Paravalvular leak (PVL) is a common serious complication associated with prosthetic valve implantation.
ObjectiveThe aim of this study was to report our single-center experience in a retrospective review and to analyze possible predictors of success.
MethodsWe performed 33 percutaneous PVL closures in 26 patients (54% female, mean age 65±13 years). All mitral prostheses were studied previously with 3D transesophageal echocardiography (TEE), and aortic prostheses with 2D/3D TEE. 3D TEE and fluoroscopy were used for the assessment, planning, and guidance of the interventions. Twelve patients also underwent computed tomography angiography for better characterization of anatomic details.
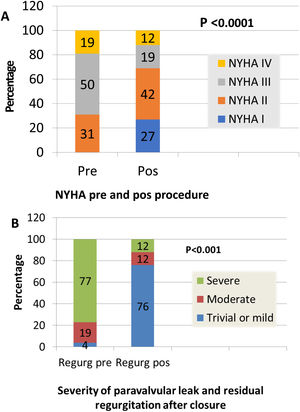
ResultsEighteen patients (69.2%) were admitted due to heart failure (New York Heart Association [NYHA] III or IV, seven (26.9%) because of heart failure and hemolysis, and one (3.8%) due to hemolysis only. Regarding the leaks, 46.2% were in aortic and 53.8% in mitral prostheses, 88.5% in mechanical and 7.7% in biological prostheses, and 3.8% in transcatheter aortic valve implants. All the aortic patients had severe aortic regurgitation. Furthermore, all mitral patients but one had moderate to severe or severe mitral regurgitation. Closure was successful in 17 patients (65.4%), partially successful in four (15.4%) and unsuccessful in five (19.2%). After the procedure, 69% were in NYHA I-II. Hemolysis worsened in three patients despite successful closure; all required further valvular surgery and two died. Regarding angiographic and echocardiographic procedural success, we analyzed age, gender, type of prosthesis (mechanical or biological), location (aortic or mitral), clinical data, maximum leak diameter, anatomic regurgitant orifice, leak location (anterior, posterior, inferior and lateral for mitral leaks and left, right and non-coronary sinus for aortic leaks), and number of devices (plugs) used for closure. No parameters presented a significant relationship with success excepting previous hemolysis. There was a relationship between clinical improvement and reduction of PVL (p=0.0001). In follow-up, cardiac-related events (new hospital admissions, cardiac valvular surgery, need for transfusion) were more frequent in patients with partially successful or unsuccessful closure (p=0.012). There was a relationship between cardiac-related events and death (p=0.029).
ConclusionPercutaneous PVL closure has emerged as an alternative treatment for PVL. Predictors of procedural success are difficult to establish. Survival is related to reduction of regurgitation and improvement in NYHA functional class.
Os leaks perivalvulares são uma complicação potencialmente grave após cirurgia protésica, com uma taxa de mortalidade ainda importante, sobretudo em doentes submetidos a reoperações sucessivas.
ObjetivoAvaliar retrospetivamente a nossa experiência e resultados e encontrar possíveis fatores preditores de sucesso.
MétodosRevimos 33 procedimentos consecutivos de encerramento de leak perivalvular efetuados em 26 doentes (dts) (54% dos dts eram de sexo feminino, idade média 65±13 anos). Todos os casos de leaks em posição mitral foram previamente estudados com ecocardiograma transesofágico 3 D (ETE), os leaks em posição aórtica foram avaliados por ETE 2D e nalguns casos 3D. Utilizaram-se a ecocardiografia transesofágica (2D/3D) e a fluoroscopia para a avaliação, planeamento, orientação e libertação dos dispositivos de encerramento; 12 dts efetuaram previamente angioTAC para melhor caracterização e definição anatómica do leak.
ResultadosClinicamente 18 dts tinham queixas de insuficiência cardíaca (New York Heart Association [NYHA] III/IV (69,2%), 7 dts tinham insuficiência cardíaca e hemólise (26,9%) e um doente apenas emólise (3,8%); 46,2% dos leaks eram aórticos e 53,8% mitrais, a maioria, 88,5%, em próteses mecânicas, 7,7% em próteses biológicas e 3,8% (um dt) em prótese percutânea aórtica (VAP). Todos os dts de leak aórtico tinham insuficiência cardíaca por regurgitação grave, os dts com leak mitral tinham insuficiência cardíaca, alguns, hemólise e um tinha apenas hemólise. O grau de regurgitação mitral era moderado a grave ou grave, com excepção do doente só com hemólise.
O encerramento foi considerado de sucesso em 17 dts (65,4%), sucesso parcial em 4 (15,4%) e insucesso em 5 (19,2%). Após o procedimento, 69% melhoraram, com melhoria das queixas de ICC (NYHA I-II), 3 dts tiveram um agravamento da hemólise apesar do encerramento ser considerado de sucesso, tendo necessitado de cirurgia, complicada em dois casos (morte).
Na tentativa de encontrar fatores preditores de sucesso de encerramento do leak, analisámos dados clínicos (idade, sexo, tipo e localização da prótese, manifestações clinicas), dados ecocardiográficos (diâmetro máximo do leak, área do orifício anatómico, localização do leak, classificando-o em anterior, posterior, inferior e lateral para as próteses mitrais e para as próteses aórticas de acordo com o seio coronário adjacente em esquerdo, direito e não coronário) e ainda o número de dispositivos de encerramento utilizados (plugs). Nenhum dos parâmetros avaliados teve correlação com o sucesso do encerramento, com excepção para a hemólise. Verificou-se relação entre melhoria clínica e redução do leak (p=0,0001). Os dts com sucesso parcial/insucesso tiveram mais eventos cardíacos (p=0,012), a ocorrência de mais eventos cardíacos (internamento hospitalar, nova cirurgia cardíaca, transfusões) se relacionou com pior prognóstico e morte (p=0,029).
ConclusãoO encerramento percutâneo de leaks protésicos pode ser uma opção ao tratamento convencional cirúrgico. Os fatores preditores de sucesso do encerramento são difíceis de estabelecer. A sobrevida destes dts relacionou-se com redução da regurgitação e melhoria clínica (NYHA).
Periprosthetic valvular regurgitation or paravalvular leak (PVL) is a common complication after surgical valve replacement, with reported incidences at follow-up of 2-10% for prosthetic valves in the aortic position and 7-17% in the mitral position.1,2 Significant PVLs have also been described after transcatheter aortic valve implantation (TAVI). Most PVLs remain clinically silent. However, 1-3% of patients with PVL require reoperation due to symptoms of congestive heart failure (CHF), hemolysis or, in most cases, both.3,4
Surgical closure remains the first-line therapy for these defects. Nevertheless, redo surgery has some limitations, associated with patient comorbidities, and has a high recurrence rate – leaks beget new leaks.3–5 Additionally, mortality increases progressively with the number of reoperations, at rates that are dismal for each successive intervention: 13% after the first, 15% after the second, and 37% after the third.3,5,6
Percutaneous PVL closure has emerged as an alternative treatment, but predictors of procedural success are unknown. We report our single-center experience, analyzing possible predictors of procedural success and survival.
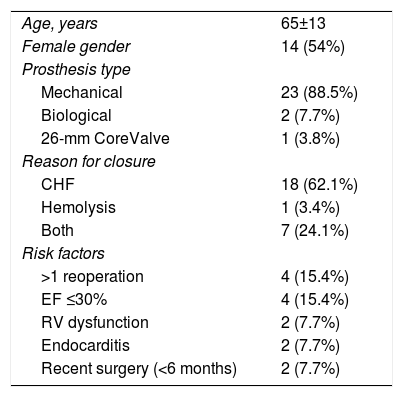
MethodsPatient populationBetween October 2010 and December 2018, 26 consecutive patients were referred to our center for possible percutaneous PVL closure. We performed 33 percutaneous closure attempts in these 26 patients. Patient demographics and medical history revealed a high surgical risk: 14 patients (54%) were refused by the surgical team due to a multitude of comorbidities (four patients had ejection fraction <30%, two had marked right ventricular dysfunction and one refused blood transfusion due to religion), redo operations (four patients had more than one previous surgery) or early leak (<6 months after surgery – two patients).
All patients were symptomatic with severe heart failure and/or mechanical hemolytic anemia requiring multiple transfusions. Hemolysis alone was the indication for PVL closure in one case (3.8%), CHF alone was the indication in 18 procedures (69.2%), and a combination of hemolysis and CHF was the indication in the other seven procedures (26.9%).
Patients’ mean age was 65±13 years (range 21-81 years), and 14 (54%) were female. Regarding the leaks, 46.2% were in aortic and 53.8% were in mitral prostheses, 88.5% (23 patients) in mechanical prostheses, 7.7% in biological and 3.8% in transcatheter aortic valve implants.
Twenty-one patients had one prosthetic valve and five had two, mostly in the mitral and aortic position, except one patient with one mitral and one biological tricuspid prosthesis. Two patients had prior endocarditis and one patient had had a fistula between the left atrium and the non-coronary sinus since the first surgical procedure (Videos 1 and 2).
Imaging dataAll mitral prostheses were studied with three-dimensional (3D) transesophageal echocardiography (TEE) (Phillips IE33, Eindhoven, Netherlands, and GE E9 and E95, GE Healthcare, Milwaukee, WI), and aortic prostheses with two-dimensional (2D)/3D TEE.
Twelve patients (46%) also underwent computed tomography angiography (CTA) on a 64-detector scanner (LightSpeed VCT, GE Healthcare, Milwaukee, WI).
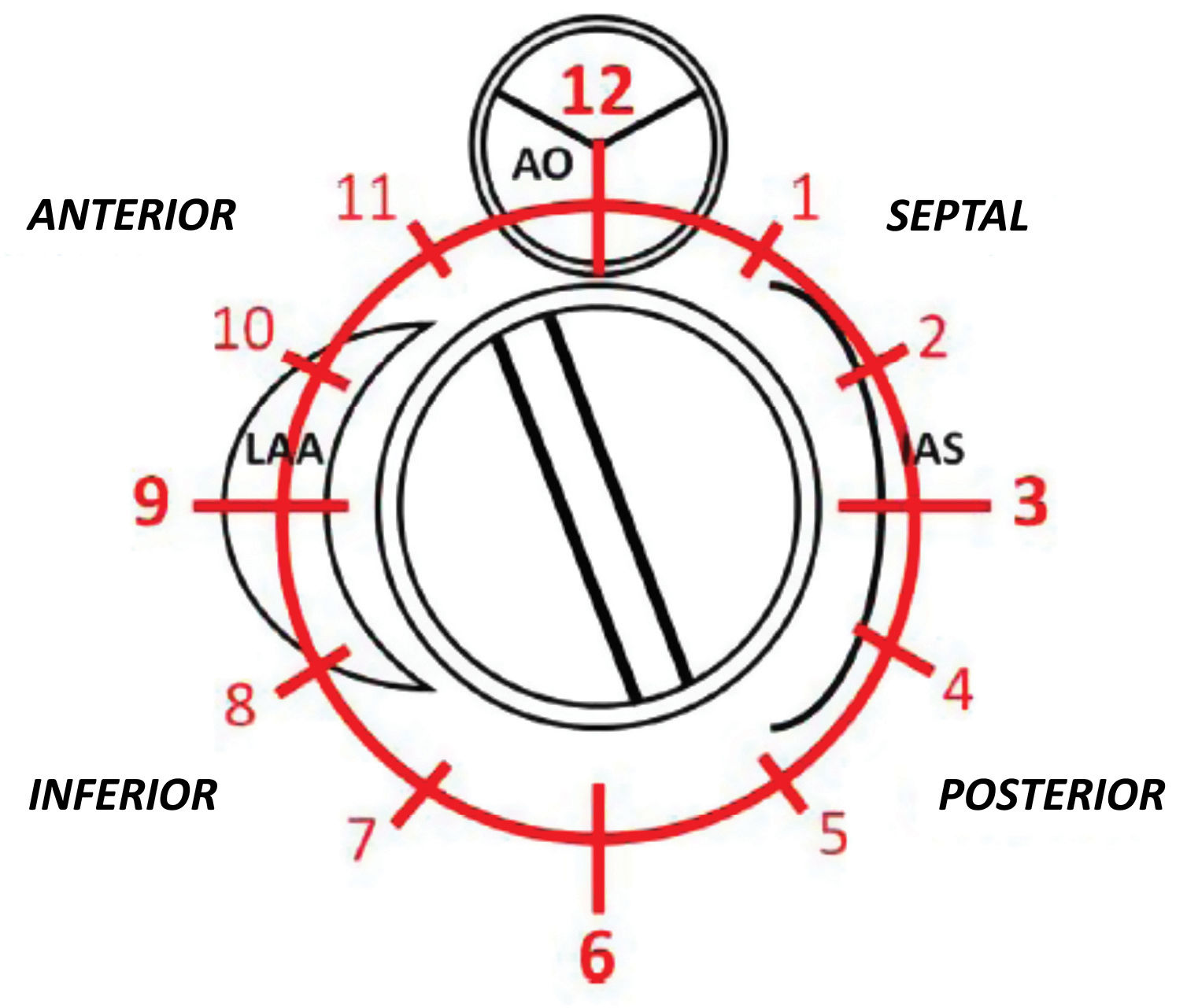
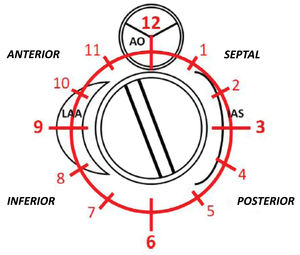
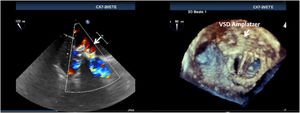
PVL was defined as a regurgitant jet, demonstrated by color Doppler echocardiography, originating between the outer margin of the prosthetic sewing ring and the native tissues around the valve. The anatomic location of the leak was defined using the clock reference for precise location in 3D TEE left atrial en-face view, with the mitral-aortic curtain at 12 o’clock, the left atrial appendage at nine o’clock, the interatrial septum at 3 o’clock and the posterolateral free wall at 6 o’clock. Mitral leaks were classified as anterior (9-12 o’clock), inferior (6-9 o’clock), posterior (3-6 o’clock) and medial/septal (0-3 o’clock). For aortic leaks the anatomic location was defined with reference to the adjacent coronary sinus (left, right and non-coronary) with the non-coronary cusp between 7 o’clock and 11 o’clock, the left coronary cusp between 11 o’clock and 3 o’clock, and the right coronary cusp between 3 o’clock and 7 o’clock (Figure 1).
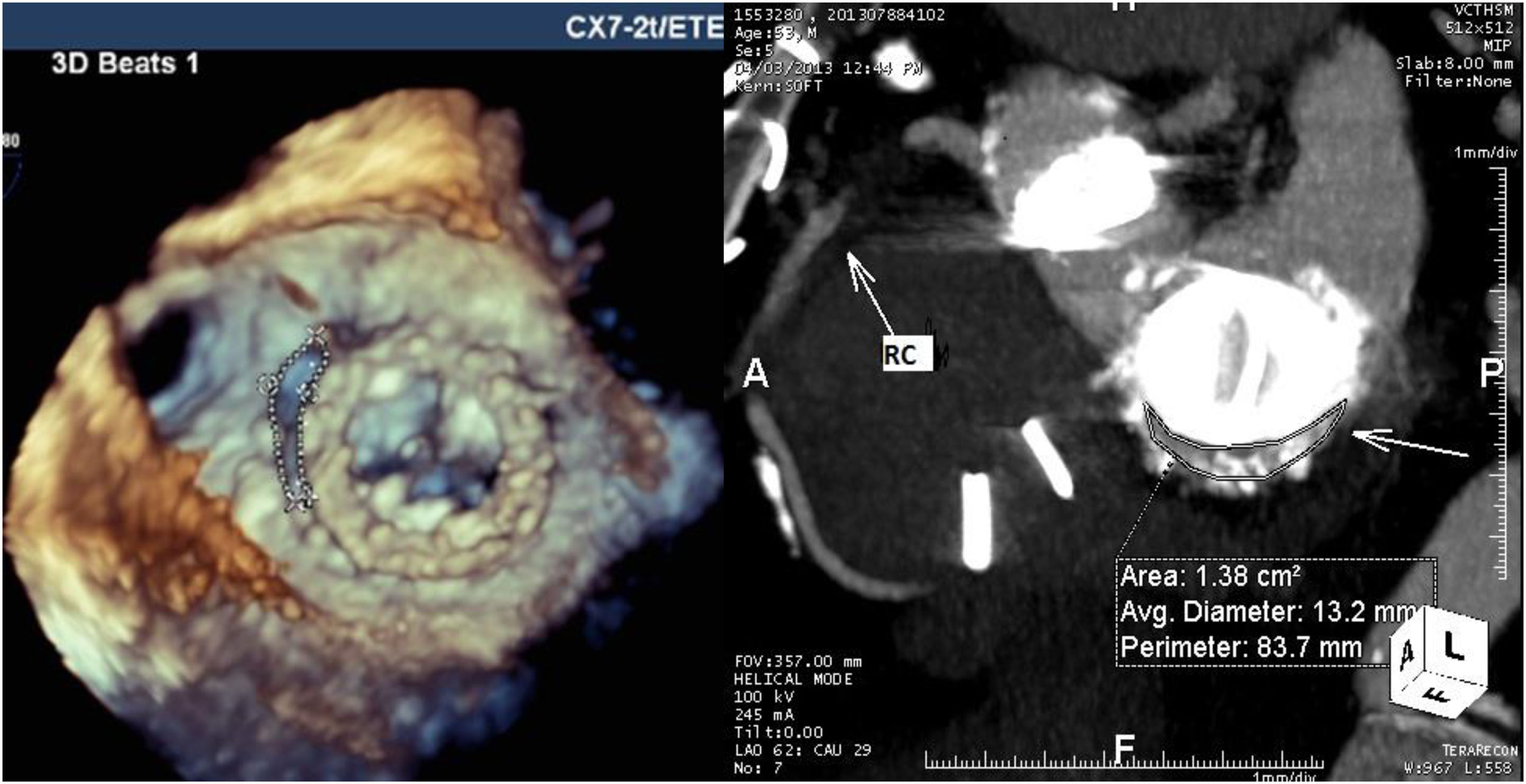
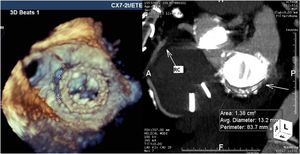
For mitral leaks the maximum leak diameter and the anatomic regurgitant orifice were measured, and the shape of the leak was classified as round or crescent-shaped (like a comma surrounding the prosthetic sewing ring). Regurgitant jets were analyzed and mitral regurgitation classified as mild, moderate or severe in accordance with the European guidelines for regurgitant prosthetic jets.7 Twelve patients with mitral and aortic prostheses underwent CTA for better anatomic definition of the leak (Figure 2).
For aortic prostheses, 2D TEE in short-axis view, biplane images and 3D TEE of the aortic valve were used to localize the leak and measure its maximum diameter.
All procedures were performed by experts in TEE and interventional procedures under TEE guidance.
Procedural aspectsLeak closure was performed under general anesthesia. TEE was used to guide transseptal puncture and passage of the guidewire and catheter across the defect, and to analyze the position of the devices and any possible interference with the disks of the prosthesis.
Mitral valve PVLs were closed via a transvenous route with transseptal puncture in the anterograde direction. In 43% of mitral PVLs a previous arteriovenous loop was necessary to support the passage of the delivery sheath. Transapical access was also used, particularly when there were double prostheses in aortic and mitral position.
Aortic valve PVLs were crossed retrogradely with femoral access. Crossing the leak was facilitated by simultaneous use of fluoroscopy and 2D/3D TEE.
PVL closure devices were selected according to physician preference and to previous measurements by 3D TEE and CTA.
Technical success was defined as successful deployment of a closure device across the leak without interfering with the valve prosthesis and without acute conversion to surgery.
Procedural success was considered to have been achieved if there was resolution of the leak with no, trivial or mild regurgitation. Partial success was defined as the regurgitant jet decreasing by one degree, but with more than mild and less than severe residual regurgitation.
Clinical success was defined as improvement of at least one New York Heart Association (NYHA) functional class within six months and/or improvement in mechanical hemolysis.
Procedural outcome events were defined as events occurring during the procedure and in the following 24 hours. Thirty-day outcome events, as assessed by telephone or during a clinic visit, included procedural outcome events and those occurring within 30 days of the procedure, including death from any cause. All follow-up events were recorded and collected by telephone or outpatient clinic visit.
Statistical analysisContinuous variables are presented as mean ± standard deviation and categorical variables as absolute numbers and percentages.
Baseline characteristics and outcomes were compared for categorical variables using the chi-square test or Fisher's exact test when appropriate. Pearson's correlation coefficient was used to test associations between continuous variables.
Changes in PVL grade and NYHA class at baseline and after closure were analyzed using the Wilcoxon rank test.
Survival estimates with 95% confidence intervals (CIs) were calculated using the Kaplan-Meier method.
Univariate and multivariate Cox regression was used to assess potential independent associations between outcome and death or major adverse cardiovascular events (MACE). The significance level was set at 0.05. Data were analyzed using IBM SPSS for Windows, version 19.0 (IBM SPSS Inc., Chicago, IL).
ResultsPatient dataPVL closure was attempted in 26 patients in 33 procedures. The procedure was first performed at our center in 2010 and began to be performed regularly 18 months later, after approval by the ethics committee.
Patients’ clinical variables are detailed in Table 1. Mean age was 65±13 years. There were no significant differences between genders (54% female). The indications for closure were heart failure (NYHA class III or IV) in 18 patients (69.2%), heart failure and hemolysis in seven patients (26.9%) and hemolysis only in one patient (3.8%) (Table 1).
Clinical characteristics.
| Age, years | 65±13 |
| Female gender | 14 (54%) |
| Prosthesis type | |
| Mechanical | 23 (88.5%) |
| Biological | 2 (7.7%) |
| 26-mm CoreValve | 1 (3.8%) |
| Reason for closure | |
| CHF | 18 (62.1%) |
| Hemolysis | 1 (3.4%) |
| Both | 7 (24.1%) |
| Risk factors | |
| >1 reoperation | 4 (15.4%) |
| EF ≤30% | 4 (15.4%) |
| RV dysfunction | 2 (7.7%) |
| Endocarditis | 2 (7.7%) |
| Recent surgery (<6 months) | 2 (7.7%) |
Values are number (percentage) or mean ± standard deviation.
CHF: congestive heart failure; EF: ejection fraction; RV: right ventricular.
Fourteen patients presented risk factors for surgical PVL closure: four had >1 previous valve surgery, four had severe left ventricular dysfunction (one after TAVI for true low-flow, low-gradient severe aortic stenosis), two had right ventricular dysfunction (due to severe tricuspid regurgitation with hepatic dysfunction, one with a dysfunctional biological tricuspid prosthesis), two had recent valve surgery (less than six months) and two had endocarditis (one after repeated piercings and cutaneous infection, the other due to intravenous drug abuse). There was a relationship between hemolysis and >1 reoperation (p=0.004). Previous hemolysis was the only parameter that correlated with technical aspects such as the number of procedures by patient (p=0.049) and partial success/failure (p=0.046).
Three procedures (11.5%) were performed in an urgent setting.
No relationship was observed between patient characteristics and technical success. NYHA>II at baseline was associated with mortality in Cox univariate analysis (p=0.008, hazard ratio [HR] 3.334, 95% confidence interval [95% CI]: 1.375-8.087).
Imaging dataAll patients underwent complete transthoracic and transesophageal echocardiographic assessment for diagnostic purposes. During the procedure all mitral PVLs were studied with 3D TEE, and aortic prostheses with 2D/3D TEE.
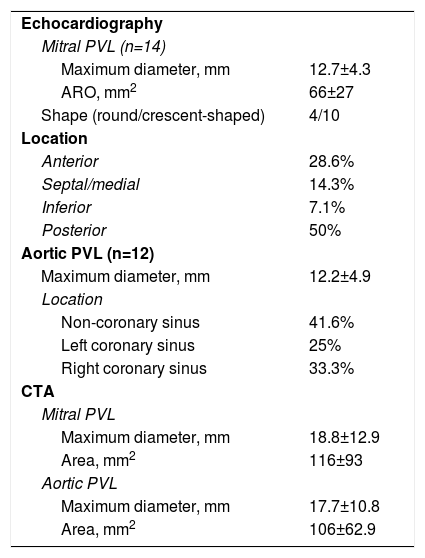
CTA was performed in 12 patients for additional characterization of the leak and TEE and CTA results were integrated for these patients. For mitral PVLs, 3D TEE gave precise anatomic details concerning the size, area and shape of the leak. Additionally, in a patient with double mitral and aortic mechanical prostheses, TEE revealed the presence of a fistula between the non-coronary sinus and left atrium that was not diagnosed by CTA. However, for aortic prostheses TEE did not provide sufficient information concerning the anatomic area of the leak and other anatomic details like outpouching (small pseudoaneurysms) of the left ventricular outflow tract, which was masked by the struts of mechanical prostheses. CTA provided more information in these cases, however the anatomic area and maximum diameter of the leak appeared oversized by CTA (Table 2).
Imaging data.
| Echocardiography | |
| Mitral PVL (n=14) | |
| Maximum diameter, mm | 12.7±4.3 |
| ARO, mm2 | 66±27 |
| Shape (round/crescent-shaped) | 4/10 |
| Location | |
| Anterior | 28.6% |
| Septal/medial | 14.3% |
| Inferior | 7.1% |
| Posterior | 50% |
| Aortic PVL (n=12) | |
| Maximum diameter, mm | 12.2±4.9 |
| Location | |
| Non-coronary sinus | 41.6% |
| Left coronary sinus | 25% |
| Right coronary sinus | 33.3% |
| CTA | |
| Mitral PVL | |
| Maximum diameter, mm | 18.8±12.9 |
| Area, mm2 | 116±93 |
| Aortic PVL | |
| Maximum diameter, mm | 17.7±10.8 |
| Area, mm2 | 106±62.9 |
Values are number (percentage) or mean ± standard deviation.
ARO: anatomic regurgitant orifice; CTA: computed tomography angiography; PVL: paravalvular leak.
Regurgitant jets were severe in 20 patients (10 mitral and 10 aortic), mild in one patient with a mitral prosthesis (hemolysis only) and moderate to severe in five (two aortic and three mitral).
No echocardiographic or CTA parameter was significantly associated with technical success. There was also no relationship between these parameters and mortality and MACE by univariate Cox proportional hazard regression.
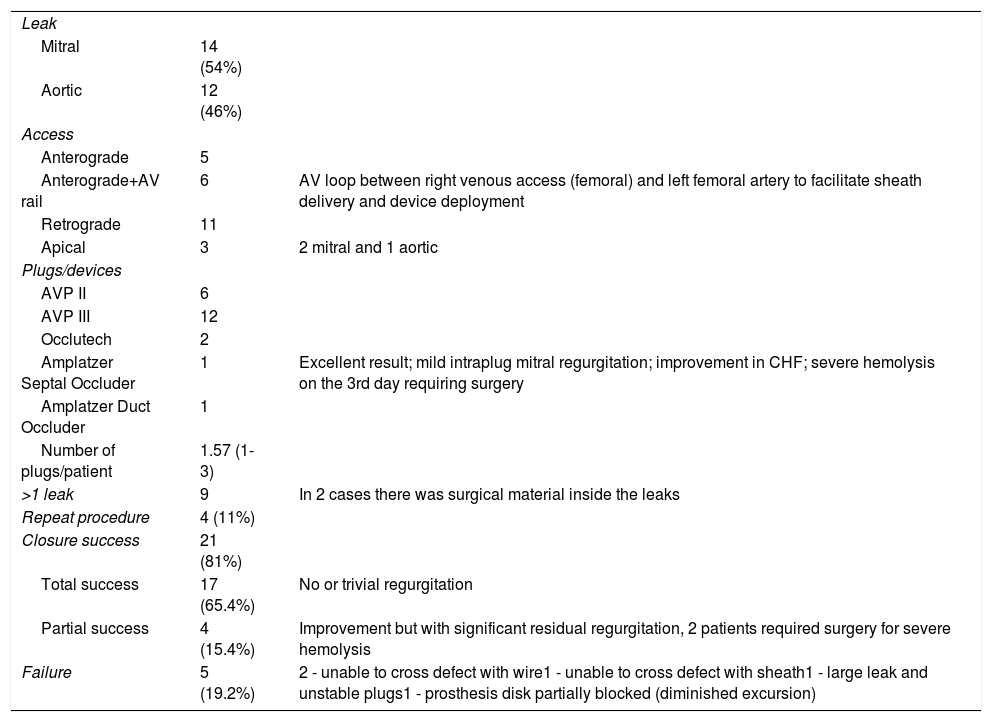
Procedural dataProcedural variables are shown in Table 3. The majority of PVLs were around mechanical valves (88.5%), and mitral PVLs accounted for 53.8% of the cases. Devices were successfully implanted in 81% patients (n=21). The approach was anterograde for mitral prostheses in 11 patients, but in six patients it was necessary to perform a arteriovenous loop between the right femoral vein, left ventricle, aorta and left femoral artery beforehand. For aortic leaks the approach was retrograde (11 patients); an apical approach was adopted in three patients (two for mitral and one for aortic prostheses) and 11% of the patients required more than one procedure.
Procedural data.
| Leak | ||
| Mitral | 14 (54%) | |
| Aortic | 12 (46%) | |
| Access | ||
| Anterograde | 5 | |
| Anterograde+AV rail | 6 | AV loop between right venous access (femoral) and left femoral artery to facilitate sheath delivery and device deployment |
| Retrograde | 11 | |
| Apical | 3 | 2 mitral and 1 aortic |
| Plugs/devices | ||
| AVP II | 6 | |
| AVP III | 12 | |
| Occlutech | 2 | |
| Amplatzer Septal Occluder | 1 | Excellent result; mild intraplug mitral regurgitation; improvement in CHF; severe hemolysis on the 3rd day requiring surgery |
| Amplatzer Duct Occluder | 1 | |
| Number of plugs/patient | 1.57 (1-3) | |
| >1 leak | 9 | In 2 cases there was surgical material inside the leaks |
| Repeat procedure | 4 (11%) | |
| Closure success | 21 (81%) | |
| Total success | 17 (65.4%) | No or trivial regurgitation |
| Partial success | 4 (15.4%) | Improvement but with significant residual regurgitation, 2 patients required surgery for severe hemolysis |
| Failure | 5 (19.2%) | 2 - unable to cross defect with wire1 - unable to cross defect with sheath1 - large leak and unstable plugs1 - prosthesis disk partially blocked (diminished excursion) |
AV: arteriovenous; AVP: Amplatzer Vascular Plug; CHF: congestive heart failure.
The devices chosen are listed in Table 3. The number of devices used per patient ranged from one to three. The Amplatzer Vascular Plug III (AVP III) accounted for 57% of the devices used. This is because the PVLs, though complex, were long and crescent-shaped in most of the series, which gives this oblong device an advantage. Occlutech has two types of device, one oblong and the other square. However, our experience with these devices is limited (two cases). Our data, comparing technical success between the AVP III and the other devices, were in favor of the AVP III (p=0.027). Procedures with more than one attempt were associated with partial success or failure (p=0.003).
OutcomeThere was one acute tamponade as an intra-procedural complication, solved using a percutaneous approach, with no adverse consequences. There were no procedure-related deaths. The only case of 30-day mortality was due to sepsis and infection, in a patient who had been in the intensive care unit for 15 days, so the procedure was urgent.
One-year mortality (one death) was due to infection (a case of endocarditis three months after leak closure).
Follow-up for patients surviving more than one year was 44±24 months (7-97 months).
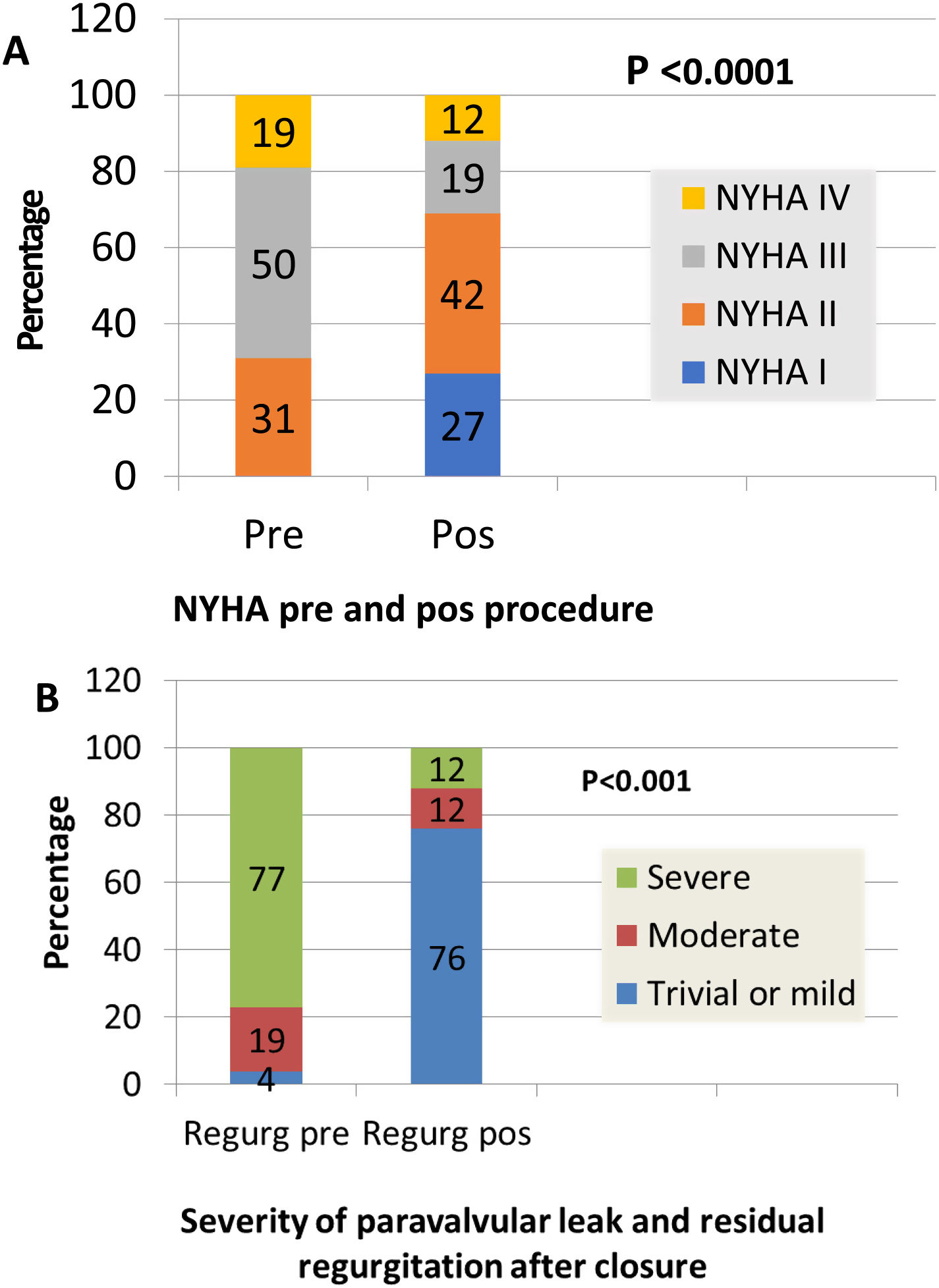
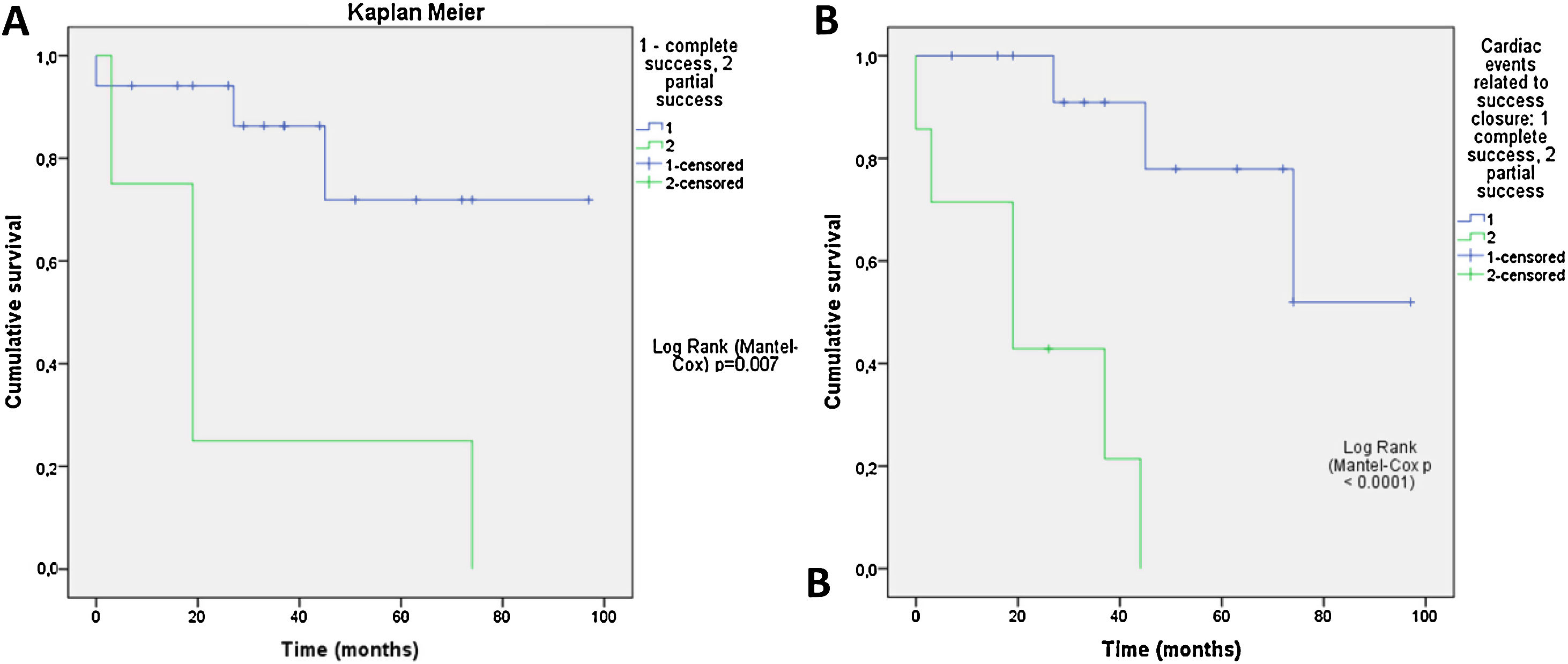
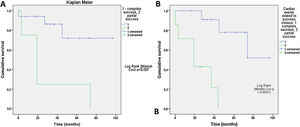
Clinical improvement, defined as a reduction in NYHA functional class, was significant and associated with reduction of the leak (p=0.0001) (Figure 3). Cardiac-related events in follow-up (new hospital admissions, new cardiac surgery, need for transfusion) were more frequent in patients with partially successful or unsuccessful closure (p=0.012). Univariate Cox regression analysis revealed that NYHA>II at follow-up (p=0.03, HR 0.17 [95% CI 0.034-0.843]), residual PVL (p=0.046, HR 0.257 [95% CI 0.68-0.961]) and cardiac-related events (p=0.029, HR 0.95 [95% CI 0.11-0.791]) were predictors of a worse prognosis. In multivariate Cox analysis only cardiac-related events were independent predictors of mortality in follow-up (p=0.029, HR 0.095 [95% CI 0.11-0.791]). Kaplan-Meier analysis showing all-cause mortality and MACE comparing patients with successful versus partially successful closure is presented in Figure 4.
Percutaneous closure of PVLs has emerged as an attractive alternative to cardiac surgery, particularly in patients who have undergone multiple previous prosthesis implantations, with lower morbidity and mortality rates compared to surgical series.8–10
PVL closure is a complex and technically demanding procedure, although the introduction of 3D TEE has led to some improvement in the delivery and deployment of devices for mitral PVLs. Furthermore, since it is not a common procedure, clinical experience in centers is limited.11–13
A recently published meta-analysis of the combined experience in the UK and Ireland revealed a success rate of 91%. In 308 PVL closure procedures in 259 patients, PVL improved post-procedure and there was no regurgitation in 33.3% of patients, mild regurgitation in 41.4%, moderate in 18.6% and severe in 6.7%. In this series, hospital mortality among elective patients was 2.9% and 6.8% for urgent cases, very low compared to the current gold standard of surgical reoperation, which is associated with in-hospital and 30-day mortality of 8.8-11.5%.13 Moreover, after repeat surgery, the probability of recurrence of PVLs is 16-37%, increasing after each redo operation.4,5 In our series, 30-day mortality was low and associated with infection, and the same was seen with one-year mortality (two deaths).
Successful transcatheter PVL reduction was associated with clinical improvement and reduction of functional NYHA class. However, in our series, clinical improvement was related to the degree of residual regurgitation and of residual leak. In the recent review of the UK and Ireland, the only factors independently associated with death were the degree of leak at follow-up and NYHA class at follow-up.13 Although our PVL closure success rate was 81%, an optimal result was obtained in only 65.4% of patients, with partial success in 15.4%. Cardiac-related events, reduction of functional class and clinical improvement were all associated with residual leak and residual regurgitation.
Patients with hemolytic anemia are the most challenging. We found a relationship between hemolysis and previous and multiple surgical procedures, and also with the number of attempts per patient and with partially successful or unsuccessful procedures. In an editorial comment by Gilchrist14 on a recent paper about prostheses and hemolysis,15 the author explained that hemolysis can be due to microjets causing fluid shear forces. The existence of such microjets could be why in our study there was one case of mild mitral periprosthetic regurgitation with severe hemolysis; the leak was too small to close since it was impossible to cross the defect with the guidewire.
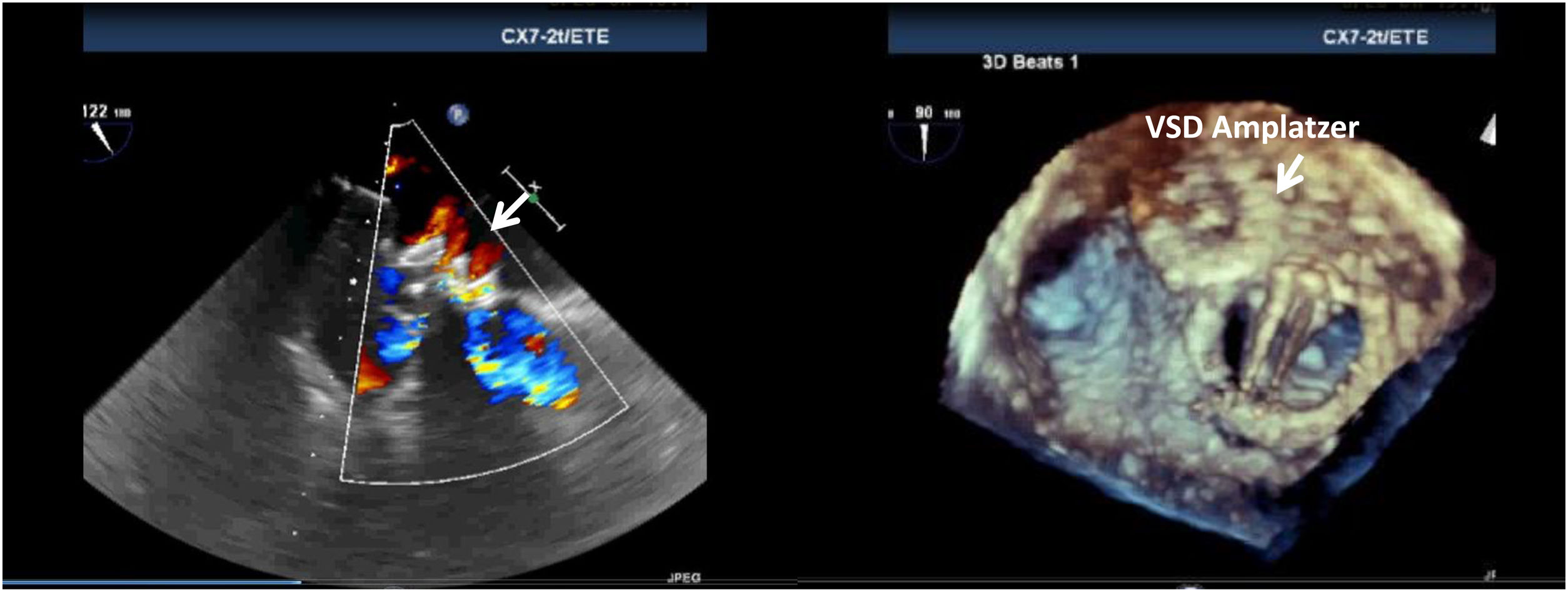
This may be also the explanation for one successful closure, the only one in which we used an Amplatzer ventricular septal defect (VSD) occluder, in a patient with severe heart failure and a very large round mitral leak that was impossible to close with two other plugs due to instability of the devices and risk of embolization. After an uneventful procedure, small peri- and intra-device jets were observed (Figure 5), and although mitral regurgitation was trivial and the patient improved clinically with reduction in brain natriuretic peptide, lactate dehydrogenase increased and haptoglobin decreased, with renal failure, prompting urgent valve surgery.16 We hypothesized that the Amplatzer VSD had some porosity that led to the hemolysis. This device is not specifically designed for leaks, and devices in mitral PVLs are under pressure between the left ventricle (higher pressure) and left atrium (low pressure), thus becoming more prone to produce these small jets with high shear stress forces.
In our series, the most commonly used device was the AVP III, which is potentially better for crescent-shaped orifices than the other devices, since it has an oval shape, multiple layers, more and thinner wires and smaller pore size. These characteristics, in comparison with the others, makes the AVP III more likely to lead to technical success.17,18 The Occlutech is a dedicated device but our experience was limited, and there are no large published series on it, mainly because it only has European CE approval.18
All of our procedures were performed under TEE guidance – 3D TEE for mitral PVLs and 2D (or 3D when available) for aortic PVLs. 3D TEE is mandatory for mitral leaks during all stages of the procedure: transseptal puncture, passage and guidance of the guidewire, passage of the sheath, release of the plug and control of the result, including the possible need for more plugs or interference with the disks of the prosthesis.11,12 In our series we found no correlation between echocardiographic parameters of anatomic characteristics of the leak and procedural success.
In our study, the procedural complication rate was low: two patients (7.6%), namely one acute tamponade and one death due to sepsis, similarly to other series.19–21 NYHA>II at follow-up affected survival, but six of our patients had compromised ventricular function (four with severe left ventricular dysfunction and two with right ventricular dysfunction), which may have influenced this result. Survival was associated with clinical improvement, reduction in NYHA functional class and optimal correction of the leak, as demonstrated by the Kaplan-Meier curves. The key to a good outcome is a good result after PVL closure with no, trivial or mild residual regurgitation22 (Videos 3 and 4).
ConclusionPercutaneous PVL closure is a challenging and demanding technique but has a reasonable success rate and low complication rates, and results are comparable to surgical treatment in high-risk patients. Predictors of procedural success are difficult to establish. Survival is associated with clinical improvement, as shown by reduction of regurgitation and improvement in NYHA functional class.
Conflicts of interestThe author has no conflicts of interest to declare.