To assess one-year outcomes in patients with persistent and long-standing persistent atrial fibrillation (AF) treated by catheter ablation.
MethodsA retrospective observational study was conducted of consecutive patients referred for catheter ablation of persistent or long-standing persistent AF between May 2016 and October 2018. Patients underwent two different ablation strategies: pulmonary vein isolation (PVI) plus complex fractionated atrial electrograms (CFAE) (from May 2016 to June 2017) or a tailored approach (from July 2017 to October 2018). The overall recurrence rate at one year was analyzed. The secondary endpoint was arrhythmia recurrence according to the type of AF (persistent vs. long-standing persistent AF) and according to the ablation strategy employed.
ResultsDuring the study period, 67 patients were included (40% with long-standing persistent AF). During a mean follow-up of 16±6 months, 27% of the patients had arrhythmia recurrence. Patients with long-standing persistent AF had a higher recurrence rate than those with persistent AF (44.4% vs. 15%, p=0.006), while patients who underwent a tailored approach presented better outcomes than those undergoing PVI plus CFAE ablation (17.5% vs. 40.7%, p=0.024). Ablation strategy (HR 6.457 [1.399-29.811], p=0.017), time in continuous AF (HR 1.191 [1.043-1.259], p=0.010) and left atrial volume index (HR 1.160 [1.054-1.276], p=0.002) were independent predictors of arrhythmia recurrence.
ConclusionCatheter ablation is an effective treatment for patients with persistent and long-standing persistent AF. Patients with persistent AF and those undergoing a tailored approach presented lower arrhythmia recurrence.
Avaliar os resultados a um ano da ablação por cateter na fibrilhação auricular (FA) persistente e persistente de longa duração.
MétodosEstudo retrospetivo observacional de doentes referenciados para a ablação de FA persistente e persistente de longa duração de maio de 2016 a outubro de 2018. Os doentes foram submetidos a duas estratégias: isolamento de veias pulmonares (PVI) e ablação de eletrogramas auriculares fracionados e complexos (CFAE) (de maio de 2016 a junho de 2017) ou a uma abordagem individualizada (de julho de 2017 a outubro de 2018). Foi avaliada a taxa de recidiva a um ano de seguimento. Os objetivos secundários consistiram na avaliação da taxa de recidiva de acordo com o tipo de FA (persistente versus persistente de longa duração) e de acordo com a estratégia de ablação realizada.
ResultadosDurante o período de estudo, 67 doentes foram incluídos (40% dos quais com FA persistente de longa duração). Durante o seguimento médio de 16±6 meses, observou-se recorrência de arritmia em 27% dos doentes. Os doentes com FA persistente de longa duração apresentaram uma maior taxa de recidiva comparativamente aos doentes com FA persistente (44,4% versus 15%, p=0,006). Por sua vez, os doentes submetidos a uma abordagem individualizada apresentaram uma menor recidiva de arritmia, em comparação com os doentes submetidos a PVI+CFAE (17,5% versus 40,7%, p=0,024). A estratégia de ablação (HR 6,457 [1,399–29,811] p=0,017), o tempo em FA (HR 1,191 [1,043–1,259] p=0,010) e o volume indexado da aurícula esquerda (HR 1,160 [1,054–1,276] p=0,002) foram preditores independentes de recidiva de arritmia.
ConclusãoA ablação por cateter é um procedimento eficaz em doentes com FA persistente e persistente de longa duração. Doentes submetidos a uma ablação individualizada e doentes com FA persistente apresentaram uma menor taxa de recidiva.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in clinical practice.1 It is independently associated with increased risk of death, heart failure (HF), stroke and hospitalization, and reduced quality of life.2–4 Catheter ablation has become a first-line treatment for symptomatic AF refractory to drugs5–8 and has recently demonstrated a particularly favorable impact in HF patients.9–14 However, there is less evidence supporting catheter ablation for persistent and long-standing persistent AF.5 Although various ablation strategies in addition to pulmonary vein isolation (PVI) have been employed, the recurrence rate remains significant in patients with non-paroxysmal AF.7 Moreover, there is a lack of national data in Portugal concerning results of catheter ablation in patients with persistent and long-standing persistent AF.
The objective of this study was to assess one-year outcomes in patients with persistent and long-standing persistent AF treated by catheter ablation.
MethodsStudy design and settingA retrospective observational study was performed of consecutive patients referred for catheter ablation of persistent or long-standing persistent AF between May 2016 and October 2018 in a tertiary referral center. Procedural endpoints and one-year follow-up results were assessed.
All patients provided written informed consent and the study complied with the Declaration of Helsinki.
Patient eligibility criteriaPatients were eligible for inclusion in the study if they presented drug-refractory persistent or long-standing persistent AF or intolerance to antiarrhythmic drug (AAD) therapy, and had undergone radiofrequency (RF) catheter ablation.
Persistent AF was defined as AF lasting between seven days and one year, and long-standing persistent AF as AF lasting for >1 year before a decision to attempt rhythm control was made.5
Exclusion criteria were age <18 years and the presence of thrombus.
Data required for cohort characterization were collected from patients’ clinical records.
Electrical cardioversion was performed in all patients before intervention to reduce the time in AF, and consequently a significant number of patients were in sinus rhythm (SR) at the time of intervention.
Ablation procedureAll procedures were conducted under general anesthesia and patients suspended AAD therapy at least five half-lives before the procedure. All patients were under oral anticoagulation for at least two months prior to the procedure. In patients under vitamin K antagonists (VKAs), the medication was continued in the periprocedural period with an international normalized ratio within the 2.0-3.0 range. In patients taking non-VKAs the last drug dose was omitted. The presence of intracardiac thrombus was excluded prior to the procedure by either transesophageal echocardiography or computed tomography (CT). During the procedure, unfractionated heparin was administered immediately after transseptal puncture and adjusted as needed for a target activated clotting time >300 s. Anatomical mapping data were collected using a 3D mapping system with CARTO 3 (Biosense Webster, Irvine, CA, USA), or NavX Precision (Abbott, IL, USA) and integrated with a CT imaging reconstruction of the left atrium, when available. Ablation lines were created with point-by-point RF energy using an irrigated tip contact force-sensing catheter, in power-controlled mode with temperature limited to 43°C. Power settings varied according to the targeted region.
During the study period, patients underwent two different AF ablation strategies: PVI plus complex fractionated atrial electrogram (CFAE) ablation (Group 1), from May 2016 to June 2017; and a tailored approach (Group 2), from July 2017 to October 2018.
Group 1: pulmonary vein isolation plus complex fractionated atrial electrogram ablationIf patients were in SR in the beginning of the procedure, bipolar voltage mapping was performed (criteria for healthy and scarred tissue >0.5 mV and <0.2 mV, respectively). Left atrial (LA) voltage mapping was considered adequate when at least 500 points were collected. Wide PVI was then performed. However, if patients were in AF at the beginning of the procedure, CFAE mapping (automatic algorithms based on bipolar recordings with acquisition of dV/dT over 5 s, CFAE being defined as a mean cycle length of <120 ms) was performed, which was used to guide a wide point-by-point PVI. The detailed technique for ablating CFAE using the automated mapping software has been described and validated previously.15 The procedure terminated if conversion to SR occurred. After performing PVI, if the patient remained in AF or if it was inducible, ablation was performed in CFAE located outside the PVI lines. When AF was converted to a regular atrial arrhythmia, this was mapped and ablated. If patients were still in AF at the end of the procedure, electrical cardioversion was performed. If typical flutter was present or previously documented, a cavotricuspid isthmus (CTI) line was also created.
Group 2: tailored approachIf patients were in SR, LA bipolar voltage mapping was performed (acquiring at least 500 points). Voltage criteria for healthy and scarred tissue were >0.5 mV and <0.2 mV, respectively. Wide point-by-point PVI was then carried out. If patients were in AF at the beginning of the procedure, PVI was performed first. Electrical cardioversion was subsequently performed if patients were still in AF after PVI, and the voltage map was obtained in SR. After PVI, low voltage areas were ablated to achieve tissue homogenization. If necessary, lines were created connecting these areas of scar. Bidirectional block was confirmed after these lines were created. If low voltage areas were absent but AF inducibility persisted, CFAE mapping was performed followed by ablation. If AF was converted to a regular atrial arrhythmia, this was mapped and ablated. Likewise, If typical flutter was present or previously documented, a CTI line was also created. If AF persisted or was still inducible at the end of the procedure, electrical cardioversion was performed.
Study endpointsThe primary endpoint was one-year arrhythmia recurrence in patients referred for non-paroxysmal AF ablation, defined by the documentation of at least 30 s of atrial arrhythmia, irrespective of symptoms, in accordance with the expert consensus statement on catheter and surgical ablation of atrial fibrillation.16
We also assessed one-year freedom from arrhythmia according to the strategy employed and the AF pattern (persistent or long-standing persistent).
Follow-upAfter the index procedure, patients were followed for a minimum of 12 months. Patients were assessed before discharge and at three, six and 12 months after the procedure. Transthoracic echocardiography and 24-hour Holter monitoring were performed before discharge. Information collected during follow-up included a 12-lead electrocardiogram and a 24-hour Holter at each appointment. Seven-day Holter monitoring was performed at least once per year and a transthoracic echocardiogram at six or 12 months. At discharge AAD therapy was prescribed according to patient characteristics and operators’ decision. The first three months post-procedure were considered as a blanking period. Recurrence of atrial fibrillation was defined as the documentation of at least 30 s of atrial fibrillation, atrial tachycardia, or atrial flutter, irrespective of symptoms, in accordance with the expert consensus statement on catheter and surgical ablation of atrial fibrillation16 and a previously ineffective but tolerated class I or class III (sotalol) drug was the preferred option. Anticoagulation strategy after the first three months was based on the CHA2DS2Vasc and HAS-BLED scores.
Statistical analysisStatistical analysis was performed using IBM SPSS Statistics version 25 (IBM, Armonk, New York). Categorical variables are expressed as frequencies and percentages and continuous variables are expressed as mean ± standard deviation or median and interquartile range (IQR) for variables with or without normal distribution, respectively. The chi-square test was used to assess differences between categorical variables and the Student's t test or the Wilcoxon test were used to compare continuous variables with or without normal distribution, respectively. The Kolmogorov-Smirnov test was used to test for normality of distribution of continuous variables. Cox regression was used to assess hazard ratios of variables regarding atrial fibrillation recurrence. Multivariate analysis was performed using the forward stepwise method. Kaplan-Meier curves and the log-rank test were used to assess and compare freedom from arrhythmia recurrence during follow-up. Statistical significance was accepted for p values <0.05.
ResultsBaseline characteristics and procedural detailsDuring the enrollment period, a total of 67 patients fulfilled our inclusion criteria and were included in this study (58% male, mean age 59±11 years). Their main baseline characteristics and echocardiographic parameters are described in Table 1. Forty percent of the patients had long-standing persistent AF. Mean LA volume index was 45±10 ml/m2 and mean left ventricular ejection fraction was 49±14%.
Characteristics of patients with persistent and long-standing persistent AF undergoing catheter ablation.
| Total (n=67) | Persistent AF (n=40, 60%) | Long-standing persistent AF (n=27, 40%) | p | Group 1 (n=27, 40% | Group 2 (n=40, 60%) | p | |
|---|---|---|---|---|---|---|---|
| Age, years | 59±11 | 58±11 | 62±10 | 0.13 | 60±12 | 60 ±10 | 0.88 |
| Male | 39 (58%) | 21 (53%) | 18 (67%) | 0.32 | 17 (63%) | 22 (55%) | 0.80 |
| Time since AF diagnosis, months | 45 (16-85) | 18 (12-37) | 85 (70-120) | <0.001 | 64 (18-83) | 44 (14-88) | 0.80 |
| Continuous AF duration, months | 10 (7-19) | 8 (6-9.8) | 20 (18-28) | <0.001 | 10 (8-24) | 10 (7-19.8) | 0.34 |
| Body mass index, kg/m2 | 29.2±4.4 | 29.3±4.6 | 29.0±4.1 | 0.81 | 28.6±5.3 | 29.3±3.7 | 0.41 |
| Hypertension | 44 (65.7%) | 23 (57.5%) | 21 (77.8%) | 0.028 | 14 (51.9%) | 30 (75%) | 0.17 |
| Dyslipidemia | 31 (46.3%) | 17 (42.5%) | 14 (51.9%) | 0.60 | 10 (37%) | 21 (52.5%) | 0.12 |
| Diabetes | 9 (13.4%) | 6 (15%) | 3 (11.1%) | 0.47 | 2 (7.4%) | 7 (17.5%) | 0.15 |
| Stroke history | 3 (4.5%) | 1 (2.5%) | 2 (7.4%) | 0.55 | 1 (3.7%) | 2 (5%) | 0.98 |
| Heart failure | 31 (46.3%) | 14 (35%) | 17 (63%) | 0.033 | 12 (44.4%) | 19 (47.5%) | 0.79 |
| NYHA I | 11 (35.5%) | 6 (42.9%) | 5 (29.4%) | 4 (33.3%) | 7 (36.8%) | ||
| NYHA II | 18 (58.1%) | 6 (42.9%) | 12 (70.6%) | 7 (58.3%) | 11 (57.9%) | ||
| NYHA III | 2 (6.5%) | 2 (14.3%) | 0 | 1 (8.3%) | 1 (5.3%) | ||
| Hyperthyroidism | 5 (7.5%) | 3 (7.5%) | 2 (7.4%) | 0.99 | 3 (11.1%) | 2 (5%) | 0.37 |
| Hypothyroidism | 7 (10.4%) | 4 (10%) | 3 (11.1%) | 0.88 | 3 (11.1%) | 4 (10%) | 0.88 |
| Creatinine clearance, ml/min | 107±39.7 | 111±37.9 | 99.9 ±42.9 | 0.32 | 112.7±51.4 | 103±30.1 | 0.40 |
| BNP at admission | 128±219 | 112±161 | 137±250 | 0.66 | 129±160 | 127±253 | 0.96 |
| CHA2DS2VASc score | 2.1±1.3 | 1.9±1.4 | 2.4±1.2 | 0.08 | 1.9±1.2 | 2.2±1.3 | 0.90 |
| HASBLED score | 1.3±0.9 | 1.1±0.8 | 1.7±0.9 | 0.002 | 1.3±1 | 1.3±0.9 | 0.46 |
| LA volume index, ml/m2 | 45±10 | 42 ±10 | 49±9 | 0.004 | 45±11 | 44±10 | 0.90 |
| LVEF, mean | 49±14% | 48±12 | 49±17 | 0.91 | 55±10 | 46±15 | 0.90 |
| • LVEF<40% | 14 (20.1%) | 9 (22.5%) | 5 (18.5%) | 0.26 | 4 (14.8%) | 10 (25%) | 0.57 |
| Only PVI performed | 21 (31%) | 14 (35%) | 7 (26%) | 0.07 | 9 (33%) | 12 (30%) | 0.32 |
| Patients with low voltage areas beyond PV | 35 (52%) | 21 (53%) | 14 (52%) | 0.73 | 12 (44%) | 23 (58%) | 0.15 |
| Atrial arrhythmia recurrence | 18 (26.9%) | 6 (15%) | 12 (44.4%) | 0.006 | 11 (40.7%) | 7 (17.5%) | 0.024 |
AF: atrial fibrillation; BNP: brain natriuretic peptide; LA: left atrial; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association class; pulmonary veins; PVI: pulmonary vein isolation.
Although electrical cardioversion had been performed before ablation, 57% of patients were in AF at the beginning of the procedure. Low voltage areas beyond the pulmonary veins were present in 52% of patients, covering a median area of 14.5 (IQR 3.2-27.1) cm2. Almost one third of patients (31%) only underwent PVI. At the end of the procedure, 73% of patients were in SR and the others required electrical cardioversion to restore SR. Approximately half of the patients (49%) were discharged without AAD. Procedural details are presented in Table 2.
Procedural characteristics.
| n (%) | |
|---|---|
| At the beginning of the procedure | |
| AF, n (%) | 38 (57%) |
| Sinus rhythm, n (%) | 29 (43%) |
| CFAE ablation, n (%) | 8 (12%) |
| Scar homogenization, n (%) | 18 (29%) |
| CTI line, n (%) | 19 (28%) |
| Lines (other than CTI), n (%) | 31 (46%) |
| Median low voltage area beyond PV, cm2 | 14.8 (3.2-27.1) |
| At the end of the procedure | |
| SR, n (%) | 49 (73%) |
| Need for cardioversion, n (%) | 18 (27%) |
| AF still induced, n (%) | 19 (33%) |
| Mean procedure time, min | 133±55 |
| Mean ablation time, min | 29±6 |
| Mean fluoroscopy time, min | 7±3 |
| Mean fluoroscopy dose, μGy/m2 | 454±283 |
AF: atrial fibrillation; CFAE: complex fractionated atrial electrograms; CTI: cavotricuspid isthmus; PV: pulmonary veins; SR: sinus rhythm.
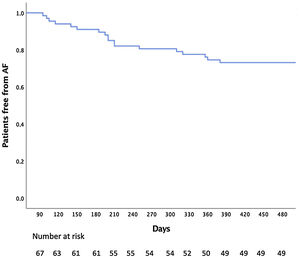
Mean follow-up was 16±6 months. After one procedure, atrial arrhythmia recurrence was observed in 27% of the patients (60% of them under AAD) (Figure 1). The majority of the recurrences were in atrial fibrillation (55%).
Persistent vs. long-standing persistent atrial fibrillationPatients with long-standing persistent AF had a longer history of AF (85 vs. 18 months, p<0.001), longer continuous AF duration (20 vs. 8 months, p<0.001), more hypertension (77.8% vs. 57.5%, p=0.028), more HF (63% vs. 35%, p=0.033), an enlarged left atrium (49 ml/m2 vs. 42 ml/m2, p=0.004) and a higher HASBLED score (1.7 vs. 1.1, p=0.002) compared with patients with persistent AF (Table 1). There were no differences between the groups concerning the presence of low-voltage areas beyond the pulmonary veins (53% for persistent AF vs. 52% for long-standing persistent AF, p=0.73). Although the proportion of patients who underwent PVI only was numerically higher in the persistent group, this did not reach statistical significance (35% for persistent AF vs. 26% for long-standing persistent AF, p=0.07).
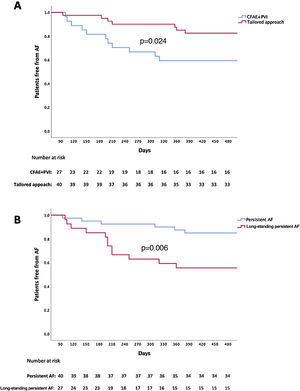
During follow-up, patients with long-standing persistent AF had a higher recurrence rate (44.4% vs. 15%, p=0.006) than patients with persistent AF. (Figure 2).
Comparison between ablation strategiesThere were no significant differences between the baseline characteristics of patients treated by the different ablation strategies, including in the proportion of long-standing persistent AF patients (44.4% for PVI plus CFAE vs. 37.5% for a tailored strategy, p=0.57), the proportion of patients who underwent PVI only (33% for PVI plus CFAE vs. 30% for a tailored strategy, p=0.32) and the presence of low-voltage areas (44% for PVI plus CFAE vs. 58% for a tailored strategy, p=0.15) (Table 1).
Patients undergoing a tailored approach had a lower arrhythmia recurrence rate than patients treated by PVI plus CFAE (17.5% vs. 40.7%, p=0.024) (Figure 2).
Predictors of recurrenceThe predictors of recurrence are detailed in Table 3. Multivariate modeling indicated that continuous duration of AF (hazard ratio [HR] 1.191 [1.043-1.259], p=0.010), ablation strategy (HR 6.457 [1.399-29.811], p=0.017) and LA volume index (HR 1.160 [1.054-1.276], p=0.002) were associated with a higher risk of arrhythmia recurrence.
Univariate and multivariate predictors of recurrence.
| Univariate model | Multivariate modela | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age | 0.986 (0.945-1.028) | 0.51 | ||
| Gender (male/female) | 1.982 (0.782-5.023) | 0.15 | ||
| Body mass index | 1.094 (0.971-1.232) | 0.14 | ||
| AF history | 1.006 (1.002-1.009) | 0.002 | ||
| Continuous duration of AF | 1.144 (1.080-1.223) | <0.001 | 1.191 (1.043-1.359) | 0.010 |
| Hypertension | 2.610 (0.756-9.009) | 0.13 | ||
| Type 2 diabetes | 1.351 (0.381-4.785) | 0.64 | ||
| Heart failure | 1.916 (0.739-4.975) | 0.18 | ||
| Hypothyroidism | 0.493 (0.066-3.705) | 0.49 | ||
| Hyperthyroidism | 4.139 (1.353-12.658) | 0.013 | ||
| CrCl | 1.015 (0.975-1.025) | 0.33 | ||
| BNP at admission | 1.016 (1.005-1.021) | 0.048 | ||
| LVEF | 1.006 (0.958-1.057) | 0.80 | ||
| LA volume | 1.121 (1.071-1.174) | <0.001 | 1.160 (1.054-1.276) | 0.002 |
| CFAE ablation | 2.811 (0.688-11.489) | 0.15 | ||
| Presence of low voltage area beyond PV | 2.714 (0.65-30.3) | 0.09 | ||
| Low voltage ablation | 1.966 (0.381-10.138) | 0.42 | ||
| Sinus rhythm at the end of the procedure | 0.267 (0.103-0.692) | 0.007 | ||
| AF induced at the end of the procedure | 8.137 (2.607-25.398) | <0.001 | ||
| Antiarrhythmic drug prescription at discharge | 0.233 (0.076-0.710) | 0.005 | ||
| PVI plus CFAE vs. tailored approach | 2.832 (1.097-7.353) | 0.031 | 6.457 (1.399-29.811) | 0.017 |
| Persistent/long-standing persistent AF | 3.597 (1.346-9.609) | 0.011 | ||
AF: atrial fibrillation; BNP: brain natriuretic peptide; CFAE: complex fractionated atrial electrograms; CI: confidence interval; CrCl: creatinine clearance; HR: hazard ratio; LA: left atrial; LVEF: left ventricular ejection fraction; PV: pulmonary veins; PVI: pulmonary vein isolation.
Variables included in Cox regression analysis with forward selection were AF history, continuous duration of AF, hyperthyroidism, BNP at admission, LA volume, sinus rhythm at the end of the procedure, AF induced at the end of the procedure, antiarrhythmic drug prescription at discharge, PVI plus CFAE vs. tailored approach and persistent/long-standing persistent AF.
There was one case of moderate pericardial effusion, without need for pericardiocentesis, only requiring more days of hospitalization.
DiscussionTo our knowledge, this is the first Portuguese study assessing the outcomes of catheter ablation in patients with persistent and long-standing persistent AF. The main findings of this study are: (i) catheter ablation is an effective treatment in these patients, with an overall success rate of 73% at 16±6 months of follow-up with one procedure; (ii) the tailored approach presented better arrhythmia-free survival; and (iii) patients with long-standing persistent AF had worse outcomes than patients with persistent AF.
The pathophysiological mechanisms responsible for electrical and structural remodeling of the atria in persistent AF are not fully understood,17 and consequently the optimal ablation strategy remains unclear. According to the recent European guidelines, PVI remains the goal even in patients with persistent AF.5 The evidence for this derives mainly from the STAR AF II study, in which performing either linear ablation or CFAE ablation in addition to PVI did not significantly improve freedom from AF, compared to PVI alone.16,18 However, in our study, overall arrhythmia-free survival with only one procedure was 73% at 16 months of follow-up, which is better than the results obtained in STAR AF II.18 This is in line with the results of other studies.12,19–22 Nevertheless, the use of contact-force sensing catheters,9,23,24 indices for predicting lesion formation,25,26 multielectrode mapping catheters27 and new activation mapping software28–30 may have contributed to the results obtained in our study.
The tailored approach was superior to the PVI plus CFAE ablation strategy. Although some studies have reported good outcomes with CFAE ablation, these results have been difficult to reproduce.16,31–33 Although our results with PVI plus CFAE ablation were superior to those obtained in other studies,34 the tailored approach led to 82.5% freedom from arrhythmia in patients with persistent and long-standing persistent AF, suggesting that an individualized approach was superior to PVI plus CFAE ablation, in line with reports from other groups.35 However, we should point out that the two strategies were performed at different times, hence the above-mentioned technological developments may have contributed to the differences observed. Nevertheless, despite this, the tailored approach remained an independent predictor of freedom from arrhythmia. Since the DECAAF study, evidence has emerged correlating the degree of atrial fibrosis with arrhythmia recurrence after AF ablation.36–40 It therefore seems logical to target these low-voltage areas when they are present. Several studies have shown improved outcomes after ablation of low-voltage areas in addition to PVI.40–43 Moreover, in our study, this approach meant that 30% of patients did not undergo ablation beyond PVI, avoiding excessive ablation, as supported by the results of the STABLE-SR trial.42
Our study also adds to the evidence that the longer in AF, the worse the outcome. Patients with long-standing persistent AF presented a significantly worst outcome than those in persistent AF, in line with the findings of previous studies.16,34,44 Also, in our study, patients with long-standing persistent AF were more likely to present hypertension, HF and an enlarged left atrium, all of which have been reported as independent predictors of recurrence.5,16
Study limitationsWe acknowledge several limitations of our work. First, this was a retrospective study involving a relatively small number of patients, and therefore comparisons between subgroups and multivariate analysis may be underpowered. However, our main goal was to assess one-year arrhythmia recurrence in patients with non-paroxysmal AF ablation. Second, although both ablation strategies were clearly defined, we recognize that scar homogenization and, more importantly, CFAE ablation can be operator-dependent and these results may not be reproduced in other centers. Third, as mentioned above, the different AF strategies were performed at different times, therefore we cannot rule out that developments in AF technology in recent years may have contributed to the results obtained in this study. Fourth, systematic monitoring using implantable loop recorders could have documented a higher rate of asymptomatic atrial fibrillation recurrence. However, to minimize this possibility, every patient underwent seven-day Holter monitoring. Also, the proportion of patients with exclusively asymptomatic AF is not expected to be consistently different between the groups.
ConclusionThis study demonstrates that catheter ablation is an effective treatment for patients with persistent and long-standing persistent AF. Patients treated by a tailored approach had better arrhythmia-free survival, while those with long-standing persistent AF had worse outcomes.
Conflicts of interestP.A.S. has received speaker fees from Biosense Webster, Boston Scientific, Medtronic and Abbott. All other authors have no conflicts of interest to declare.
This article is the subject of two Editorial Comments, which reflect the views of two arrhythmologists and are published in alphabetical order of their authors.