Homozygous familial hypercholesterolemia (HoFH) is a rare disease (OMM #143890) characterized by low-density lipoprotein cholesterol receptor gene mutations and progressive atherosclerosis, leading to death before the age of 30 if not treated. The high cardiovascular risk is hard to manage.1
Fondazione Toscana Gabriele Monasterio is a cardiopulmonary tertiary-level Institute with 123 beds; >5000 hospital admissions per year, a cath-lab hub for acute coronary syndrome, adult and pediatric cardiac surgery center, referral facility for heart failure, primitive pulmonary hypertension patients and reference center for inherited dyslipidemias, rare lipid disorders with lipoprotein apheresis unit), HoFH clinical follow-up includes annually carotid artery ultrasound with intima-media thickness evaluation and stress echocardiography wall motion criteria with Doppler-derived coronary flow reserve of the mid-distal left anterior descending artery (LAD). This test is a valid tool for assessing inducible myocardial ischemia, LAD stenosis, and coronary microvascular dysfunction.2
In view of the availability of new lipid lowering therapy (LLT), their role in coronary plaque and heart valves calcification is a particularly intriguing issue. HoFH represents per se a clinical model to assess these LLT interactions.
Since 2013, we have followed four siblings with compound heterozygotes for LDL-receptor gene mutations.3 All patients were asymptomatic for angina and/or dyspnea, a satisfactory lipid profile was obtained adding evolocumab to high-intensity statin therapy.4 During follow-up, two subjects developed significant coronary artery disease treated with percutaneous coronary revascularization while the 37-year old female twins presented, on a dipyridamole stress echocardiography, progressive CRF reduction and increased carotid calcification. Both subjects underwent computed tomography coronary angiography with evidence of diffuse non-critical calcific atherosclerotic disease (see Figure 1 and Figure 2).
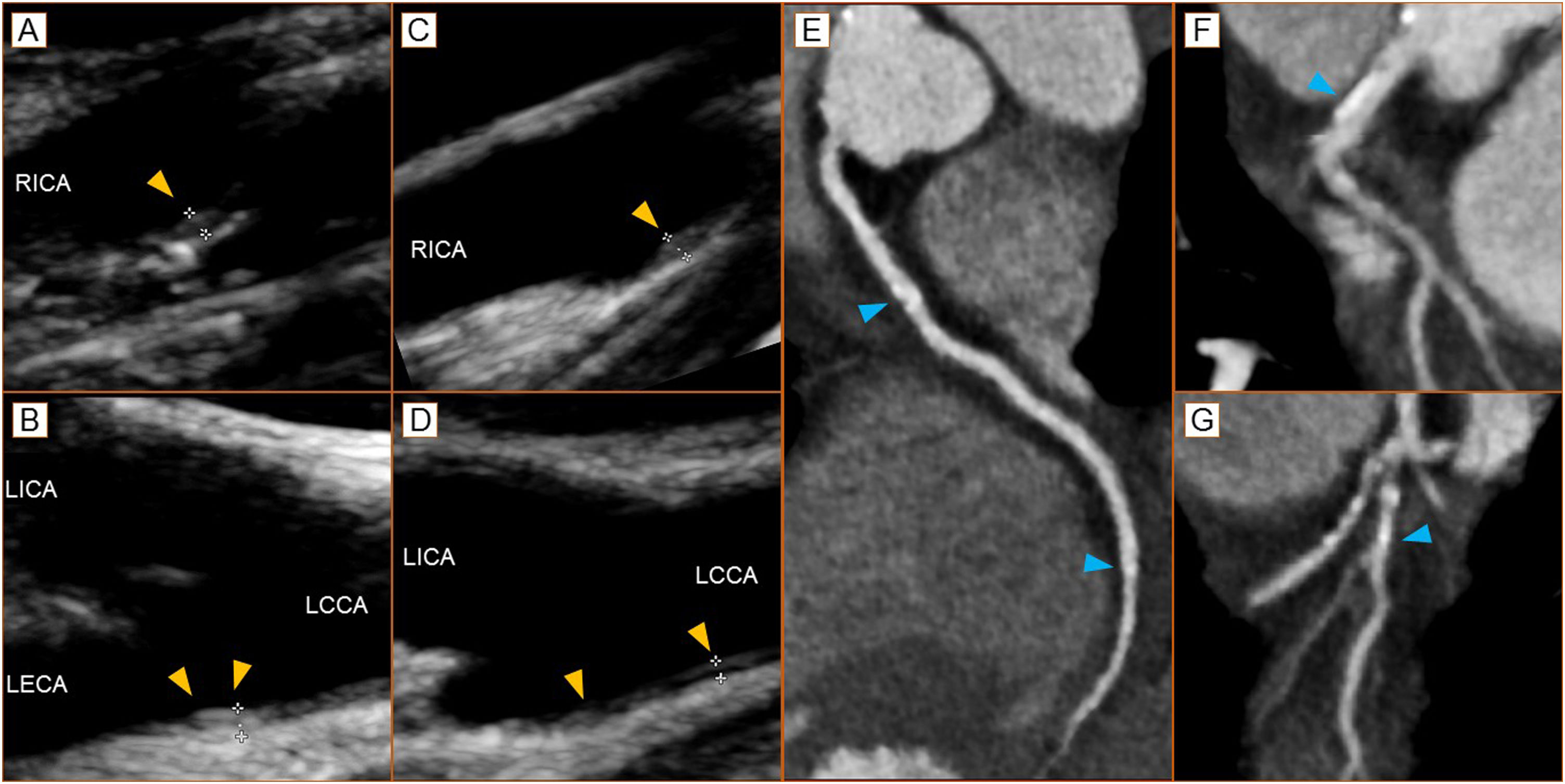
Panel A - baseline focal intimal hyperplasia (1 mm - arrowhead) of the right carotid bulb (long axis view); Panel B - baseline focal intimal hyperplasia (1.1 mm - arrowheads) of the left carotid bulb (long axis view); Panel C - 2 years’ follow-up of focal intimal hyperplasia (1 mm - arrowhead) of the right carotid bulb (long axis view); Panel D - 2 years follow-up with hyper-echogenicity and dimensional reduction (0.8 mm - arrowheads) at left carotid bulb (long axis view). We recorded a significant reduction in Doppler-derived coronary flow reserve (from 2.57 to 1.90). Panel E - right coronary artery at CT coronary angiography with focal, eccentric calcified plaques (arrowheads); Panel F - calcified eccentric plaque of the common trunk of the left coronary artery (arrowhead); Panel G - calcified eccentric plaque of the diagonal branch of the left coronary artery (arrowheads). Agatson score 93.
LCCA: left common carotid artery; LECA: left external carotid artery; LICA: left internal carotid artery; RICA: right internal carotid artery.
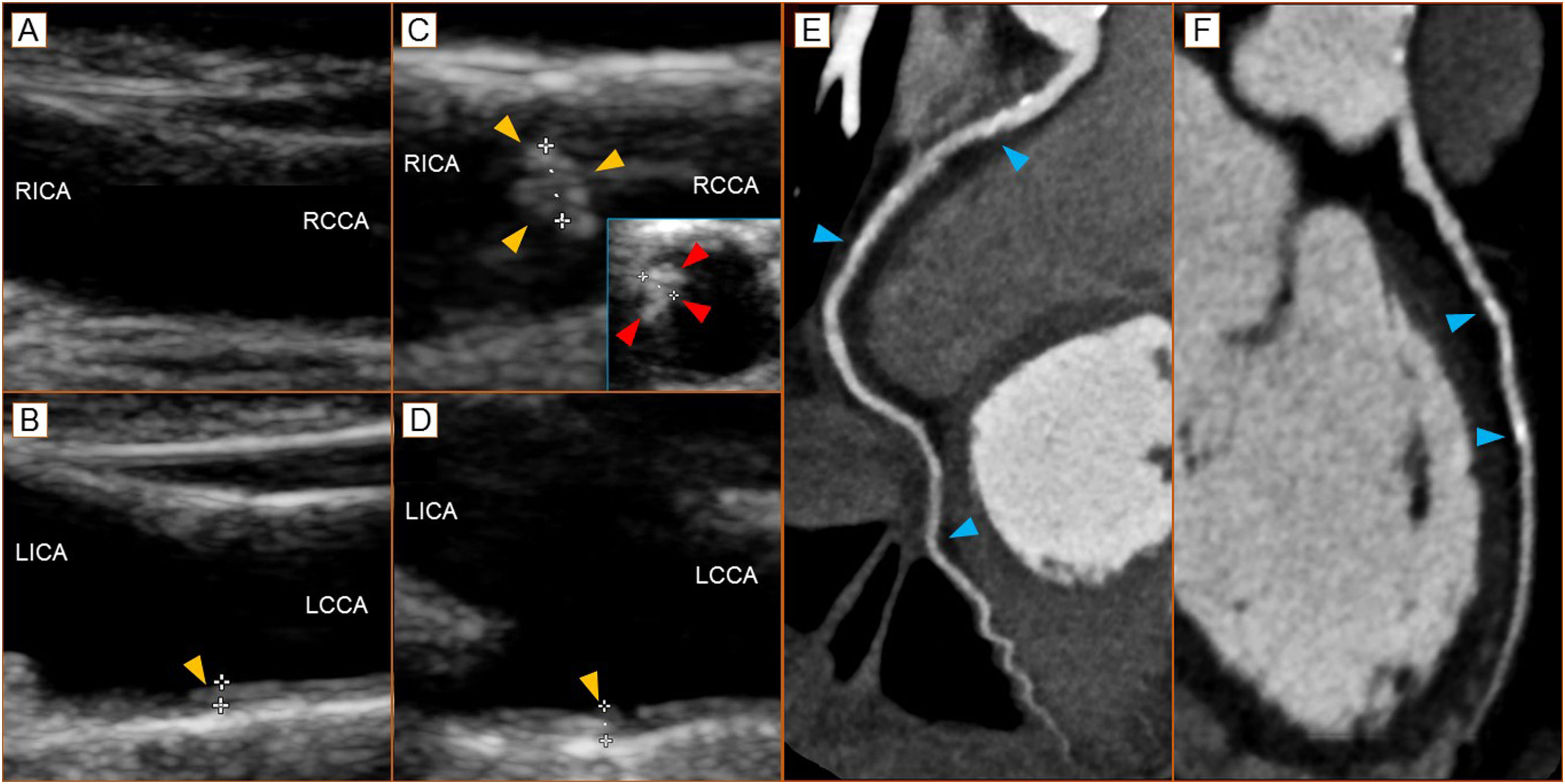
Panel A - baseline right carotid bulb (long axis view); Panel B - baseline focal intimal hyperplasia (1.0 mm - arrowhead) of the left carotid bulb (long axis view); Panel C - 2 years’ follow-up of the right carotid bulb with presence of eccentric calcified plaque (1.4 mm - long axis - yellow arrowheads; short axis view - red arrowheads); Panel D - 2 years follow-up of focal intimal hyperplasia (1.1 mm - arrowhead) of the lest carotid bulb (long axis view). To note the we recorded a significant reduction in Doppler-derived coronary flow reserve (from >2.00 to 1.74). Panel E - right coronary artery at CT coronary angiography with focal, eccentric calcified plaques (arrowheads); Panel F - calcified eccentric plaque of the anterior descending artery of the left coronary artery (arrowheads). Agatson score 69.
LCCA: left common carotid artery; LICA: left internal carotid artery; RCCA: right common carotid artery; RICA: right internal carotid artery.
Familial hypercholesterolemia shows altered endothelial function and reduced coronary vasodilatation capacity with subsequent coronary atherosclerosis due to vascular calcification. While the role of lipoproteins is well characterized in the pathogenesis of atherosclerotic plaques, their roles in plaque calcification are not well understood. In the arterial intima calcification process it is assumed that a role of spotty early-stage calcification perpetuates the inflammatory cycle leading to plaque instability followed by plaque macrocalcification.5 Statin therapy, which reduces cardiovascular mortality, has been shown to exert its dual actions on plaque morphology, i.e., regression of atheroma and increment of macroscopic calcium deposits,6 while the PCSK9i effect is not univocal: for evolocumab there is evidence of inverse correlation between changes in LDL cholesterol and coronary plaque calcification.7 Meanwhile alirocumab has been able to progressively reduce carotid plaque lipid core increasing the plaque fibrous tissue.8
We decided to intensify the high-intensity statin therapy opting for rosuvastatin because, compared to atorvastatin, it might be more effective at reducing coronary atherosclerotic plaque volume, even if there is a lack of long-term evidence on cardiovascular outcomes.9
The cases reported herein underline the difficulty of managing patients at high risk to develop coronary heart disease as HoFH, with respect to “null receptor” HoFH in which, instead, the lipoprotein apheresis treatment, keep the patient free of cardiovascular events and allows the complete regression of lipid deposition.10
Funding sourcesNo financial support was received.
Conflicts of interestThe authors have no conflicts of interest to declare.
None.