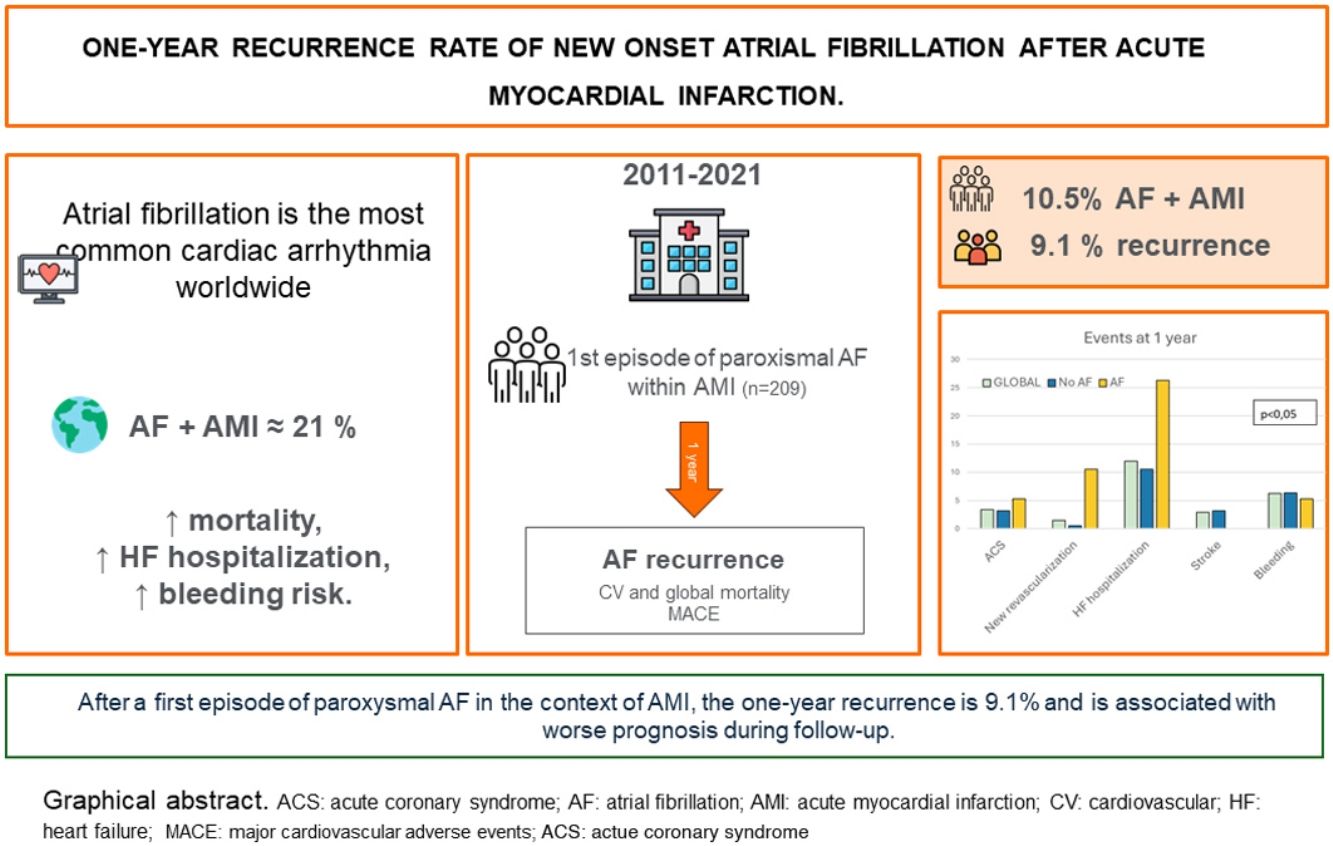
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide, with a prevalence of up to 21% in the early phase of acute myocardial infarction (AMI). Data on new-onset AF in this context are limited, and long-term prognosis remains unclear.
MethodsWe conducted a retrospective observational cohort study from December 2011 to May 2021, including patients who experienced a first episode of paroxysmal AF during hospitalization for AMI. The primary outcome was the recurrence of AF within the first year post-discharge. Secondary outcomes included all-cause mortality, cardiovascular mortality, and a composite of major adverse cardiovascular events.
ResultsA total of 209 patients were included. There was AF recurrence in 19 patients, 9.1% (95% CI 5.2–13.0%) with a median time to recurrence of 84 days (interquartile range 27.5–157.5). While mortality in the AF recurrence group was numerically higher than in the non-AF recurrence group, this difference did not achieve statistical significance (15.8% vs. 7.4%, p=0.19). Patients with AF recurrence had significantly worse prognosis (47.4% vs. 23.7%, p=0.04), primarily due to increased heart failure (HF) hospitalizations.
ConclusionsIn patients with a first episode of paroxysmal AF during AMI, one-year recurrence is relatively low (9.1%); however, AF recurrence is associated with significantly worse prognosis, driven largely by HF hospitalizations.
A fibrilhação auricular (FA) é a arritmia cardíaca mais comum a nível mundial, com uma prevalência de até 21% na fase inicial do enfarte agudo do miocárdio (EAM). Os dados relativos à FA de início recente neste contexto são limitados, e o seu prognóstico a longo prazo permanece incerto.
MétodosRealizámos um estudo de coorte observacional retrospetivo entre dezembro de 2011 e maio de 2021, incluindo doentes que apresentaram um primeiro episódio de FA paroxística durante a hospitalização por EAM. O resultado primário foi a taxa de recorrência de FA no primeiro ano após a alta hospitalar. Os resultados secundários incluíram mortalidade por todas as causas, mortalidade cardiovascular e um composto de eventos cardiovasculares adversos major.
ResultadosUm total de 209 doentes foi incluído. A recorrência de FA ocorreu em 19 doentes (9,1%, IC 95% 5,2-13,0%), com um tempo mediano até à recorrência de 84 dias [IQR 27,5-157,5]. Apesar da mortalidade no grupo com recorrência de FA ter sido numericamente superior à do grupo sem recorrência de FA, essa diferença não alcançou significância estatística (15,8% vs. 7,4%, p=0,19). Os doentes com recorrência de FA apresentaram um prognóstico significativamente pior (47,4% versus 23,7%; p=0,04), principalmente devido ao aumento das hospitalizações por insuficiência cardíaca.
ConclusõesEm doentes com um primeiro episódio de FA paroxística durante o EAM, a taxa de recorrência em um ano é relativamente baixa (9,1%), mas a recorrência de FA está associada a um prognóstico significativamente pior, principalmente devido ao aumento das hospitalizações por insuficiência cardíaca.
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide,1 and its occurrence in the setting of acute myocardial infarction (AMI) may impact prognosis. Although the prevalence of AF during AMI can reach up to 21%,2 data on new-onset AF (NOAF) in this context remain limited, and its long-term impact is not well understood. These patients are particularly complex, since both conditions confer an elevated risk of thromboembolic events, which may lead to ischemic complications. Furthermore, the use of antiplatelet and anticoagulant therapies, which are necessary to manage this risk, can exacerbate the likelihood of hemorrhagic complications.1,3
Several studies have reported a significant increase in mortality, heart failure (HF) hospitalization, and clinically relevant bleeding in patients with AMI and AF compared to those without AF.4,5 However, data specifically on NOAF in the context of AMI are limited and sometimes contradictory.2,4,6–11 Some authors suggest that NOAF may be transient and self-limiting,12 which makes the long-term antithrombotic management of these patients controversial.
The introduction of direct oral anticoagulants (DOACs) has improved the management of embolic risk in AF patients. However, anticoagulant therapy –even with the safer profiles of DOACs– carries a significant risk of bleeding, which can have consequences comparable to recurrent ischemic events in terms of morbidity, mortality, and quality of life.13 While several randomized trials have evaluated anticoagulant and antiplatelet strategies in patients with a prior history of AF and AMI,14–18 there is limited evidence on how to manage patients who develop AF for the first time during hospitalization for acute coronary syndrome (ACS).
This paucity of robust evidence is reflected in current clinical guidelines, where recommendations for antithrombotic management in this setting carry a weak level of evidence (IIaC).1
ObjectivesThe primary objective of this study was to evaluate the AF recurrence within the first year after discharge in patients with AMI who experienced a first episode of paroxysmal AF. For this study, the ‘AF recurrence group’ refers to patients who experienced a recurrence of AF during follow-up, while the ‘non-AF recurrence group’ refers to those who did not. To enhance readability, these groups will be referred to as the ‘AF group’ and ‘non-AF group’ from this point onward.
Secondary objectives include comparisons between the AF group and the non-AF group in terms of all-cause mortality, cardiovascular mortality, and a composite endpoint comprising HF hospitalization new ACS, new revascularization, stroke, and major bleeding according to the BARC criteria (see S1. Secondary objectives).19 These outcomes were chosen based on prior evidence indicating their relevance in the prognosis of patients with AF and AMI.4,5,8,20
MethodsPatients and study designA retrospective observational cohort study was conducted including consecutive patients admitted to the Cardiovascular Critical Care Unit (CCCU) of a tertiary care center from December 2011 to May 2021 with ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation myocardial infarction (NSTEMI) diagnosis who developed a first episode of paroxysmal AF during hospitalization.
Atrial fibrillation was defined according to current clinical practice guidelines1 as documented by a standard 12-lead electrocardiogram (ECG) or a single-lead ECG tracing more than 30 seconds showing a cardiac rhythm with indiscernible P waves followed by irregular RR intervals. Paroxysmal AF was defined as AF that reverted spontaneously or required intervention within the first seven days after diagnosis.1 Myocardial infarction was diagnosed by the attending physician according to current guidelines,21,22 based on evidence of myocardial damage (elevated cardiac troponin levels >99th percentile of the upper reference limit), with evidence of necrosis in a clinical context consistent with myocardial ischemia. No restrictions were made regarding the type of AMI including types 1–5 as defined by the Universal Definition of Myocardial Infarction.23 Coronary anatomy was assessed using diagnostic coronary angiography, and the number of diseased vessels and treated vessels was recorded. Complete revascularization was defined as the treatment of all vessels with a diameter >2 mm and stenosis >70%, or >50% in the case of the left main coronary artery.
Exclusion criteria included patients with a history of prior AF (paroxysmal, permanent, or persistent, even if they presented with sinus rhythm at admission), those who remained in AF at the time of discharge, and those for whom one-year follow-up was not possible. This included patients who died during hospitalization and those who were treated in another healthcare system, as their medical records could not be reliably followed.
Demographic, laboratory, and echocardiographic data, along with interventional and pharmacological treatments during hospitalization and at discharge, were collected. All variables are defined in the supplementary material (see S2. Variable definitions). A minimum follow-up of one year from the date of hospital discharge was conducted by reviewing patients’ medical records through the healthcare information system of the Castilla-La Mancha Health Service (SESCAM).
Statistical analysisCategorical variables were expressed as numbers and percentages. Quantitative variables were expressed as median and interquartile range (IQR 25–75%). Normal distribution of quantitative variables was assessed using the Shapiro–Wilk test. Categorical variables were compared between groups using the χ2 test or Fisher's exact test, as appropriate. For normally distributed continuous variables, comparisons were made using Student's t-test, while the Mann-Whitney U test was used for non-normally distributed variables. Comparisons of continuous variables across independent groups were conducted using analysis of variance with Bonferroni correction for multiple comparisons. A two-sided p-value <0.05 was considered statistically significant. Survival outcomes were analyzed using Kaplan–Meier survival curves and Cox proportional hazards regression.
Multivariate regression analysis was performed, including variables related to the index event as reported in the literature,6,24 as well as those variables that showed p<0.2 in the bivariate analysis. An additional regression analysis powered by bootstrap technique on 1000 samples was performed ad hoc. All statistical analyses were performed using the Statistical Package for the Social Sciences software (SPSS v. 23.0 for Windows, SPSS Inc., Chicago, IL, USA). This article follows the STROBE recommendations25 for reporting observational studies.
EthicsThe study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice guidelines. It was approved by the hospital's Ethics Committee, which granted an exemption from the requirement to obtain informed consent from patients.
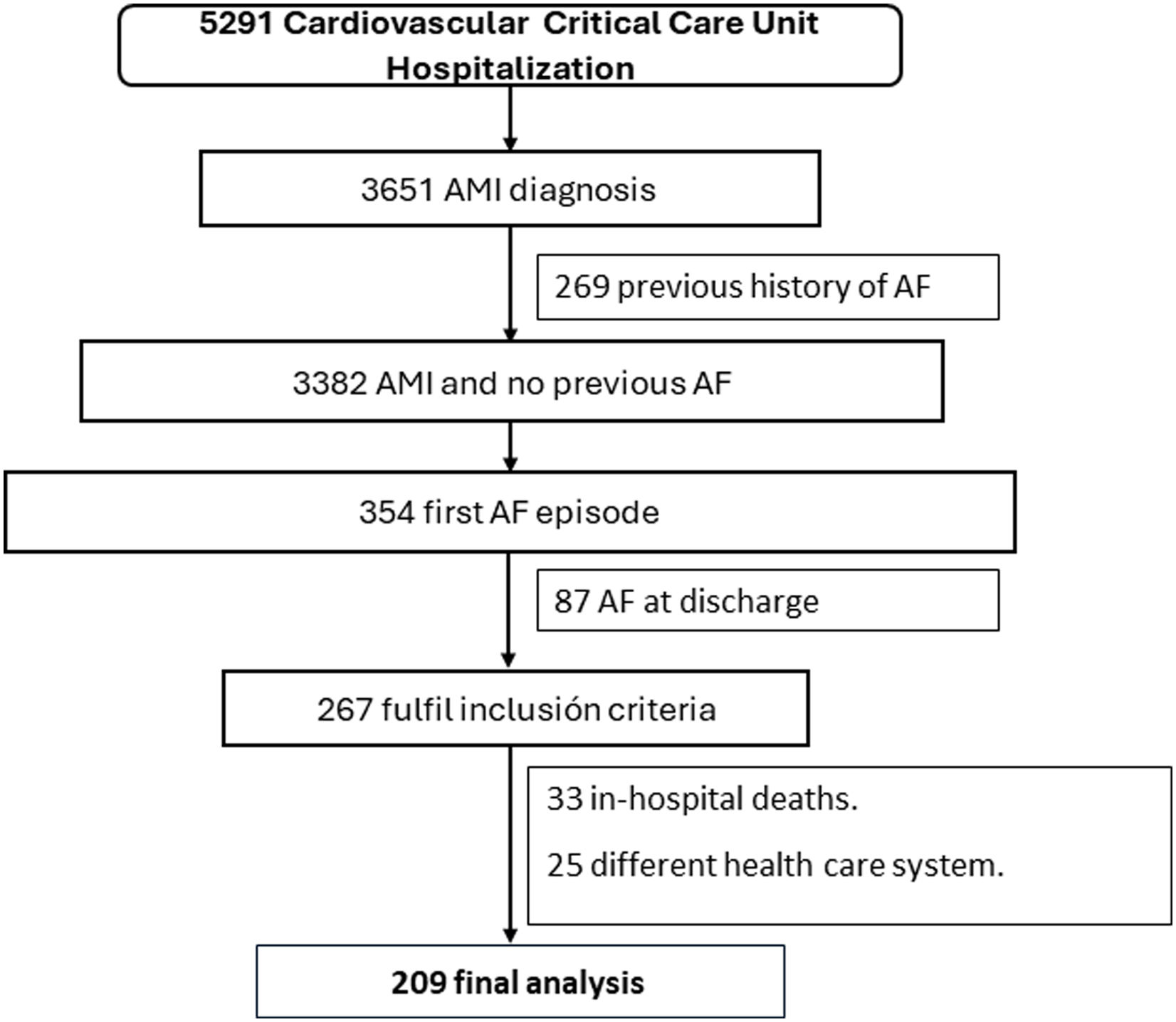
ResultsBetween December 2011 and May 2021, a total of 5291 patients were admitted to the CCCU, of whom 3651 had a diagnosis of AMI. Among these, 269 patients with previous AF were excluded, leaving 3382 patients. From these, 354 developed a first episode of AF (NOAF). After excluding patients with AF at discharge (n=87), in-hospital death (n=33), and those who were expected to be lost to follow-up due to being part of a different healthcare system (n=25), a total of 209 patients were included in the final analysis (Figure 1). Of these, 69.9% (146) were male, with a median age of 74 years [IQR 64–80].
Regarding the primary objective, 19 patients, 9.1% (confidence interval [CI] 5.2–13.0) presented a new episode of AF, of which 13 (68.4%) were paroxysmal. The median time to AF recurrence was 84 days [IQR 28–158] (S3. Kaplan–Meier analysis for AF recurrence). The clinical characteristics, ischemic event features, and treatments are detailed in Tables 1–3. Coronary angiography was performed in 98.6% of patients and percutaneous coronary intervention was the preferred revascularization strategy in both groups.
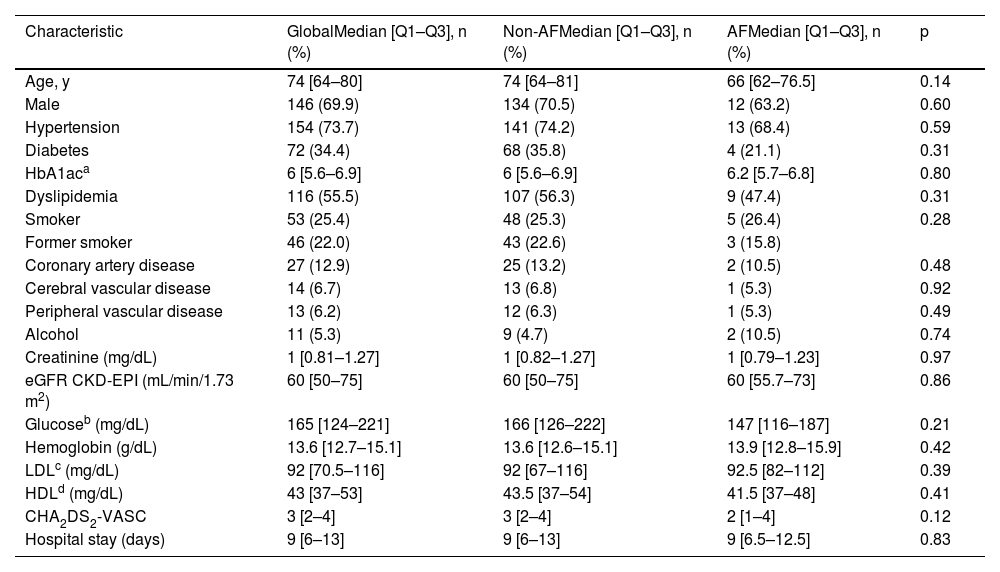
Baseline characteristics of the patients according to main objective.
| Characteristic | GlobalMedian [Q1–Q3], n (%) | Non-AFMedian [Q1–Q3], n (%) | AFMedian [Q1–Q3], n (%) | p |
|---|---|---|---|---|
| Age, y | 74 [64–80] | 74 [64–81] | 66 [62–76.5] | 0.14 |
| Male | 146 (69.9) | 134 (70.5) | 12 (63.2) | 0.60 |
| Hypertension | 154 (73.7) | 141 (74.2) | 13 (68.4) | 0.59 |
| Diabetes | 72 (34.4) | 68 (35.8) | 4 (21.1) | 0.31 |
| HbA1aca | 6 [5.6–6.9] | 6 [5.6–6.9] | 6.2 [5.7–6.8] | 0.80 |
| Dyslipidemia | 116 (55.5) | 107 (56.3) | 9 (47.4) | 0.31 |
| Smoker | 53 (25.4) | 48 (25.3) | 5 (26.4) | 0.28 |
| Former smoker | 46 (22.0) | 43 (22.6) | 3 (15.8) | |
| Coronary artery disease | 27 (12.9) | 25 (13.2) | 2 (10.5) | 0.48 |
| Cerebral vascular disease | 14 (6.7) | 13 (6.8) | 1 (5.3) | 0.92 |
| Peripheral vascular disease | 13 (6.2) | 12 (6.3) | 1 (5.3) | 0.49 |
| Alcohol | 11 (5.3) | 9 (4.7) | 2 (10.5) | 0.74 |
| Creatinine (mg/dL) | 1 [0.81–1.27] | 1 [0.82–1.27] | 1 [0.79–1.23] | 0.97 |
| eGFR CKD-EPI (mL/min/1.73 m2) | 60 [50–75] | 60 [50–75] | 60 [55.7–73] | 0.86 |
| Glucoseb (mg/dL) | 165 [124–221] | 166 [126–222] | 147 [116–187] | 0.21 |
| Hemoglobin (g/dL) | 13.6 [12.7–15.1] | 13.6 [12.6–15.1] | 13.9 [12.8–15.9] | 0.42 |
| LDLc (mg/dL) | 92 [70.5–116] | 92 [67–116] | 92.5 [82–112] | 0.39 |
| HDLd (mg/dL) | 43 [37–53] | 43.5 [37–54] | 41.5 [37–48] | 0.41 |
| CHA2DS2-VASC | 3 [2–4] | 3 [2–4] | 2 [1–4] | 0.12 |
| Hospital stay (days) | 9 [6–13] | 9 [6–13] | 9 [6.5–12.5] | 0.83 |
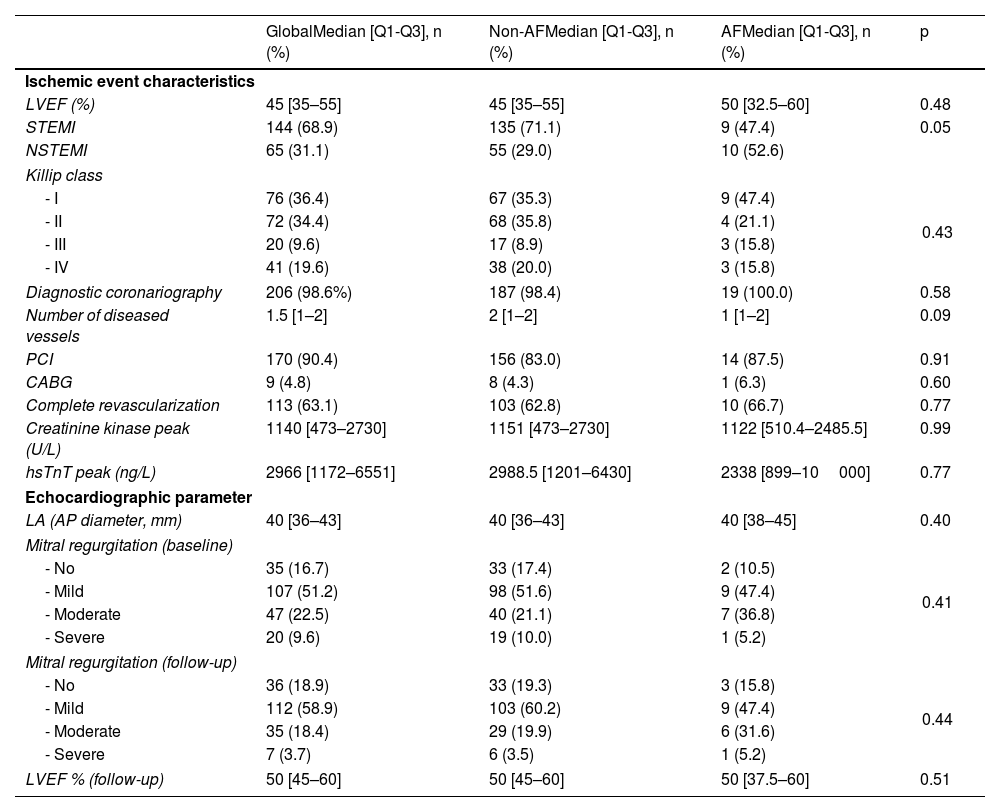
Characteristics related to ischemic event and echocardiographic parameters according to main objective.
| GlobalMedian [Q1-Q3], n (%) | Non-AFMedian [Q1-Q3], n (%) | AFMedian [Q1-Q3], n (%) | p | |
|---|---|---|---|---|
| Ischemic event characteristics | ||||
| LVEF (%) | 45 [35–55] | 45 [35–55] | 50 [32.5–60] | 0.48 |
| STEMI | 144 (68.9) | 135 (71.1) | 9 (47.4) | 0.05 |
| NSTEMI | 65 (31.1) | 55 (29.0) | 10 (52.6) | |
| Killip class | ||||
| - I | 76 (36.4) | 67 (35.3) | 9 (47.4) | 0.43 |
| - II | 72 (34.4) | 68 (35.8) | 4 (21.1) | |
| - III | 20 (9.6) | 17 (8.9) | 3 (15.8) | |
| - IV | 41 (19.6) | 38 (20.0) | 3 (15.8) | |
| Diagnostic coronariography | 206 (98.6%) | 187 (98.4) | 19 (100.0) | 0.58 |
| Number of diseased vessels | 1.5 [1–2] | 2 [1–2] | 1 [1–2] | 0.09 |
| PCI | 170 (90.4) | 156 (83.0) | 14 (87.5) | 0.91 |
| CABG | 9 (4.8) | 8 (4.3) | 1 (6.3) | 0.60 |
| Complete revascularization | 113 (63.1) | 103 (62.8) | 10 (66.7) | 0.77 |
| Creatinine kinase peak (U/L) | 1140 [473–2730] | 1151 [473–2730] | 1122 [510.4–2485.5] | 0.99 |
| hsTnT peak (ng/L) | 2966 [1172–6551] | 2988.5 [1201–6430] | 2338 [899–10000] | 0.77 |
| Echocardiographic parameter | ||||
| LA (AP diameter, mm) | 40 [36–43] | 40 [36–43] | 40 [38–45] | 0.40 |
| Mitral regurgitation (baseline) | ||||
| - No | 35 (16.7) | 33 (17.4) | 2 (10.5) | 0.41 |
| - Mild | 107 (51.2) | 98 (51.6) | 9 (47.4) | |
| - Moderate | 47 (22.5) | 40 (21.1) | 7 (36.8) | |
| - Severe | 20 (9.6) | 19 (10.0) | 1 (5.2) | |
| Mitral regurgitation (follow-up) | ||||
| - No | 36 (18.9) | 33 (19.3) | 3 (15.8) | 0.44 |
| - Mild | 112 (58.9) | 103 (60.2) | 9 (47.4) | |
| - Moderate | 35 (18.4) | 29 (19.9) | 6 (31.6) | |
| - Severe | 7 (3.7) | 6 (3.5) | 1 (5.2) | |
| LVEF % (follow-up) | 50 [45–60] | 50 [45–60] | 50 [37.5–60] | 0.51 |
AP: anteroposterior; CABG: coronary artery bypass grafting; hsTnT: high-sensitivity T troponin; LA: left atrial; LVEF: left ventricle ejection fraction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction.
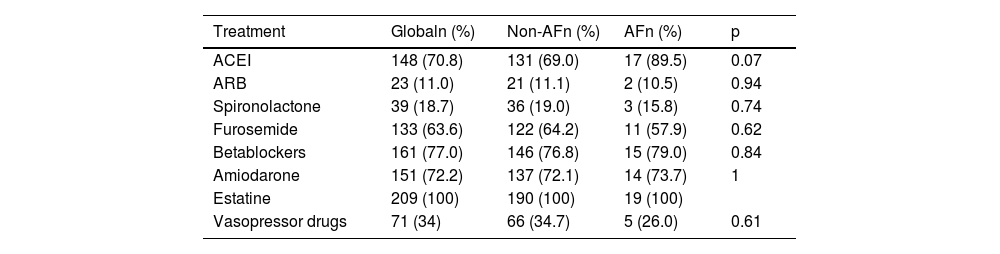
Drug treatment according to main objective.
| Treatment | Globaln (%) | Non-AFn (%) | AFn (%) | p |
|---|---|---|---|---|
| ACEI | 148 (70.8) | 131 (69.0) | 17 (89.5) | 0.07 |
| ARB | 23 (11.0) | 21 (11.1) | 2 (10.5) | 0.94 |
| Spironolactone | 39 (18.7) | 36 (19.0) | 3 (15.8) | 0.74 |
| Furosemide | 133 (63.6) | 122 (64.2) | 11 (57.9) | 0.62 |
| Betablockers | 161 (77.0) | 146 (76.8) | 15 (79.0) | 0.84 |
| Amiodarone | 151 (72.2) | 137 (72.1) | 14 (73.7) | 1 |
| Estatine | 209 (100) | 190 (100) | 19 (100) | |
| Vasopressor drugs | 71 (34) | 66 (34.7) | 5 (26.0) | 0.61 |
| Antiplathelet drug | Globaln (%) | Non-AFn (%) | AFn (%) | p |
|---|---|---|---|---|
| ASA | 208 (99.5) | 189 (99.5) | 19 (100) | 0.75 |
| Clopidogrel | 174 (83.3) | 160 (84.2) | 14 (73.7) | 0.24 |
| Prasugrel | 38 (18.2) | 33 (17.4) | 5 (26.3) | 0.34 |
| Ticagrelor | 28 (13.4) | 24 (12.6) | 4 (21.1) | 0.30 |
| Anticoagulant | Globaln (%) | Non-AFn (%) | AFn (%) | p |
|---|---|---|---|---|
| No | 132 (63.2) | 123 (64.7) | 9 (47.4) | 0.60 |
| Dabigatran | 12 (5.7) | 10 (5.3) | 2 (10.5) | |
| Apixaban | 30 (14.4) | 26 (13.7) | 4 (21.1) | |
| Rivaroxaban | 4 (1.9) | 3 (1.6) | 1 (5.3) | |
| Edoxaban | 1 (0.5) | 1 (0.5) | 0 | |
| VKA | 30 (14.4) | 27 (14.2) | 3 (15.89) |
ACEI: angiotensin-converting enzyme inhibitors; blockers; ARB: angiotensin receptor blocker; ASA: acetylsalicylic acid; VKA: vitamin K antagonist.
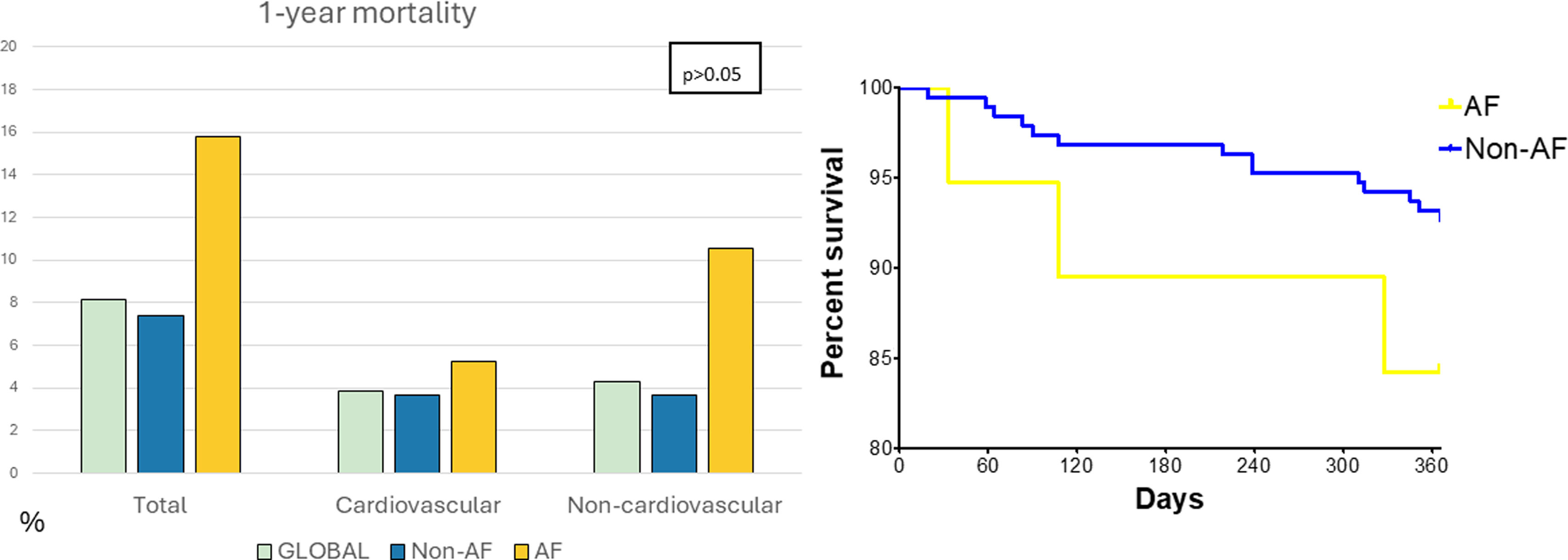
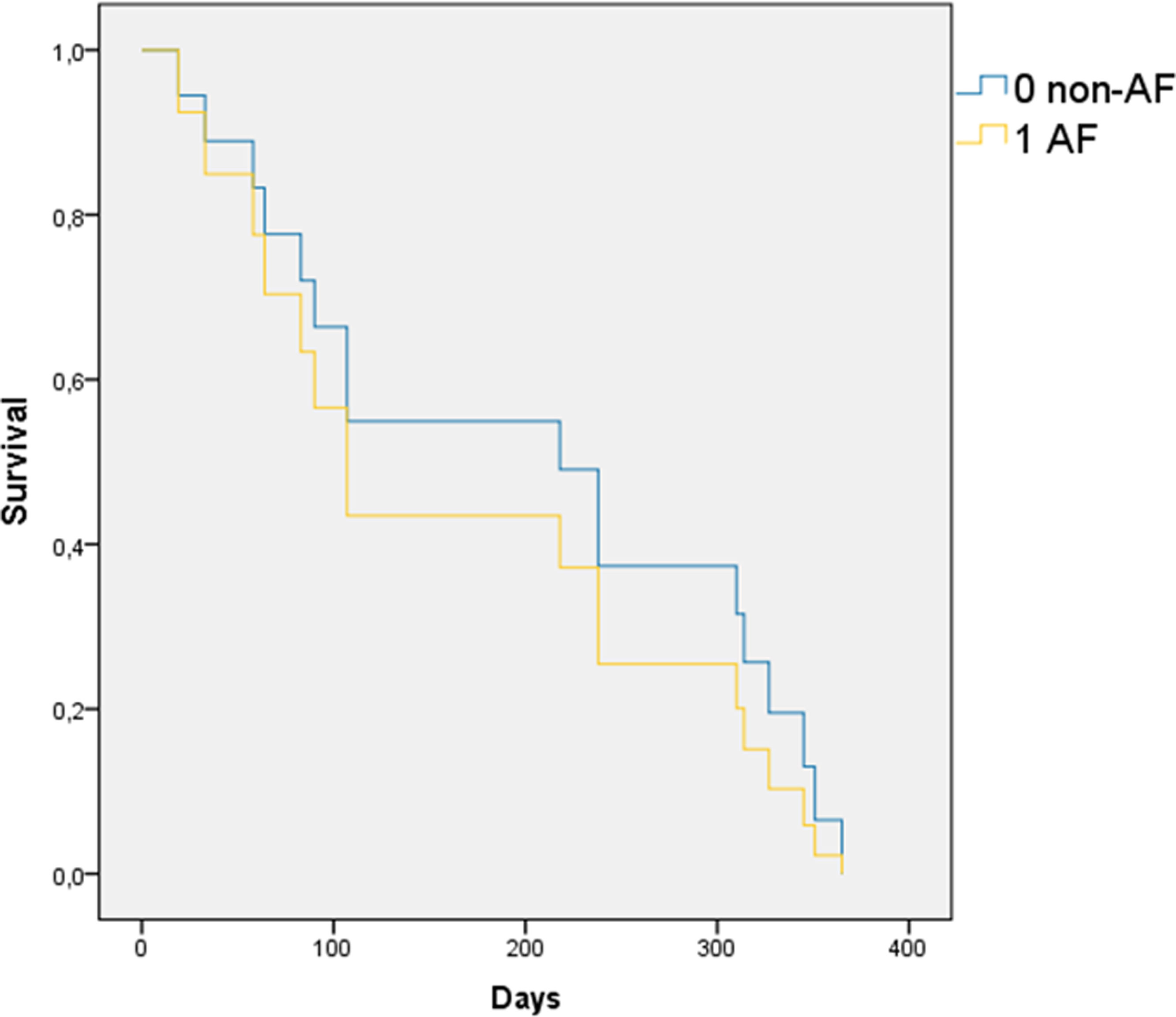
Within one year of follow-up, 17 patients (8.1%) died, including 14 (7.4%) in the non-AF group and 3 (15.8%) in the AF group (p=0.19). Cardiovascular mortality was 3.8% (8 patients), with no significant differences between groups (p=0.35) (Figure 2). Cardiovascular deaths were primarily due to HF (4 patients) and sudden cardiac death (4 patients), while non-cardiovascular causes included infections (4 patients), cancer (4 patients), and pulmonary embolism (1 patient). Cox regression and other survival analyses showed no statistically significant differences in mortality (Figure 3). The Breslow and Tarone-Ware tests were also applied to detect significant differences at the beginning or end of the curves, but did not provide significant results.
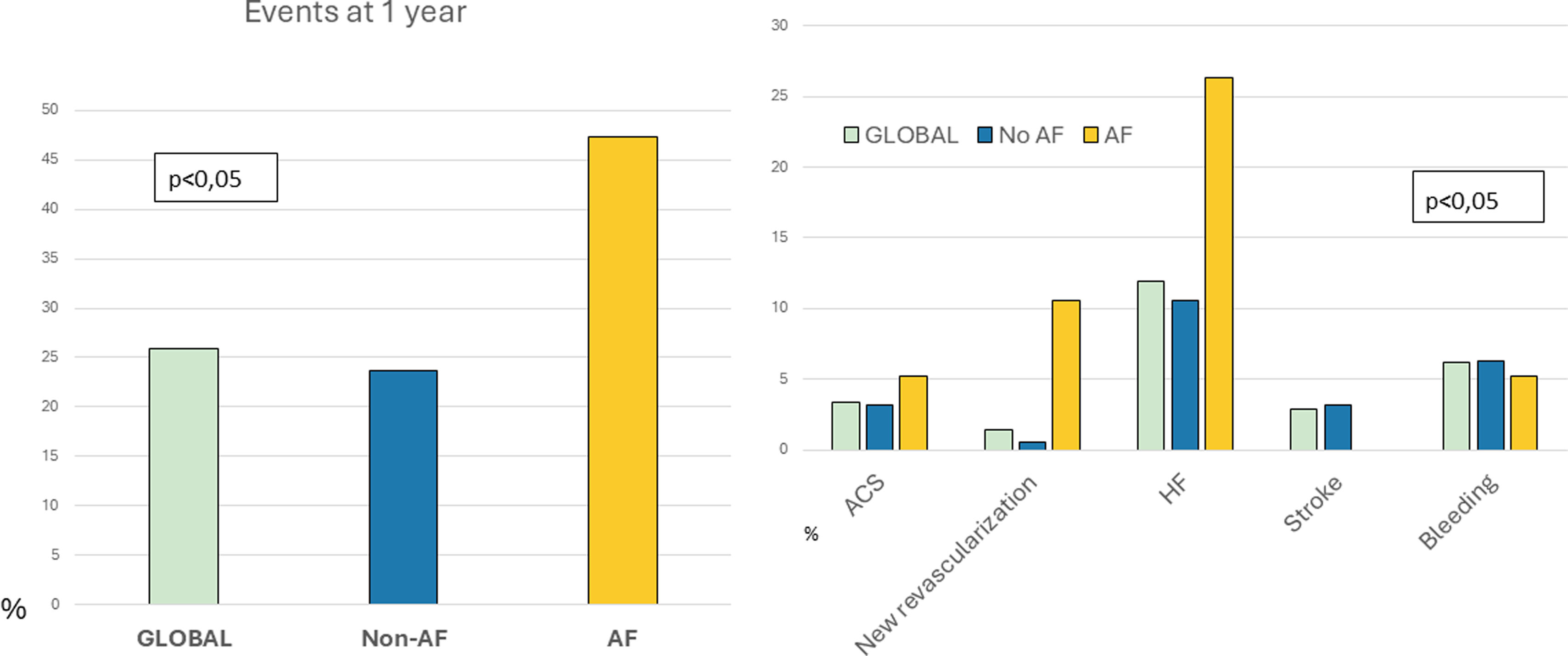
Regarding major adverse cardiovascular events, 25.8% (54 patients) experienced at least one event. Stratification by AF recurrence showed that 47.4% of patients in the AF group had a cardiovascular event within the first year, compared to 23.7% in the non-AF group (p=0.003) (Figure 4).
Outcomes at 1 year follow-up. Incidence of events at one year follow-up after AMI discharge. Left: overall event rate (%) in the global cohort, non-AF, and AF groups (p<0.05). Right: breakdown by event type—acute coronary syndrome (ACS), new revascularization, heart failure (HF), stroke, and major bleeding—according to group (p<0.05). AF, atrial fibrillation.
Multivariate regression analysis identified a potential protective effect of angiotensin-converting enzyme inhibitors (ACEis) (b=−2.82; p=0.02) on AF recurrence (S4. Regression analysis). Additional ad hoc analysis using the bootstrap technique to artificially increase the sample size suggested a relationship between diabetes and AF recurrence (b=1.47; p=0.044), as well as protective effects of ACEis and mineralocorticoid receptor blockers (MRBs) (b=−2.77; p=0.002 and b=−1.94; p=0.047, respectively) (S5. Regression analysis with bootstrapping over 1000 samples).
DiscussionThe main findings of our study can be summarized as follows: (1) prevalence of NOAF in the context of AMI in this cohort is approximately 10%; (2) patients with paroxysmal AF (sinus rhythm at discharge) experienced recurrence of around 10% during the first year; (3) AF recurrence was associated with worse prognosis within one year of discharge.
(1) Prevalence of new onset atrial fibrillation in the context of acute myocardial infarction is approximately 10%The relationship between AF and AMI has been widely studied due to the high prevalence of both conditions and their significant clinical and socioeconomic impact.8,26,27 However, data on NOAF are more limited. The prevalence of NOAF in the context of AMI in our cohort was 10.5% (354 patients who developed a first episode of AF during hospitalization out of 3382 patients without a prior history of AF). This finding is similar to previous studies such as the RIMA registry (8.8%) and Batra et al. (7.6%).9,10 A key distinction in our study is the inclusion of both STEMI and NSTEMI patients, whereas previous studies often focused solely on STEMI. Furthermore, we excluded other supraventricular arrhythmias, ensuring that our analysis was specific to NOAF. Additionally, we provided information on patients’ heart rhythm at discharge, finding that most were cardioverted during hospitalization (spontaneously or actively), with 24.6% remaining in AF at discharge. These findings are consistent with other studies, while also offering additional insights into rhythm management during hospitalization.
(2) Patients with paroxysmal atrial fibrillation (sinus rhythm at discharge) present recurrence near to 10% within one yearTo our knowledge, this is the first study specifically analyzing the recurrence of paroxysmal AF in the context of AMI. We observed a recurrence rate of 9.1% during the first year. Although continuous electrocardiographic monitoring was not used –which could lead to missed self-limiting arrhythmic episodes– our results provide valuable insights into the clinical course of NOAF following AMI. It is well established that acute ischemic events can act as triggers for AF through mechanisms such as atrial ischemia, increased left ventricular filling pressures, and subsequent atrial dilation.6 These factors may resolve after the acute phase, potentially preventing long-term structural damage, which could explain why many patients remain in sinus rhythm during follow-up. This recurrence raises important questions about the need for long-term anticoagulation in patients who maintain sinus rhythm, especially since most of these patients receive dual antiplatelet therapy for 6–12 months post-AMI. Identifying predictors of recurrence is crucial to guiding early anticoagulation strategies. In our study, multivariate logistic regression suggested a protective effect of ACEis on AF recurrence. Given the limitations of our sample size, a secondary analysis using bootstrapping was performed, which further suggested an association between diabetes and AF recurrence and confirmed the protective effect of ACEis and ARBs.
It is worth noting that there were no significant differences in baseline characteristics or treatment between patients with and without AF recurrence, which suggests that the perceived cardiovascular risk by the treating physicians was similar across groups. However, changes in clinical practice guidelines over the decade-long study period may have introduced some bias, particularly regarding newer treatments such as neprilysin inhibitors and SGLT2 inhibitors, which were not widely available at the start of the study.
(3) Arrhythmic recurrence is associated with worse prognosis in the first year after dischargeA prior history of AF has been historically associated with worse outcomes in patients with AMI, including higher mortality, hospitalizations, and reduced quality of life.8,9,20,28 However, NOAF which occurs in response to acute ischemic events may have a different prognosis. Studies have reported conflicting results regarding the impact of NOAF recurrence on outcomes.4,9 The CREDO-Kyoto study4 reported that both NOAF and chronic AF were associated with increased long-term mortality in AMI patients at five years, regardless of whether sinus rhythm was restored. However, in the RIMA registry,9 NOAF was associated with worse cardiovascular outcomes only in the short-term, particularly in the first year following AMI (HR 2.49; 95% CI 1.32–4.67; p=0.004).
In our study, AF recurrence during the first year was associated with worse clinical outcomes, especially HF hospitalizations, compared to patients who maintained sinus rhythm. Although we did not find significant differences in overall mortality, likely due to the small sample size, there was a trend toward higher mortality in the AF group. This highlights the need for closer monitoring and management of HF in patients with AF recurrence following AMI.
Larger studies are necessary to confirm the long-term impact of AF recurrence and to determine whether early rhythm control or anticoagulation strategies could improve outcomes in this high-risk group.
LimitationsOur study has certain limitations inherent to its design. Firstly, as a retrospective observational study, the data were limited to the information available in the electronic medical records, which may introduce bias or incomplete data. Additionally, as it was a single-center study, the sample size was restricted, which could limit the ability to achieve statistical significance in some analyses. As such, these results should be interpreted as hypothesis-generating. A prospective multicenter study would be valuable to further investigate the questions raised in this analysis. Moreover, the study spans a ten-year period, during which updates to clinical practice guidelines were made as new evidence emerged. This may have introduced variability in the management of patients over time, potentially affecting the consistency of treatments and outcomes.
ConclusionsOur study provides valuable data on the prevalence of NOAF in the context of AMI over a 10-year period, confirming its frequent occurrence in routine clinical practice. Importantly, we focused on patients discharged in sinus rhythm, a cohort underrepresented in the literature. We found that arrhythmic recurrence occurred in approximately 10% of these patients within the first year. Although cardiovascular, non-cardiovascular, and overall mortality were higher in patients with AF recurrence, statistical significance was not reached likely due to sample size limitations. Nonetheless, these patients had a significantly worse prognosis at one year, mainly driven by a higher incidence of HF hospitalizations.
Larger multicenter studies are needed to better understand the factors that predispose patients to arrhythmic recurrence and worse outcomes, facilitating closer monitoring and tailored management for high-risk individuals.
Ethical approvalEthics Committee of the Integrated Care Management of Albacete, study no. 2022-091.
Conflict of interestsThe authors have no conflicts of interest to declare.