Oral anticoagulation (OAC) with non-vitamin K antagonist oral anticoagulants (NOACs) after surgical mitral valve repair (MVR) or bioprosthetic valve replacement (BVR) in mitral position remains a controversial topic among the cardiovascular community, in particular in the early postoperative period. This study aimed to evaluate the efficacy and safety of NOACs in the first three months after MVR or mitral BVR compared to vitamin K antagonists (VKAs).
MethodsThis was a single-center retrospective study with prospectively collected peri-intervention outcomes between 2020 and 2021. Records were retrieved and all participants were contacted by telephone. Patients were divided into groups according to OAC strategy. The primary outcome was a composite of death, rehospitalization, myocardial infarction, stroke or transient ischemic attack, systemic embolism, mitral thrombosis, or bleeding during the first three months after surgery.
ResultsA total of 148 patients were enrolled, with a mean age of 65.5±12.2 years, 56.8% male. On discharge, 98 (66.2%) patients were on VKAs and 50 (33.8%) were on DOACs for at least three months. The primary outcome occurred in 22 (22.4%) patients in the VKA group and in three (6%) in the NOAC group (p=0.012), mainly driven by more bleeding events in the former. Independent predictors of the primary outcome were smoking (p=0.028) and OAC with VKAs at discharge, the latter predicting three times more events (p=0.046, OR 3.72, 95% CI 1.02–13.5).
ConclusionsNOACs were associated with fewer events, supporting their efficacy and safety during the first three months after surgical MVR or mitral BVR.
A anticoagulação oral (OAC) com anticoagulantes orais não antagonistas da vitamina K (NOACs) após reparação cirúrgica da valva mitral (MVR) ou substituição cirúrgica por prótese valvular biológica (BVR) em posição mitral permanece um tópico controverso entre a comunidade cardiovascular, em particular no período pós-operatório inicial. O objetivo do estudo foi avaliar a eficácia e segurança dos NOACs nos primeiros três meses após MVR ou BVR em posição mitral, comparando esta estratégia com antagonistas da vitamina K (AVKs).
MétodosAnálise retrospetiva de um único centro com resultados peri-intervenção colhidos prospetivamente entre 2020/2021. Os registos foram analisados e todos os participantes foram contatados por telefone. Os pacientes foram divididos em grupos de acordo com a estratégia OAC. O outcome primário composto foi definido como morte, re-hospitalização, enfarte do miocárdio, acidente vascular cerebral ou ataque isquémico transitório, embolia sistémica, trombose mitral ou hemorragia durante os primeiros três meses após a cirurgia.
ResultadosForam incluídos 148 doentes, com uma idade média de 65,5 ± 12,2 anos e 56,8% eram do sexo masculino. À data da alta, 98 (66,2%) pacientes foram medicados com AVKs e 50 (33,8%) com NOACs, mantidos pelo menos durante três meses. O outcome primário ocorreu em 22 (22,4%) pacientes no grupo AVKs e em 3 (6%) no grupo NOACs (p = 0,012), principalmente sobre a influência de um maior número de eventos hemorrágicos no primeiro. Os preditores independentes do outcome primário foram tabagismo (p = 0,028) e OAC com AVKs à data da alta, este último prevendo três vezes mais eventos (p = 0,046, OR 3,72, IC 95% 1,02 a 13,5).
ConclusõesOs NOACs foram associados a menos eventos, nomeadamente hemorrágicos, apoiando a sua eficácia e segurança durante os primeiros três meses em doentes após MVR ou BVR em posição mitral.
Valvular heart disease affects more than 100 million people worldwide and an estimated 300000 prosthetic heart valves are implanted every year. This rate is increasing, mainly driven by intrinsic valvular degeneration in aging populations.1
The use of bioprosthetic valve replacement (BVR) has also increased in the last 20 years compared to mechanical heart valves. This shift is difficult to explain, but may be related to the drawbacks associated with long-term oral anticoagulation (OAC) in younger patients as well as the higher burden of side effects, particularly in the elderly.2 Even with appropriate OAC therapy after valve implantation, there is a higher lifelong risk of thromboembolic events (estimated at 1–4%) and bleeding (2–9%) compared to the general population.3,4
Non-vitamin K antagonist oral anticoagulants (NOACs) have been shown to be safe and at least as effective as vitamin K antagonists (VKAs) in patients with atrial fibrillation (AF) and without moderate to severe mitral stenosis or rheumatic valvular disease (RVD).5 However, patients with mechanical heart valves should be anticoagulated with VKAs, given the harmful effects of dabigatran noted in the RE-ALIGN phase II trial, and more recently, the premature termination of the PROACT Xa trial of apixaban versus warfarin.5–7
However, the ideal OAC strategy in patients with mitral BVR or mitral valve repair (MVR) remains a matter of debate in the cardiovascular community, especially regarding the first three months after surgery. The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend initiating OAC with VKAs within three months of index valvular surgery regardless of rhythm or valve position, and the European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines also recommend VKAs for biological heart valves in mitral position within three months, adding that NOACs may be considered for patients with AF and a bioprosthesis in mitral position (class of recommendation IIb, level of evidence C). These recommendations are mainly based on observational studies.8–12
Nevertheless, off-label use of NOACs within three months of index mitral surgery is growing, supported by small studies and meta-analyses.4,13–15
In the RIVER trial, the subgroup of patients taking rivaroxaban in the first months after index valvular surgery showed fewer primary endpoint events, but only 18.8% of the patients in the main study were randomized within three months.16 Similarly, the ENAVLE trial showed that edoxaban was non-inferior to warfarin regarding the primary efficacy endpoint as well as for major bleeding. However, this was a small trial which included patients with bioprosthetic aortic valve replacement (49%), which may limit the applicability of these results.17
Regarding thromboembolic and bleeding events, NOACs appear to be as safe and as effective as VKAs in patients with surgical mitral BVR or MVR within three months of surgery. However, stronger evidence from observational studies and larger randomized trials are needed to further sustain these findings.
ObjectivesThis study aimed to evaluate the efficacy and safety of NOACs in the first three months after MVR or mitral BVR compared to VKAs.
MethodsEthical statementThe study abided by the principles stated in the 1975 Helsinki Declaration and was approved by the institutional ethics committee of the hospital center in which the study took place.
Patient selectionThis was a single-center retrospective cohort study, with prospectively collected peri-intervention outcomes between 2020 and 2021, which included patients undergoing either surgical MVR or mitral BVR.
Patients were eligible for the study if they underwent MVR or mitral BVR. Patients who underwent mechanical prosthetic valve implantation or concomitant ascending aorta replacement or other valvular procedure, or who died during the index hospitalization, were excluded. Patients treated by other antithrombotic strategies such as concomitant use of antiplatelets or heparin were also excluded.
Patients were then divided into two groups according to OAC strategy (NOACs or VKAs). In patients receiving VKAs on discharge, the recommended target international normalized ratio (INR) was between 2 and 3. They were entered into a follow-up period of at least three months, with monitoring at specific time points (either at the hospital or with their primary care provider). Baseline patient demographic data, cardiovascular risk factors, and clinical, laboratory, echocardiographic and surgical data were recorded. All patients were contacted by telephone. Switching OAC therapy only occurred during hospitalization, and it was maintained in the postoperative period.
OutcomesThe primary outcome (net clinical benefits) was a composite of death, rehospitalization, myocardial infarction, stroke or transient ischemic attack, systemic embolism, mitral thrombosis, or bleeding during the first three months after surgery. Bleeding events were defined according to the Bleeding Academic Research Consortium (BARC) scale.18 A separate analysis aimed to assess bleeding (on the BARC scale) and stroke events in the AF population during the first three months after surgery.
Statistical analysisContinuous variables were presented as mean±standard deviation or median and interquartile range. These were compared between groups using the independent samples t test or the Mann–Whitney U test, based on their distribution. Categorical variables were presented as frequencies and percentages and were compared using the chi-square test or Fisher's exact test, as appropriate.
Separate analyses were conducted for net clinical benefits (primary outcome). Independent predictors of the primary outcome were assessed by multivariate logistic regression analyses using the forward stepwise method. The Hosmer–Lemeshow test was used to calibrate the regression model. The effects of the variables were assessed by estimating odds ratios (OR) and 95% confidence intervals (CI). The variables entered into the model were gender, age, smoking status, history of type 2 diabetes, and VKAs at discharge.
In the AF subpopulation, a separate analysis for bleeding events (on the BARC scale) was also conducted. The variables entered into the model were gender, age, anticoagulation, and switching OAC therapy at discharge. Statistical differences between the presence of AF, type of surgery, and presence of RVD were assessed by analysis of variance (ANOVA). A p-value for interaction of <0.05 was taken to indicate statistical significance.
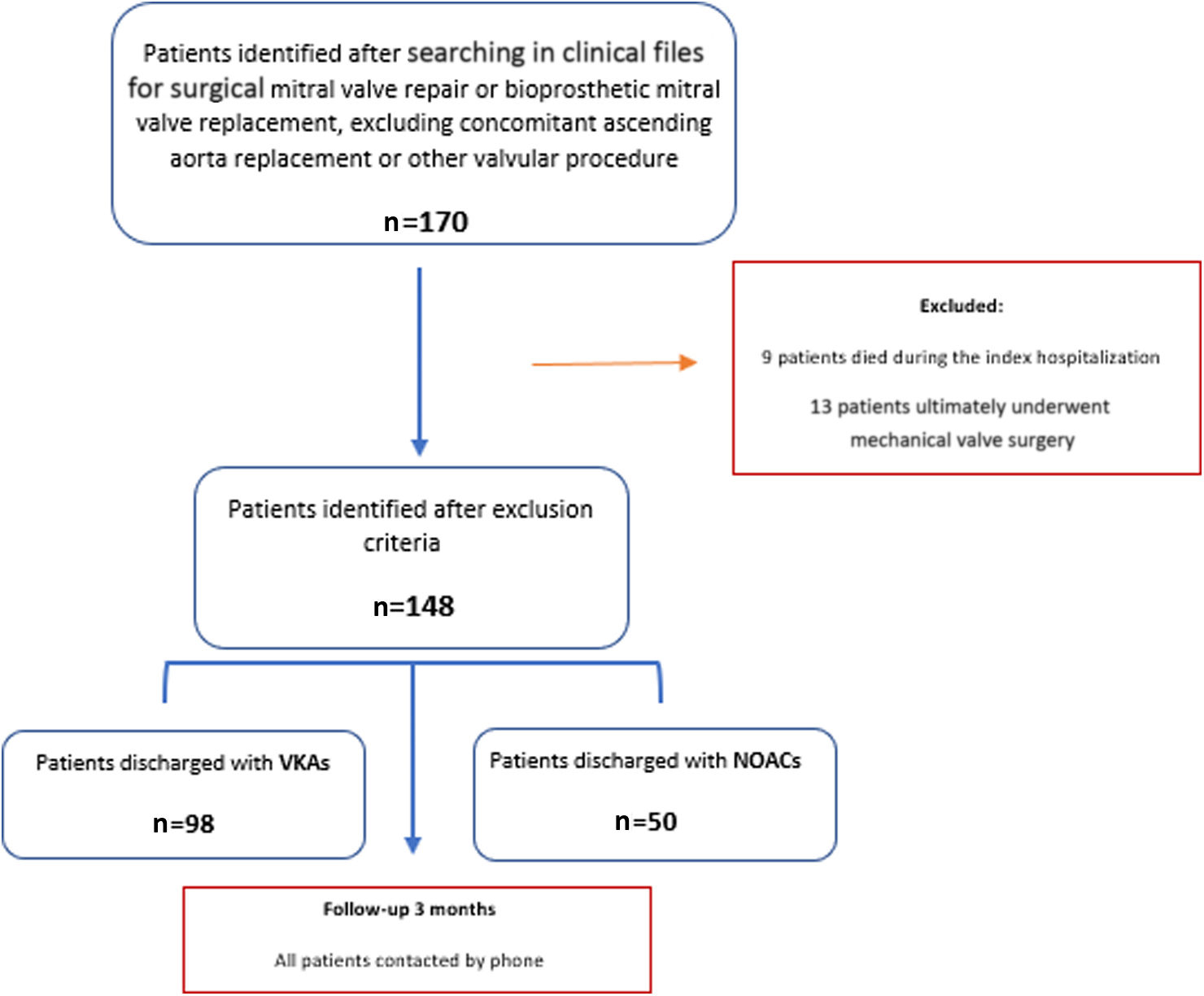
ResultsBaseline patient dataA total of 170 patients were initially identified, of whom 22 were excluded due to death during hospitalization (n=9) or mechanical mitral valve surgery (incorrectly classified) (n=13) (Figure 1). A total of 148 patients were therefore eligible and were contacted.
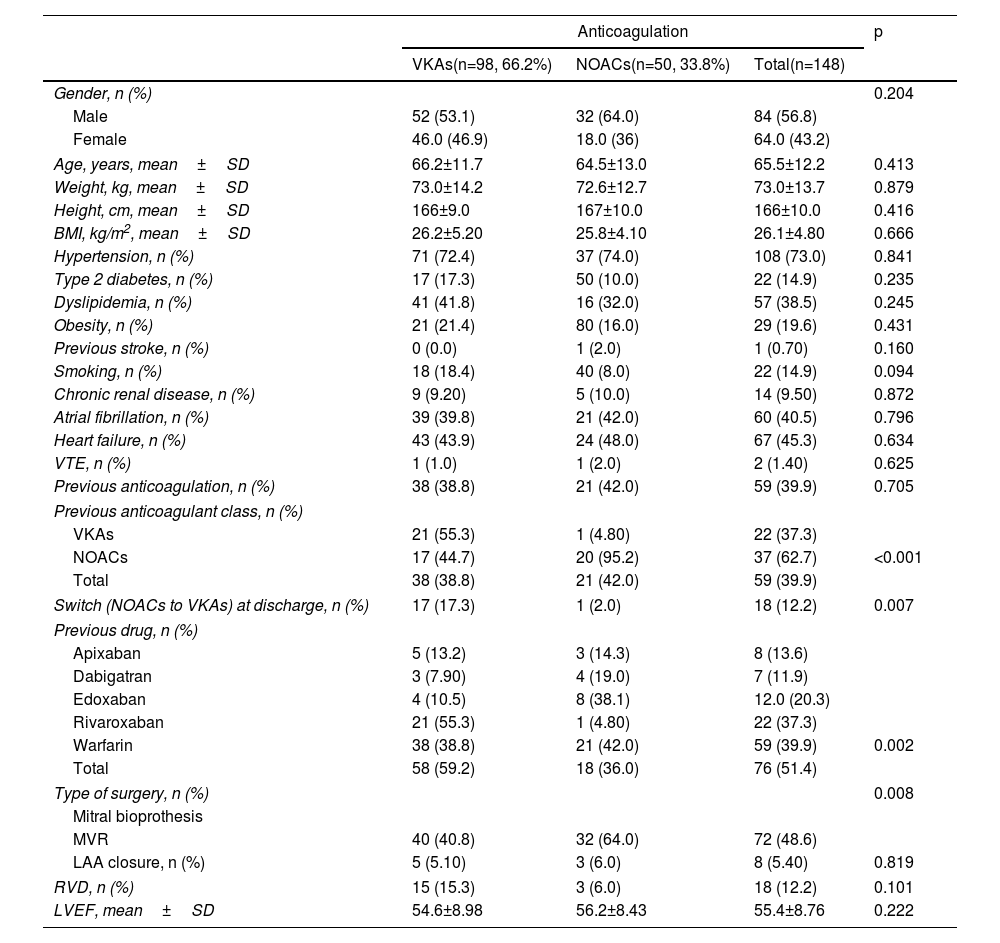
Participants’ mean age was 65.5±12.2 years and 56.8% of the sample were male. In terms of comorbidities, 73% were hypertensive, 14.9% were diabetic, 19.6% were obese, 9.5% had chronic kidney disease and 45.3% had heart failure. Clinically, AF was previously diagnosed in 60 (40.5%) patients. Fifty-nine (39.9%) were on OAC therapy before surgery: 22 (14.9%) with VKAs and 37 (25%) with DOACs. RVD was present in 12.2%. From a surgical standpoint, 76 (51.4%) patients underwent surgical mitral BVR and 72 (48.6%) MVR. On discharge, 98 (66.2%) were on VKAs and 50 (33.8%) were on NOACs maintained for at least three months (Table 1).
Patient demographic, clinical, and surgical characteristics compared by anticoagulation strategy at discharge.
| Anticoagulation | p | |||
|---|---|---|---|---|
| VKAs(n=98, 66.2%) | NOACs(n=50, 33.8%) | Total(n=148) | ||
| Gender, n (%) | 0.204 | |||
| Male | 52 (53.1) | 32 (64.0) | 84 (56.8) | |
| Female | 46.0 (46.9) | 18.0 (36) | 64.0 (43.2) | |
| Age, years, mean±SD | 66.2±11.7 | 64.5±13.0 | 65.5±12.2 | 0.413 |
| Weight, kg, mean±SD | 73.0±14.2 | 72.6±12.7 | 73.0±13.7 | 0.879 |
| Height, cm, mean±SD | 166±9.0 | 167±10.0 | 166±10.0 | 0.416 |
| BMI, kg/m2, mean±SD | 26.2±5.20 | 25.8±4.10 | 26.1±4.80 | 0.666 |
| Hypertension, n (%) | 71 (72.4) | 37 (74.0) | 108 (73.0) | 0.841 |
| Type 2 diabetes, n (%) | 17 (17.3) | 50 (10.0) | 22 (14.9) | 0.235 |
| Dyslipidemia, n (%) | 41 (41.8) | 16 (32.0) | 57 (38.5) | 0.245 |
| Obesity, n (%) | 21 (21.4) | 80 (16.0) | 29 (19.6) | 0.431 |
| Previous stroke, n (%) | 0 (0.0) | 1 (2.0) | 1 (0.70) | 0.160 |
| Smoking, n (%) | 18 (18.4) | 40 (8.0) | 22 (14.9) | 0.094 |
| Chronic renal disease, n (%) | 9 (9.20) | 5 (10.0) | 14 (9.50) | 0.872 |
| Atrial fibrillation, n (%) | 39 (39.8) | 21 (42.0) | 60 (40.5) | 0.796 |
| Heart failure, n (%) | 43 (43.9) | 24 (48.0) | 67 (45.3) | 0.634 |
| VTE, n (%) | 1 (1.0) | 1 (2.0) | 2 (1.40) | 0.625 |
| Previous anticoagulation, n (%) | 38 (38.8) | 21 (42.0) | 59 (39.9) | 0.705 |
| Previous anticoagulant class, n (%) | ||||
| VKAs | 21 (55.3) | 1 (4.80) | 22 (37.3) | |
| NOACs | 17 (44.7) | 20 (95.2) | 37 (62.7) | <0.001 |
| Total | 38 (38.8) | 21 (42.0) | 59 (39.9) | |
| Switch (NOACs to VKAs) at discharge, n (%) | 17 (17.3) | 1 (2.0) | 18 (12.2) | 0.007 |
| Previous drug, n (%) | ||||
| Apixaban | 5 (13.2) | 3 (14.3) | 8 (13.6) | |
| Dabigatran | 3 (7.90) | 4 (19.0) | 7 (11.9) | |
| Edoxaban | 4 (10.5) | 8 (38.1) | 12.0 (20.3) | |
| Rivaroxaban | 21 (55.3) | 1 (4.80) | 22 (37.3) | |
| Warfarin | 38 (38.8) | 21 (42.0) | 59 (39.9) | 0.002 |
| Total | 58 (59.2) | 18 (36.0) | 76 (51.4) | |
| Type of surgery, n (%) | 0.008 | |||
| Mitral bioprothesis | ||||
| MVR | 40 (40.8) | 32 (64.0) | 72 (48.6) | |
| LAA closure, n (%) | 5 (5.10) | 3 (6.0) | 8 (5.40) | 0.819 |
| RVD, n (%) | 15 (15.3) | 3 (6.0) | 18 (12.2) | 0.101 |
| LVEF, mean±SD | 54.6±8.98 | 56.2±8.43 | 55.4±8.76 | 0.222 |
BMI: body mass index; LAA: left atrial appendage; LVEF: left ventricular ejection fraction; MVR: mitral valve repair; NOACs: non-vitamin K antagonist oral anticoagulants; RVD: rheumatic valve disease; SD: standard deviation; VKAs: vitamin K antagonists; VTE: venous thromboembolism.
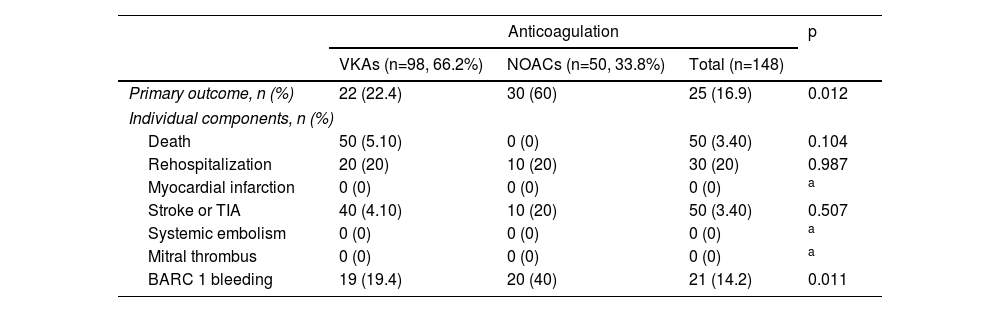
The primary composite outcome occurred in 22 patients (22.4%) in the VKA group and in three patients (6%) in the NOAC group (p=0.012). Analysis of the individual components of the primary outcome shows that there were no differences in mortality (p=0.104), rehospitalization (p=0.987), myocardial infarction (no events in either group), stroke or transient ischemic attack (p=0.507), systemic embolism (no events in either group) or mitral thrombosis (no events in either group). The main difference in the primary outcome was driven by more bleeding events (BARC 1 bleeding) in the VKA group (n=19, 19.4% versus n=2, 4% in the NOAC group) (p=0.011) (Table 2). No BARC >1 events were recorded during the follow-up period.
Primary outcome and its individual components compared by anticoagulation strategy at discharge.
| Anticoagulation | p | |||
|---|---|---|---|---|
| VKAs (n=98, 66.2%) | NOACs (n=50, 33.8%) | Total (n=148) | ||
| Primary outcome, n (%) | 22 (22.4) | 30 (60) | 25 (16.9) | 0.012 |
| Individual components, n (%) | ||||
| Death | 50 (5.10) | 0 (0) | 50 (3.40) | 0.104 |
| Rehospitalization | 20 (20) | 10 (20) | 30 (20) | 0.987 |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | a |
| Stroke or TIA | 40 (4.10) | 10 (20) | 50 (3.40) | 0.507 |
| Systemic embolism | 0 (0) | 0 (0) | 0 (0) | a |
| Mitral thrombus | 0 (0) | 0 (0) | 0 (0) | a |
| BARC 1 bleeding | 19 (19.4) | 20 (40) | 21 (14.2) | 0.011 |
BARC: Bleeding Academic Research Consortium criteria; NOACs: non-vitamin K antagonist oral anticoagulants; TIA: transient ischemic attack; VKAs: vitamin K antagonists.
In a subgroup analysis, no statistically significant differences were found for the presence of AF (p for interaction=0.823), type of surgery (p for interaction=0.954) or presence of RVD (p for interaction=0.171) with regard to the primary outcome.
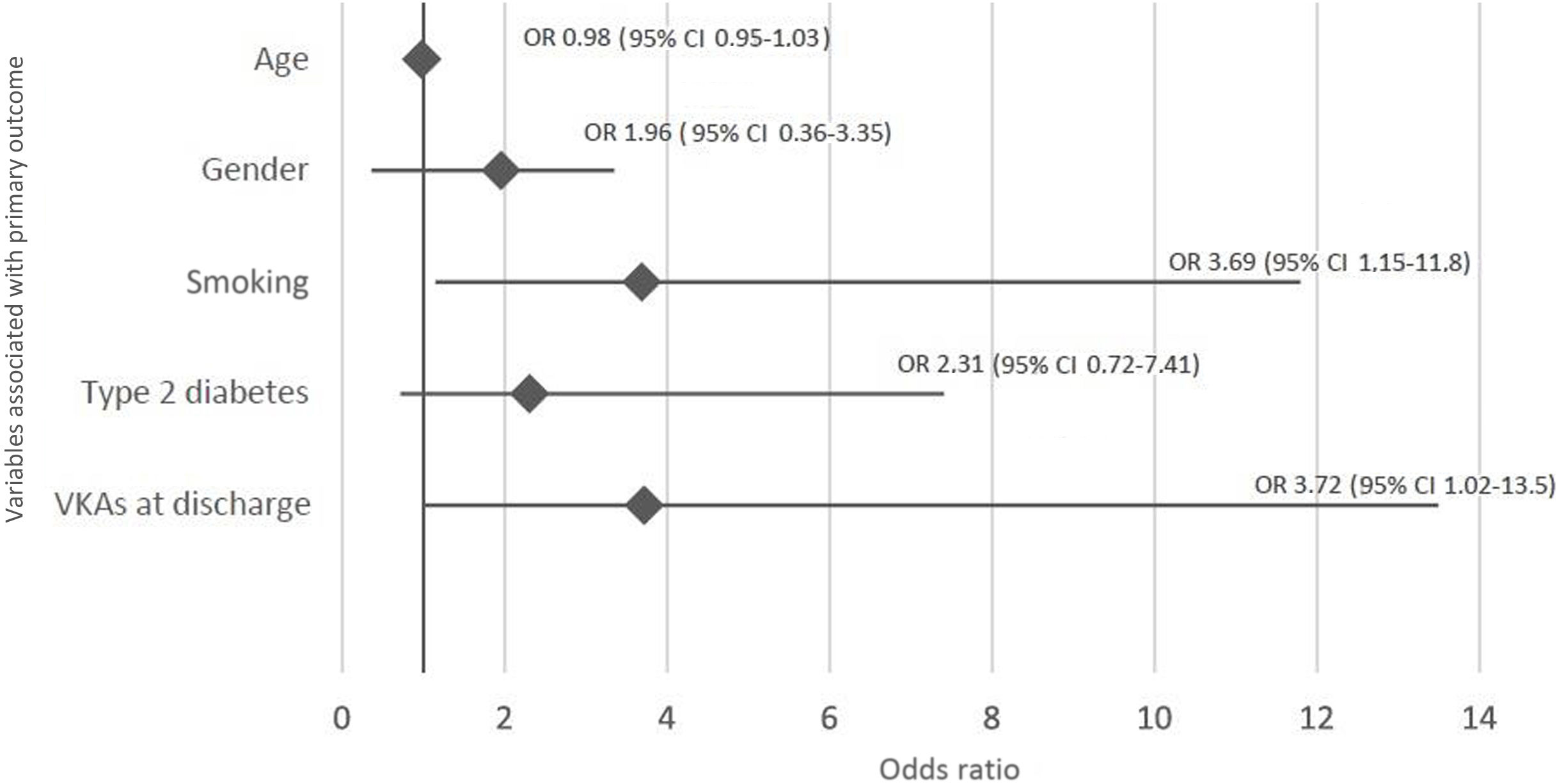
Independent predictors of the primary outcomeIn logistic regression analysis, independent predictors of the primary outcome were smoking (p=0.028) and OAC with VKAs on discharge (p=0.046); the latter was predicted to increase the chance of primary outcome events threefold (p=0.046, OR 3.72, 95% CI 1.02–13.5) (Figure 2).
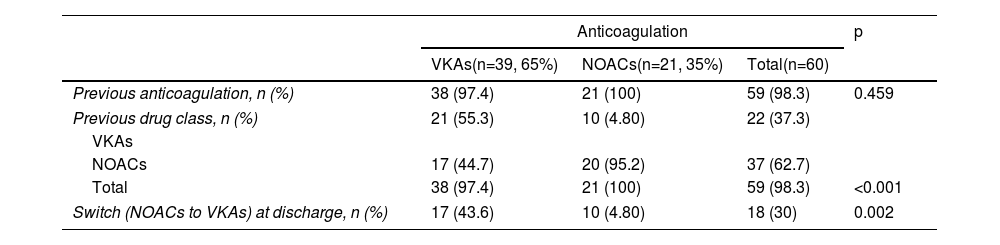
Outcomes in atrial fibrillation patientsA total of 60 (40.5%) patients with AF were identified, of whom 59 (98.3%) were on OAC therapy before surgery, 22 (37.3%) with VKAs and 37 (62.7%) with NOACs. At discharge, 39 (65%) were on VKAs and 21 (35%) were on NOACs maintained for at least three months. Switching OAC therapy on discharge occurred more frequently in the VKA group: of the 39 patients on VKAs at discharge, 17 (43.6%) patients were previously treated with NOACs. By contrast, of the 21 patients discharged with NOACs, only one (4.8%) was previously on VKAs (p=0.002) (Table 3). RVD was present in 16 (26.7%) patients, of whom 14 (87.5%) were discharged on VKAs (p=0.028).
Anticoagulation regimes of atrial fibrillation patients undergoing surgical mitral valve repair or mitral bioprosthetic valve replacement, compared by anticoagulation strategy at discharge.
| Anticoagulation | p | |||
|---|---|---|---|---|
| VKAs(n=39, 65%) | NOACs(n=21, 35%) | Total(n=60) | ||
| Previous anticoagulation, n (%) | 38 (97.4) | 21 (100) | 59 (98.3) | 0.459 |
| Previous drug class, n (%) | 21 (55.3) | 10 (4.80) | 22 (37.3) | |
| VKAs | ||||
| NOACs | 17 (44.7) | 20 (95.2) | 37 (62.7) | |
| Total | 38 (97.4) | 21 (100) | 59 (98.3) | <0.001 |
| Switch (NOACs to VKAs) at discharge, n (%) | 17 (43.6) | 10 (4.80) | 18 (30) | 0.002 |
NOACs: non-vitamin K antagonist oral anticoagulants; VKAs: vitamin K antagonists.
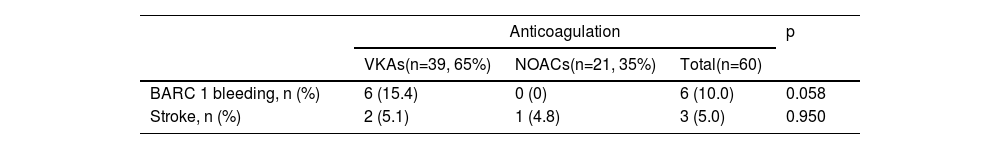
Bleeding events classified as BARC 1 occurred in the VKA group only (six patients, 15.4%) (p=0.058). Ischemic stroke in the three-month postoperative period occurred in two patients (5.1%) on VKAs and in one patient (4.8%) in the NOAC group (p=0.950) (Table 4). There was no statistical difference for bleeding (p for interaction=0.119) or stroke events (p for interaction=0.789) regarding the presence of RVD.
Bleeding and stroke events in atrial fibrillation patients undergoing surgical mitral valve repair or mitral bioprosthetic valve replacement, compared by anticoagulation strategy at discharge.
| Anticoagulation | p | |||
|---|---|---|---|---|
| VKAs(n=39, 65%) | NOACs(n=21, 35%) | Total(n=60) | ||
| BARC 1 bleeding, n (%) | 6 (15.4) | 0 (0) | 6 (10.0) | 0.058 |
| Stroke, n (%) | 2 (5.1) | 1 (4.8) | 3 (5.0) | 0.950 |
BARC: Bleeding Academic Research Consortium criteria; NOACs: non-vitamin K antagonist oral anticoagulants; VKAs: vitamin K antagonists.
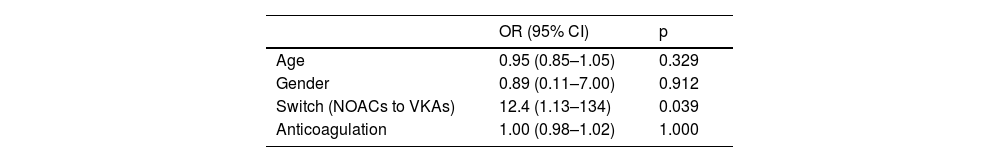
Previous anticoagulation (p=0.002) and switching OAC therapy on discharge from NOACs to VKAs were associated with bleeding (p=0.039), the latter predicting 12 times more events (p=0.039, OR 12.4, 95% CI 1.13–134) (Table 5).
Multivariate analysis (logistic regression) for bleeding events in atrial fibrillation patients.
| OR (95% CI) | p | |
|---|---|---|
| Age | 0.95 (0.85–1.05) | 0.329 |
| Gender | 0.89 (0.11–7.00) | 0.912 |
| Switch (NOACs to VKAs) | 12.4 (1.13–134) | 0.039 |
| Anticoagulation | 1.00 (0.98–1.02) | 1.000 |
CI: confidence interval; NOACs: non-vitamin K antagonist oral anticoagulants; OR: odds ratio; VKAs: vitamin K antagonists.
This study found that treatment with NOACs within three months of bioprosthetic mitral valve implantation or mitral repair was associated with fewer net clinical events, mainly driven by less bleeding. In addition, switching OAC strategy from NOACs to VKAs at discharge in AF patients was associated with more bleeding events. Therefore, these results suggest that NOACs were as safe and as effective as VKAs in this patient cohort.
In the present study, cardiovascular risk factors such as hypertension, dyslipidemia and diabetes resembled those of the groups studied in the RIVER and ENAVLE trials.16,17 From a surgical standpoint, 51.4% of patients underwent mitral BVR and 48.6% MVR; AF was previously diagnosed in 40.5% of patients.
At discharge, 98 (66.2%) patients were on VKAs and 50 (33.8%) on NOACs for at least three months. In the subgroup of AF patients with previous indication for OAC, therapy on admission influenced the OAC strategy on discharge, with 21 patients (55.3%) in the VKA group and 20 (95.2%) in the NOAC group discharged under the same therapy prescribed on admission. Switching OAC on discharge was infrequent (18 patients, 30%), albeit more common in the VKA group (43.6% patients switched from NOACs to VKAs on discharge, p=0.002).
The primary outcome occurred in 22 (22.4%) patients in the VKA group and in three (6%) in the NOAC group (p=0.012), mainly driven by more bleeding events in the former (19.4%, p=0.011). No differences were found with regard to death, rehospitalization, myocardial infarction, stroke or transient ischemic attack, systemic embolism or mitral thrombosis. Similarly, no statistically significant difference was found for the presence of AF (p for interaction=0.823).
Bleeding events were classified as 1 on the BARC criteria scale, and OAC with VKAs on discharge was predicted to generate three times more events. The bleeding rate in the VKA group was similar to the subgroup of patients with bioprosthetic valves included in the trials that compared NOACs to VKAs, although driven by minor bleeding events.16,19–22 This was probably related to the short follow-up period (within three months of surgery) in the study, since NOAC-related bleeding events tend to occur later in the course of treatment.23
The primary efficacy outcome in the ENAVLE trial (death, clinical thromboembolic event, or asymptomatic intracardiac thrombus) favored edoxaban (noninferior and superior) over warfarin, as did safety outcomes (bleeding events) and net clinical outcome, but without statistical difference (noninferior).17 These results were consistent with those in the present study, with some differences: our study showed NOACs to have comparable efficacy and better safety properties compared to VKAs, whereas ENAVLE showed better efficacy and similar safety in patients treated with NOACs; the lower rate of edoxaban use in the present study does not permit a direct comparison between the studies.
Analysis of the subpopulation of AF patients in the present study showed no differences in bleeding or stroke events in the NOAC group compared to VKA patients, although it is suggestive of fewer bleeding events in the former. In the RIVER trial,16 the primary outcome (net clinical outcome) was noninferior for rivaroxaban versus warfarin, and bleeding events also showed no differences between groups, although only 18.8% of patients were enrolled and randomized within three months of surgery. These findings were corroborated by the present subanalysis of AF patients.
Transition between different types of oral OAC may represent a period of increased risk of thromboembolism or bleeding, especially when switching from NOACs to VKAs.24,25 In the ROCKET-AF trial,22 there was an increase in thrombotic events after discontinuation of rivaroxaban and transition to warfarin at the end of the study. At the end of the ARISTOTLE trial26 (apixaban versus warfarin in AF patients), the blinded study drug was stopped, and open label warfarin was recommended. Patients who switched from apixaban to warfarin had more thrombotic and bleeding events. The higher risk of stroke and bleeding in these patients was probably associated with initiation of VKAs (comparable with the group on stable warfarin) rather than with stopping apixaban.24 Similarly, in the present study, switching OAC therapy on discharge in AF patients from NOACs to VKAs was associated with 12-fold higher odds of more bleeding (p=0.039).
While the current 2020 ACC/AHA guidelines provide no recommendation on OAC after MVR, the ESC/EATCS guidelines recommend thromboprophylaxis with VKAs in the first three months, as well as in patients undergoing mitral BVR.8,9 The clinical evidence for antithrombotic therapy after MVR is limited, based mainly on expert opinion, but the use of an annuloplasty ring and the possible occurrence of AF after surgery may justify short-term therapy.27 From a surgical standpoint, the present analysis showed that 64% of those in the NOAC group underwent MVR and 59.2% of the VKA group underwent mitral BVR (p=0.008), and the primary outcome showed no statistical difference between the type of surgery (p for interaction=0.954). NOACs thus appear to be a potential alternative to VKAs.
Although rate and rhythm management of rheumatic AF after valve intervention are suggested in the literature, understanding of the management of antithrombotic therapy after MVR or mitral BVR in RVD patients is limited.28 In this study, RVD was present in 26.7% patients, of whom 87.5% were discharged on VKAs (p=0.028). Nevertheless, bleeding (p for interaction=0.119) and stroke events (p for interaction=0.789) did not differ depending on the presence of RVD.
LimitationsThis was an observational study based on information obtained from medical records and by telephone consultation with patients, and is thus limited in the scope of collectable data. For example, lack of reporting of INR and time in therapeutic range is an acknowledged limitation. The presence of only minor bleeding events (BARC 1) may also limit the significance of the study's conclusions. Finally, the findings from this study, which recruited a small sample from a single center, may not be generalizable to other cardiac centers.
ConclusionNOACs were associated with fewer events within three months of surgical MVR or mitral BVR, supporting their efficacy and safety compared to VKAs. In addition, recent data from large trials predict a promising role for NOACs used in the immediate postoperative period in these patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to express their gratitude to all colleagues who cooperated in providing data for the current analysis.