Primary percutaneous coronary intervention (PPCI) has become the treatment of choice in patients with ST-segment elevation myocardial infarction (STEMI). Drug-eluting stents (DES) reduce restenosis compared to bare-metal stents (BMS) but there is conflicting data concerning their use in the setting of STEMI. We aimed to evaluate the influence of the type of stent on the outcomes of PPCI.
MethodsThis was a single-center longitudinal study including 213 consecutive patients (76% men, mean age 60±12 years) with STEMI undergoing PPCI between 2003 and 2007, divided into two groups: BMS (43.7%) and DES (56.3%). We assessed clinical and demographic features as well as angiographic and electrocardiographic signs of myocardial reperfusion. The composite outcome of death, myocardial infarction (MI) or target-lesion revascularization (TLR) was evaluated.
ResultsAt a median follow-up of 26 months there were no differences in the composite outcome of death/MI/TLR (BMS 18.3% vs DES 15.8%) or in the incidence of stent thrombosis. Angiographic results of the procedure were also similar. Independent predictors of the composite outcome were age (HR=1.06, 95% CI [1.02-1.11], left anterior descending artery as infarct-related vessel (HR=2.69, 95% CI [1.17-6.19]) and use of glycoprotein IIb/IIIa inhibitors (HR=0.33, 95% CI [0.13-0.83]).
ConclusionsThere was no benefit in angiographic outcomes or major cardiac events after treatment with drug-eluting stents compared to bare-metal stents in this group of patients with STEMI.
A angioplastia primária (ICPP) é o tratamento de eleição para enfarte agudo do miocárdio com elevação de ST (EAM ST). Os stents farmacológicos (DES) permitem reduzir a taxa de restenose coronária, sendo controverso o seu uso no contexto de EAM ST. O objectivo deste estudo foi avaliar os resultados clínicos da ICPP em função do tipo de stent usado (não revestido versus DES).
População e métodosEstudo longitudinal de centro único, incluindo 213 doentes consecutivos, idade média 60±12 anos, 76% homens, submetidos a ICPP no contexto de EAM ST, entre 2003 e Novembro 2007. Foram considerados 2 grupos: stent não revestidos (BMS) (43,7%) e DES (56,3%). Analisaram-se as características clínicas e demográficas dos 2 grupos, comparando-se também variáveis angiográficas, de perfusão miocárdica, grau de resolução de segmento ST pós-ICPP e pico de troponina. Determinou-se no seguimento a incidência do evento combinado: morte, enfarte do miocárdio (EAM) ou revascularização de lesão alvo (TLR).
ResultadosNo seguimento mediano de 26 meses não se encontraram diferenças no evento combinado Morte/EAM/TLR (BMS 18,3% versus 15,8%) nem na trombose de stent. Os resultados angiográficos foram também semelhantes. Os preditores independentes de morte/EAM/TLR foram a frequência cardíaca (HR=1.06 95% IC [1.02-1.11], descendente anterior como vaso culprit (HR=2.69 95% IC [1.17-6.19]) e utilização de inibidores da glicoproteína IIbIIIa (HR=0.33 95% IC [0.13-0.83]).
ConclusãoO tipo de stent utilizado não parece ter influência na ocorrência de eventos cardíacos em doentes submetidos a angioplastia primária, no contexto de EAM ST.
The use of stents in primary and rescue percutaneous coronary intervention (PCI) is superior to balloon angioplasty, with lower rates of repeat revascularization and lower mortality1,2. Drug-eluting stents (DES) have been shown to be safe and more effective than bare-metal stents (BMS) in reducing restenosis and the frequency of repeat interventions in patients undergoing elective PCI3,4. However, there are conflicting results regarding the efficacy of DES as compared to BMS in the setting of primary PCI for ST-elevation acute myocardial infarction (STEMI). In particular, late stent thrombosis due to delayed endothelization and malapposition after DES implantation raises safety concerns, since there are studies suggesting that DES are associated with an increased rate of this event compared to BMS5,6.
The aim of this study was to compare the clinical outcomes of patients presenting with STEMI treated with DES or BMS as part of primary PCI.
MethodsStudy design and data collectionThis was a single-center, longitudinal, observational study which included 213 patients treated with primary PCI for STEMI between January 2003 and November 2007. Of these, 93 were treated only with BMS and 120 were treated only with DES. All data were collected prospectively by the study investigators. Procedural data, including adjunctive pharmacology, device utilization, reference vessel diameter, pre- and post-procedural TIMI flow, lesion length and lesion characteristics were assessed by the operating interventional cardiologist.
All the angiography films were reviewed to assess the following variables: pre- and post-procedural TIMI frame count, thrombus grade and myocardial blush grade at the end of the procedure.
The electrocardiograms on admission and after the procedure were also reviewed to evaluate the degree of ST-segment resolution. Peak troponin I and CK-MB were recorded.
Patients were prospectively followed for the occurrence of major adverse cardiac events (defined as a composite of all-cause death, non-fatal myocardial infarction [MI] or target lesion revascularization [TLR]).
Post-discharge clinical follow-up was conducted by telephone interview. All data were entered into a centralized database (CardioBase®).
DefinitionsReinfarction was defined as a clinical event with any new elevation of troponin I or creatine kinase-MB above the upper reference limit and included both ST- and non ST-elevation myocardial infarction. Target vessel and target lesion revascularization were defined as any revascularization procedure of the target vessel or target lesion (from 5mm distally to the stent up to 5mm proximally to the stent), respectively. Stent thrombosis was classified as definite if a thrombus was angiographically or echographically documented within the stent, as probable in the case of unexplained death within one month of the index procedure, or as possible in the case of unexplained death within one year of the index procedure. Stent thrombosis was classified as acute if it occurred within 24hours of the index procedure, subacute if it occurred between 1 and 30 days, and late if it occurred after 30 days.
Study outcomesThe primary objective of the study was the composite outcome of all-cause death, non-fatal MI or TLR. Secondary outcomes were target vessel revascularization (TVR), stent thrombosis, final TIMI flow, final TIMI frame count, myocardial blush grade and degree of ST-segment resolution.
Statistical analysisBaseline and outcome variables were compared using the Student's unpaired t-test or the Mann-Whitney test for continuous variables, and the chi-square test or Fisher's exact test for categorical variables. The normality assumption for continuous variables was assessed by the Kolmogorov-Smirnov test. Event-free survival was computed using Kaplan-Meier estimates and compared between groups with the log-rank test. The predictors of the primary objective of the study were found by multivariate analysis with a Cox proportional hazard regression model. All p values were 2-sided, and a p value of less than 0.05 was considered statistically significant. All analyses were conducted with SPSS version 17 statistical analysis software (SPSS Inc, Chicago, Illinois).
ResultsBaseline clinical, angiographic and procedural variablesThe study population were 75.6% male, 17.5% diabetic and with a mean age of 60±12 years. Patients treated with DES were younger. All the other baseline demographics were similar between the groups (Table 1).
Baseline characteristics according to type of stent.
| Total | BMS | DES | p | |
| n | 213 | 93 (43.7%) | 120 (56.3%) | |
| Mean age±SD (years) | 60±12 | 62.2±12.3 | 58.3±11.8 | 0.014 |
| Male | 161 (75.6%) | 67 (72%) | 94 (78.3%) | 0.289 |
| Cardiovascular risk factors | ||||
| Hypertension | 118 (55.4%) | 53 (57%) | 65 (54.2%) | 0.681 |
| Diabetes | 37 (17.5%) | 14 (15.2%) | 23 (19.2%) | 0.453 |
| Smoking | 122 (57.3%) | 49 (52.7%) | 73 (60.8%) | 0.233 |
| Hyperlipidemia | 107 (50.2%) | 43 (46.2%) | 64 (53.3%) | 0.304 |
| Body mass index (median and interquartile range) | 26.2 (24-29) | 26 (24-29) | 26.4 (24.3-29) | 0.319 |
| Normal LVEF (n=200) | 167 (83.5%) | 72 (80%) | 95 (86.4%) | 0.228 |
| Previous history | ||||
| PCI | 39 (18.3%) | 18 (19.4%) | 21 (17.5%) | 0.728 |
| CABG | 8 (3.8%) | 2 (2.2%) | 6 (5%) | 0.278 |
| MI | 39 (18.3%) | 16 (17.2%) | 23 (19.2%) | 0.713 |
| Killip-Kimball 1 on admission | 171 (80.3%) | 71 (76.3%) | 100 (83.3%) | 0.204 |
| Heart rate on admission | 75.6±17.4 | 75.5±18.8 | 75.7±16.3 | 0.925 |
| Systolic arterial pressure (median and interquartile range) | 132.5 (115-159) | 135 (118.5-156) | 130 (110-160) | 0.468 |
| CKD on hemodialysis | 4 (1.9%) | 3 (3.2%) | 1 (0.8%) | 0.202 |
MI: myocardial infarction; CABG: coronary artery bypass graft; CKD: chronic kidney disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention.
DES patients more often had the left anterior descending artery as the infarct-related vessel (45.8% vs 32.3%, p=0.045) and complete revascularization (60% vs 41%, p=0.006). There were fewer coronary lesions in DES patients (2.21±1.33 vs 2.60±1.47, p=0.036) but on average more vessels were stented in this group (1.24±0.48 vs 1.10±0.30, p=0.017). The median implanted stent diameter was larger in the BMS group (3.30mm vs 3.00mm, p<0.001) (Table 2).
Procedure characteristics according to type of stent.
| Total (n=213) | BMS (n=93) | DES (n=120) | p | |
| Pain-to-balloon time, median (h:m) and interquartile range | 4:30 (3:10-7:56) | 4:20 (3:04-7:24) | 4:44 (3:16-8:09) | 0.439 |
| Infarction-related vessel=LAD | 85 (40%) | 30 (32.3%) | 55 (45.8%) | 0.045 |
| TIMI flow pre-procedure=0 | 129 (60.6%) | 60 (64.5%) | 69 (57.5%) | 0.299 |
| Number of diseased vessels | 1.83±0.82 | 1.94±0.84 | 1.75±0.80 | 0.103 |
| Number of treated vessels | 1.18±0.42 | 1.10±0.30 | 1.24±0.48 | 0.017 |
| Complete revascularization | 113 (53.1%) | 40 (43%) | 73 (60.8%) | 0.010 |
| Number of lesions | 2.38±1.40 | 2.60±1.47 | 2.21±1.33 | 0.036 |
| Number of stents implanted | 1.15±0.46 | 1.17±0.46 | 1.14±0.46 | 0.689 |
| Median stent diameter (mm) and interquartile range | 3.00 (2.75-3.50) | 3.30 (3-00-3.50) | 3.00 (2.75-3.50) | <0.001 |
| Median total stent length (mm) and interquartile range | 23 (18-28) | 19 (16-24) | 23 (18-28) | 0.133 |
| Use of GP IIb/IIIa inhibitors | 151 (71.2%) | 62 (66.7%) | 89 (74.8%) | 0.195 |
LAD: left anterior descending artery; GP IIb/IIIa: glycoprotein IIb/IIIa.
No significant differences were found in the following variables: final TIMI flow grade=3 (80.6% in BMS vs 84.2% in DES, p=0.501), corrected final TIMI frame count ≤21 (62% in BMS vs 56% in DES, p=0.088), myocardial blush grade=3 (43.5% in BMS vs 50% in DES, p=0.432), degree of ST-segment elevation resolution ≥70% (45.2% in BMS vs 45% in DES, p=0.981), peak troponin I (median and interquartile range: 74; 34-123μg/l in BMS vs 72; 22-125μg/l in DES, p=0.422) (Table 3).
Angiographic, electrocardiographic and laboratory results according to type of stent.
| Total (n=213) | BMS (n=93) | DES (n=120) | p | |
| Median troponin I peak (μg/l) and interquartile range | 73 (28-123) | 74 (34-123) | 72 (22-125) | 0.422 |
| Corrected final TIMI frame count ≤21 | 167 (78.4%) | 78 (83.9%) | 89 (74.2%) | 0.088 |
| Final TIMI flow=3 | 176 (82.6%) | 75 (80.6%) | 101 (84.2%) | 0.501 |
| Myocardial blush grade=3 (n=145) | 68 (46.9%) | 30 (43.5%) | 38 (50%) | 0.432 |
| ST-segment resolution ≥70% | 96 (45.1%) | 42 (45.2%) | 54 (45%) | 0.981 |
At 30 days, the primary outcome of death/reinfarction/TLR occurred in 12 patients (5.6%), with no differences between the two groups (5.4% in BMS vs 5.8% in DES, p=0.886). At one year the primary outcome was similar (14% in BMS vs 9.2% in DES, p=0.271). The occurrence of TLR was not statistically different (5.4% in BMS vs 2.5% in DES, p=0.273) (Table 4).
Clinical outcomes according to type of stent.
| Total (n=213) | BMS (n=93) | DES (n=120) | p | |
| Median follow-up (months) and interquartile range | 26.2 (15.5-38.4) | 17.3 (12.8-36.1) | 29.5 (18.3-39.1) | 0.004 |
| Death | 20 (9.4%) | 8 (8.6%) | 12 (10%) | 0.729 |
| Death at 30 days | 8 (3.8%) | 4 (4.3%) | 4 (3.3%) | 0.713 |
| Death at 1 year | 13 (6.1%) | 6 (6.5%) | 7 (5.8%) | 0.852 |
| Reinfarction | 18 (8.5%) | 9 (9.7%) | 9 (7.5%) | 0.571 |
| Reinfarction at 30 days | 6 (2.8%) | 1 (1.1%) | 5 (4.2%) | 0.176 |
| Reinfarction at 1 year | 14 (6.6%) | 6 (6.5%) | 8 (6.7%) | 0.950 |
| TLR | 11 (5.2%) | 5 (5.4%) | 6 (5%) | 0.902 |
| TLR at 30 days | 3 (1.4%) | 1 (1.1%) | 2 (1.7%) | 0.716 |
| TLR at 1 year | 8 (3.8%) | 5 (5.4%) | 3 (2.5%) | 0.273 |
| Death, reinfarction or TLR | 36 (17%) | 17 (18.3%) | 19 (15.8%) | 0.637 |
| Death, reinfarction or TLR at 30 days | 12 (5.6%) | 5 (5.4%) | 7 (5.8%) | 0.886 |
| Death, reinfarction or TLR at 1 year | 24 (11.3%) | 13 (14%) | 11 (9.2%) | 0.271 |
| Stent thrombosis | 6 (2.8%) | 3 (3.2%) | 3 (2.5%) | 0.731 |
TLR: target lesion revascularization.
Patients treated with DES had a longer median follow-up (29.5 vs 17.3 months, p=0.004). In the entire follow-up period there were no differences in the primary outcome (18.3% in BMS vs 15.8% in DES, p=0.637). There were also no differences between the two groups when these variables were analyzed individually (Table 4).
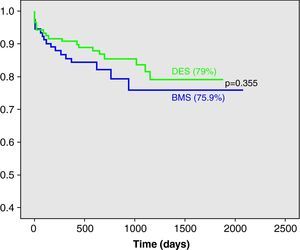
Kaplan-Meier estimates of survival free from death, MI or TLR are shown in Fig. 1; there was no significant difference between the groups during follow-up (75.9% in BMS vs 79% in DES, p=0.355 by the log rank test).
There were six cases (2.8%) of stent thrombosis, of which three occurred in the DES group (2.5%) and three in the BMS group (3.2%) (Table 5).
Characteristics of patients with stent thrombosis.
| Patient | Gender | Age | Type of stent | Days to stent thrombosis | Acute, subacute or late | Probable, possible or definite | Direct cause of death |
| #1 | Female | 61 | DES | 0 | Acute | Probable | Yes |
| #2 | Male | 52 | DES | 0 | Acute | Definite | No |
| #3 | Male | 50 | DES | 3 | Subacute | Definite | No |
| #4 | Male | 60 | BMS | 3 | Subacute | Definite | No |
| #5 | Male | 75 | BMS | 210 | Late | Definite | No |
| #6 | Male | 74 | BMS | 94 | Late | Possible | Yes |
BMS: bare-metal stent; DES: drug-eluting stent.
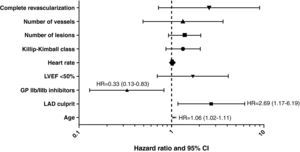
The independent predictors of the composite outcome (death/MI/TLR) in the overall population were: age (hazard ratio=1.06, 95% confidence interval [CI] 1.02-1.11), LAD as infarct-related vessel (hazard ratio=2.69, 95% CI 1.17-6.19) and use of glycoprotein IIb/IIIa inhibitors (hazard ratio=0.33, 95% CI 0.13-0.83) (Fig. 2).
Independent predictors of the primary outcome. All the predictors by univariate analysis are shown and were included in the model, but only age, LAD as the infarct-related vessel and use of GP IIb/IIIa inhibitors remained as predictors by multivariate analysis. GP: glycoprotein; LAD: left anterior descending artery; LVEF: left ventricular ejection fraction.
The data from this registry show that DES are safe in the setting of STEMI, although it demonstrates no benefit in efficacy, since there was no significant difference in the incidence of the primary outcome of death, MI or TLR at one year and during the entire follow-up.
The incidence of stent thrombosis and the angiographic and electrocardiographic variables that were assessed showed that the procedure results were similar regardless of type of stent. Although the groups were well matched in most clinical and angiographic characteristics, patients in the DES group tended to be younger and had a greater incidence of the LAD as the infarct-related vessel.
Results from registries and randomized trials have shown conflicting results. The main proven benefit of DES over BMS is a reduction in restenosis, which may be outweighed by the need for prolonged dual antiplatelet therapy and the potentially higher risk of very late stent thrombosis. Recent robust data from the HORIZONS-AMI trial demonstrate that in patients with STEMI undergoing primary angioplasty, implantation of paclitaxel-eluting stents, compared with bare-metal stents, significantly reduced angiographic evidence of restenosis and ischemia-driven repeat revascularization procedures, with no differences in death, stent thrombosis, reinfarction or stroke7. However, the PASSION trial, which compared 619 patients treated with paclitaxel-eluting stents or BMS in the setting of STEMI, found no significant difference in death, reinfarction, stent thrombosis or TLR at one year8, which is in line with the results of our study. Trials that randomized patients to sirolimus-eluting stents or BMS, such as STRATEGY, TYPHOON and SESAMI, have different results from our registry, showing a lower rate of repeat revascularization procedures, but no difference in other clinical outcomes9–11. It should be emphasized, however, that these studies on sirolimus-eluting stents all had angiographic follow-up, which may have biased the operator to intervene in the BMS group.
Late stent thrombosis is a matter of concern when DES are used in patients with STEMI, since there are published data indicating that late healing and re-endothelization may be impaired when DES are implanted at the site of a ruptured plaque, and this may lead to late stent thrombosis12. In fact, incomplete expansion and undersizing may occur more often in the setting of STEMI because of the presence of coronary thrombus. This may be more frequently associated with DES, where there is little tissue in-growth, compared with BMS, where more extensive tissue in-growth occurs and may prevent malapposition. The MISSION intervention study showed that acquired stent malapposition at 9 months was more common with DES than with BMS (25% vs 5%, p<0.001) 13. Our study found no difference in stent thrombosis between DES and BMS, which is similar to the results from several randomized trials, as shown by De Luca et al. in a recent meta-analysis of randomized trials on the efficacy and safety of DES in STEMI (1.6% in DES vs 2.2% in BMS, p=NS, at 12-month follow-up) 14. The incidence of this event in our registry is higher than in this meta-analysis but is similar to the results of HORIZONS-AMI and TYPHOON7,10. In our study most stent thromboses occurred within the first days after STEMI, which suggests that they were related to the procedure rather than the type of stent. The only two cases of late stent thrombosis were observed in the BMS group, which is surprising given the concern regarding this event and the use of DES.
A parallel finding of our study concerns the protective effect of glycoprotein IIb/IIIa inhibitors on the occurrence of death, MI and TLR in this population. Randomized trials have shown conflicting results regarding the use of glycoprotein IIb/IIIa inhibitors in this setting. A previous meta-analysis showed significant benefits in mortality and reinfarction15, but more recent large randomized trials with the adjunctive administration of clopidogrel (BRAVE-3 and HORIZONS trials) failed to demonstrate the same results16,17. One striking feature is the proportion of patients (over 70%) undergoing this therapy, in contrast to the data in the Portuguese Registry of Acute Coronary Syndromes, in which only 20% of patients with STEMI were treated with glycoprotein IIb/IIIa inhibitors18.
Our study has the limitations of a single-center registry and as such the outcomes may be influenced by confounders. For instance the larger stent diameter in the BMS group may mean that these patients had a lower risk of restenosis than the patients in the DES group, which would underestimate the benefit of DES use. Moreover, the results are based on a relatively small patient cohort and may lack power. Nevertheless the study provides outcomes of a cohort of patients with STEMI treated with BMS or DES and in addition to having a long follow-up, it takes into account important reperfusion variables such as TIMI frame count, myocardial blush grade and ECG ST-segment elevation resolution.
ConclusionsThis study found no differences in major adverse cardiovascular events or angiographic outcomes after treatment with DES or BMS in this group of patients with STEMI, which validates the safety of coated stents but shows no improvement in efficacy.
Concomitant administration of glycoprotein IIb/IIIa inhibitors still confers protection in this setting.
Conflicts of interestThe authors declare they have no conflicts of interest.