatment (NO) (no., %) | 46 (80.7) | 8 (53.3) | 0.029 |
| (YES) | 11 (19.3) | 7 (46.7) | |
| Inhaled bronchodilator treatment (NO) (no. %) | 45 (78.8) | 8 (53.3) | 0.045 |
| (YES) | 12 (21.1) | 7 (46.7) | |
| Diuretic treatment (NO) (no., %) | 54 (94.7) | 9 (60.0) | 0.000 |
| (yes) | 3 (5.3) | 6 (40.0) | |
| Intraventricular hemorrhage (NO) (no., %) | 35 (61.4) | 4 (26.7) | 0.016 |
| (yes) | 22 (38.6) | 11 (73.3) | |
| HIV-associated PVVI (NO) (no., %) | 55 (96.5) | 12 (80.0) | 0.025 |
| (yes) | 2 (3.5) | 3 (200) | |
| Retinopathy of prematurity (NO) (no., %) | 21 (38.2) | 2 (14.3) | 0.002 |
| (yes) | 33 (60.0) | 8 (57.1) |
CPAP: continuous positive airway pressure ventilation; GA: gestational age; HC: head circumference; HIV: human immunodeficiency virus; PVVI: periventricular venous infarction; IVH: intraventricular hemorrhage; RBC: red blood cell.
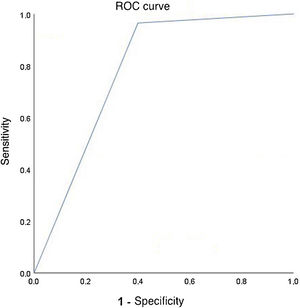
Multivariable logistic regression (forward selection) explored models that predicted the efficacy of medical treatment of PDA with ibuprofen, from the associations found in the univariate analysis (p<0.25). The logistic regression model that considered the effect of the variables: gestational age, type of delivery, need for diuretic treatment and platelet transfusion was statistically significant, χ2(4)=35.947, p<0.0001. The model explained 64.3% (Nagelkerke R2) of the variance in the efficacy of medical treatment of PDA with ibuprofen and correctly classified 89.7% of cases. The sensitivity of the model was 98.1%, the specificity 57.1%, and a positive predictive value of 89.8% and a negative predictive value of 88.8% were obtained. The discriminative ability of the model given by the area under the receiver operating characteristic curve was 0.782 (95% confidence interval, 0.624-0.941), which is an acceptable discrimination level according to the Hosmer and Lemeshow test (Figure 2).18
Discriminative ability of the model predictive of the occurrence of ibuprofen treatment efficacy in ductus arteriosus closure (area under the receiver operating characteristic curve=0.782; 95% confidence interval 0.624 to 0.941) in preterm newborns with gestational age between 23 and 32 weeks.7
Survival of preterm infants has increased significantly over the last two decades19 due to early identification of preterm infant-specific diseases and improvements in care. The early diagnosis and treatment of PDA have led to improvements in preterm infant survival. However, it remains unclear whether the various comorbidities identified arise as a result of prematurity, the existence of PDA with hemodynamic significance, or the therapeutic interventions. Additionally, the optimal point for therapeutic intervention after PDA diagnosis remains uncertain, since waiting for spontaneous closure may prevent overtreatment and associated adverse effects, and ibuprofen treatment itself seems to be effective even when applied relatively later.7 Nevertheless, aggressive and early treatment of PDA has been recommended to prevent further complications. The knowledge and characterization of the different variables, including prenatal history, birth and resuscitation in the delivery room, other comorbidities and treatments performed, may allow the stratification of preterm infants with PDA and thus improve intervention and outcomes for these premature infants.
In clinical practice, not all preterm infants diagnosed with PDA respond to treatment with COX inhibitors. Lago et al. observed an effective response to ibuprofen treatment in 86% of the subjects included in their study.20 It is therefore essential to determine the predictors of therapeutic success for PDA closure with ibuprofen to improve the individual approach to this group of preterm infants, thus avoiding unnecessary drug exposure and possibly considering a surgical approach earlier in the decision algorithm. Effective closure of DA after ibuprofen treatment occurred in 80.6% of the preterm infants included in the study. However, at the end of the first cycle of ibuprofen treatment, the efficacy of closure was 62.5%. The values found are slightly lower than the efficacy of closure described both overall and at the end of the first cycle of treatment (86% and 73%, respectively), which17 may be related to the lower gestational age range included in this study (up to 31 weeks and 6 days).
Additionally, in this study, superiority in the efficacy of PDA closure with ibuprofen treatment was observed for higher GA, birth weight, length, and head circumference at birth. Other studies have linked the efficacy of treatment with COX inhibitors to DA maturation, and it has been shown that lower birth weight and GA are associated with larger ductal diameter, thus making pharmacological closure more difficult.21–23
Our data show that preterm infants born by cesarean section responded better to treatment with ibuprofen. This observation is supported by some studies that have estimated the protective effects of cesarean delivery, particularly in very low birth weight preterm infants compared with spontaneous vaginal delivery. However, large-scale prospective studies are needed to establish this association.24
Some authors have suggested that platelets play an important role in physiological ductus arteriosus closure after the initial functional constriction of the DA.25 In this study, in PIs requiring platelet transfusion, the efficacy of closure with ibuprofen treatment was only 50%, and the mean number of platelet transfusions was higher in the group in which this treatment was not effective. Previous studies have described higher initial platelet counts in preterm infants who responded effectively to indomethacin treatment compared with those who did not.26 Whereas Akar et al. noted that platelet mass does not affect the efficacy of ibuprofen treatment for PDA in premature infants.27 The data from this study show that the need for platelet transfusions is associated with a worse response to ibuprofen treatment, which may be due to these preterm infants being critically ill, justifying platelet transfusions and the closure of the PDA. Nevertheless, in the subgroup of preterm infants that required platelet transfusions (n=20; GA between 23 and 29 weeks and weight between 410 and 1110 g), six required surgical ligation and nine died, which corroborates the worse prognosis of this subgroup of preterm infants. However, the relationship of platelet count and transfusion requirements with other factors, such as infection, that may contribute to the worse prognosis and mortality in this subgroup is highlighted.
Hyaline membrane disease not only increases the incidence of PDA in preterm infants but has also been associated with the efficacy of response to treatment of PDA with COX inhibitors.28 Additionally, surfactant administration causes a rapid decrease in pulmonary vascular resistance, contributing to increased left-to-right flow through the ductus arteriosus.29 However, in the present study we observed the presence of hyaline membrane disease in 90.1% of the sample, and surfactant was used in 84% of the preterm infants included in the study, making it difficult to assign an association for these variables.
Bronchopulmonary dysplasia (BPD) is defined as oxygen dependence at 36 weeks GA, and has a higher incidence the lower the birth weight of newborns.30 The pathogenesis of BPD is multifactorial and associated with some risk factors of prematurity.30 In the postnatal period, several approaches have been employed to prevent and treat BPD, including the use of loop diuretics, such as furosemide, to improve lung function and decrease pulmonary vascular resistance.31 However, the use of furosemide has been associated with several side effects, including inhibition of the response to treatment with COX inhibitors.32 In our sample, treatment with diuretics was statistically associated with reduced efficacy of PDA closure after treatment. Additionally, in this study, bronchodilators and inhaled corticosteroids, which are also used to improve gas exchange and lung mechanics in the prevention of BPD,33 were associated with a less effective response to ibuprofen treatment. Several other studies associate the presence of PDA with the development of BPD,34 thus highlighting the need to clarify the relationship between these variables.
Additionally, the use of mechanical ventilation at birth was associated with an increased risk of hemodynamically significant PDA.35 The need for more aggressive mechanical ventilation may thus relate the existence of PDA and the increased risk of bronchopulmonary dysplasia in preterm infants. Conversely, the pharmacological closure of PDA is associated with a decrease in pulmonary edema and therefore with a decrease in BPD and the need for ventilation.36 In this study, we observed a lower efficacy of PDA closure in patients on invasive mechanical ventilation, probably because they were more unstable patients, both from a ventilatory and hemodynamic perspective.
In this study, we observed an association between the efficacy of ibuprofen treatment and hemoglobin and globular volume values at birth.37 It is noteworthy that all preterm infants who did not require RBC transfusion during hospitalization (n=20) responded to ibuprofen treatment. It is known that RBC transfusion improves cardiorespiratory status by increasing circulating hemoglobin and consequently improving tissue oxygenation. Some studies, however, associate RBC transfusion with adverse clinical outcomes in preterm infants, such as NEC, BPD, HIV, and ROP.38
In the series under study, paracetamol was exceptionally used for the treatment of PDA in three preterm infants. Recent studies on the use of this drug in the closure of PDA have shown that it is equally safe and effective when compared to treatment with ibuprofen or indomethacin, with few adverse effects.39 Thus, it may be a safe solution in situations that contraindicate the use of ibuprofen.40 However, several studies are currently underway to investigate the possibility of paracetamol use more widely, as some studies suggest an association with prerenal lesions and neurodevelopmental complications.39
In the event of non-response to medical treatment after the second cycle, a third cycle may be considered in exceptional cases.16 In our series, this was performed in five preterm infants and was effective in all cases. This sample included preterm infants with GA between 24 and 31 weeks and birth weight between 685 and 1925 grams. These results highlight the importance of clinical census when deciding the treatment algorithm for PDA.
Surgical closure of PDA reduces time on mechanical ventilation, improves hemodynamic response and pulmonary compliance. However, it is not without adverse effects and is therefore reserved for cases in which medical treatment fails.40 In our sample, in 13 cases surgical ligation of the ductus arteriosus had to be performed and there were several postoperative complications, including three cases of pneumothorax, four cases of vocal cord lesions, one case of diaphragmatic hemiparesis, two cases of hemorrhage, seven newborns required inotropic treatment in the immediate postoperative period, and seven newborns had transient renal failure after surgery. These data thus emphasize the need to define the subgroup of preterm infants with hemodynamically significant PDA in whom surgery is most likely to be beneficial.
This study has the disadvantages associated with being a retrospective study and, as such, there is limited control over the sample variables and the clinical data files are incomplete; however, we managed to obtain a robust sample for the intended analysis. Despite the limitation of the study having been conducted at a single center, there is also the advantage of it being a level III center and a reference center for congenital heart diseases. This means the center provides differentiated and integrated care to preterm infants, ensuring the accessibility, effectiveness, safety and excellence of the care according to the highest international ethical and scientific standards.41
ConclusionPatent ductus arteriosus is a frequently observed condition in preterm infants and therapeutic approaches remain a challenge. Closure of PDA using medical or surgical treatment is not without complications and the decision remains controversial. COX inhibitors, such as ibuprofen, are frequently used for the treatment of PDA in preterm infants, however, in clinical practice not all preterm infants with PDA respond to ibuprofen treatment. Some studies have shown that variables such as GA, birth weight, and ductal diameter may help predict the therapeutic response to COX inhibitors.
In this study, we aimed to characterize the population of preterm infants diagnosed with PDA and describe the factors that may predict the therapeutic success of ibuprofen use in the closure of PDA. According to the multivariate logistic regression model that considers the effect of variables including gestational age, type of delivery, need for diuretic treatment and platelet transfusion, it is possible to provide a high sensitivity prediction of the efficacy of the response to medical treatment. However, further studies are needed to improve this model to optimize the therapeutic strategies offered to preterm infants diagnosed with PDA.
Conflicts of interestThe authors have no conflicts of interest to declare.