We examined the potential role of polymorphisms of the platelet genes GP1BA (rs2243093, rs6065 and VNTR), ITGB3 (rs5918), ITGA2 (rs938043469) and P2RY12 (rs2046934, rs6801273 and rs6798347) as risk factors for myocardial infarction (MI).
MethodsThe study population was divided into three groups: controls (n=235), MI at age ≤45 years (MI ≤45, n=44), and MI at age >45 years (MI >45, n=78). The control group was further divided into two subgroups (control ≤45 and >45), and subgroups including only men were also considered for statistical analysis. Polymorphisms were detected by polymerase chain reaction and restriction fragment length polymorphism analysis.
ResultsRegarding non-genetic risk factors, the control group differed statistically from the MI ≤45 group (p<00.5) in terms of smoking, hypertension, diabetes and obesity, and from the MI >45 group (p<0.05) in terms of hypertension, diabetes, obesity, family history of thrombosis and high cholesterol. For the studied ITGA2 polymorphism, a statistical difference was found when MI >45 was compared with the control group, with a higher risk of MI in the TT genotype (OR 2.852; 95% CI: 1.092-7.451; p=0.032). In the GP1BA rs6065 polymorphism, a statistically significant difference was found between control ≤45 only men and MI ≤45 only men, with a higher risk in the CT genotype (OR 5.568; 95% CI: 1.421-21.822; p=0.016), despite the low numbers included. The other polymorphisms studied did not show any statistically significant correlations.
ConclusionThere is a statistically significant association between the TT genotype of the ITGA2 rs938043469 polymorphism and increased risk for MI >45.
Determinou-se o papel de polimorfismos dos genes de expressão plaquetária GP1BA (rs2243093, rs6065 e o VNTR p.Ser415_Thr428(0_4)), ITGB3 (rs5918), ITGA2 (rs938043469) e P2RY12 (rs2046934, rs6801273 e rs6798347) como fatores de risco para o enfarte agudo do miocárdio (EAM).
MétodosA amostra foi dividida em três grupos: Controlo (n=235), EAM ≤45 anos (n=44) e EAM >45 anos (n=78). O grupo Controlo foi ainda dividido em dois subgrupos (Controlo ≤45 e >45). Subgrupos incluindo somente homens também foram considerados para fins estatísticos. Os polimorfismos foram estudados através de PCR e RFLP.
ResultadosEm relação aos fatores de risco não genéticos, o grupo Controlo diferia estatisticamente do grupo EAM ≤ 45 anos (p<0,05) em termos de hábitos tabágicos, hipertensão, diabetes e obesidade e também diferia do grupo EAM >45 anos (p<0,05) nas variáveis hipertensão, diabetes, obesidade, antecedentes familiares de trombose e colesterol. Para o polimorfismo estudado do gene ITGA2, verificou-se uma diferença estatisticamente significativa quando se compararam os grupos EAM >45 anos e Controlo, associando-se o genótipo TT a aumento de risco de EAM (OR 2,852; IC 95% de 1,092 a 7,451; p=0,032). No polimorfismo rs6065 do gene GP1BA foi encontrada uma diferença estatística quando comparados os grupos Controlo ≤ 45 só homens e EAM ≤ 45 só homens, associando-se o genótipo C/T a um maior risco de EAM (OR 5,568; IC 95% de 1,421 a 21,822; p=0,016), apesar do baixo n. Os outros polimorfismos não apresentaram correlação significativa.
ConclusãoExiste uma associação estatística significativa entre o genótipo T/T do polimorfismo rs938043469 do gene ITGA2 e o risco de EAM >45.
Platelet receptors are central to physiological platelet responses. They are involved in platelet thrombus formation after vascular injury, being responsible for platelet adhesion to damaged vessel walls and aggregation. Four platelet receptors – glycoprotein (GP) Ib-IX-V, integrin α2β1 (GPIa-IIa), integrin α2bβ3 (GPIIb-IIIa), and the P2Y12 ADP receptor – are crucial for normal hemostasis.1–4
GPIb-IX-V initiates platelet adhesion to subendothelial von Willebrand factor (vWF) under high shear stress conditions.5 The GPIb subunit is composed of two disulfide-linked polypeptides, alpha and beta. The GPIb alpha subunit contains the binding sites for vWF and alpha-thrombin, both of which activate platelets,6 and is encoded by the GP1BA gene.1
Integrin α2β1 is a specific collagen receptor found in platelets and other cell types that mediates platelet adhesion to collagen following platelet activation.7 It is composed of two subunits, alpha2 and beta1, that are encoded by the ITGA2 and ITGB1 genes, respectively.2
Integrin α2bβ3 plays a pivotal role in platelet aggregation. The major ligands for this glycoprotein are fibrinogen and vWF, and interaction between these ligands and integrin α2bβ3 begins platelet plug formation.8 Integrin alpha2b and beta3 are encoded by the ITGA2B and ITGB3 genes, respectively.3
The purinergic receptor P2Y12 is a Gi-coupled seven-membrane-spanning protein encoded by the P2RY12 gene4 that interacts with ADP.4 ADP stimulates P2Y12-mediated inhibition of adenylyl cyclase and activates intracellular signal transduction pathways that stabilize platelet aggregation.4,9 The fact that this receptor is the target for prasugrel and clopidogrel, both P2Y12 inhibitors used as antiplatelet therapy for patients with acute coronary syndromes, is of clinical relevance.10
These four platelet membrane receptors present genetic polymorphisms that can affect platelet responsiveness.
Three polymorphisms that influence the function and expression of GPIb-IX-V have been described: a variable number of tandem repeats (VNTR) polymorphism; rs6065 (also known as HPA-2); and rs2243093 (Kozak). All of them occur in the GPIb alpha subunit. The VNTR polymorphism, a molecular weight polymorphism within the mucin-like macroglycopeptide region of GPIb alpha, is a 13-amino-acid sequence repeat in which the A allele gives rise to four repeats, B to three repeats, C to two repeats, D to one repeat, and E lacks the 13-amino acid sequence.11 The rs6065 polymorphism is a threonine-to-methionine substitution at position 161 that forms the basis of the HPA-2 platelet alloantigen system.12 The rs2243093 polymorphism is a variation at position -5 from the ATG start codon in which either T or C is present.13
The T allele of the rs938043469 polymorphism (C807T) within the coding region of the alpha2 subunit of integrin α2β1 (GPIa-IIa) is associated with high expression of the receptor and may lead to platelet hyperreactivity, even though this polymorphism does not alter the amino acid sequence of the protein.14,15
At least nine polymorphisms have been described in the beta3 subunit of integrin α2bβ3. Incompatibility for HPA-1 (PlA) alloantigens is the most common cause of fetal and neonatal alloimune thrombocytopenia in Caucasians.16 The rs5918 polymorphism is a T>C transition creating a missense Leu59Pro variant. Studies on the functional consequences of this polymorphism in thrombotic diseases have yielded conflicting results,17,18 but an increase in platelet reactivity has been reported in C allele carriers (PlA2) compared with TT genotype individuals (PlA1/A1).19
Various single nucleotide polymorphisms (SNPs) in intron regions of the P2RY12 gene have been described, some of them associated with greater ADP-induced platelet aggregation, including rs2046934 (C>T), rs6801273 (T>C) and rs6798347 (G>A).20
As platelets play an important role in the pathophysiology of myocardial infarction (MI)21 and there is controversy regarding the possible association of platelet polymorphisms and increased cardiovascular risk,22 further studies in specific populations are necessary to clarify the status of this relationship. In this study, we assessed the potential role of eight polymorphisms, one VNTR and seven SNPs, of four genes coding for platelet receptors (GP1BA, ITGB3, ITGA2 and P2RY12) as risk factors for MI. To this end, two groups of patients were analyzed according to the age at which they suffered MI: 45 years or younger (MI ≤45) and more than 45 years (MI >45).
MethodsPopulationThe study population included only Portuguese Caucasians. Subjects were divided into three groups: controls, MI ≤45 and MI >45. The control group consisted of 235 subjects (57 male and 178 female) with ages ranging from 15 to 69 years (mean 27 years). The characteristics of the control group have been described previously,23 except for polymorphisms of the ADP receptor gene P2RY12. At the time of blood sample collection, subjects had no clinical signs of hemorrhagic or thrombotic disease. The MI ≤45 group consisted of 44 patients (40 male and four female) who had suffered an MI at or before the age of 45 years, with ages ranging from 27 to 45 years (mean 42 years). The MI >45 group consisted of 78 patients (67 male and 11 female) who had suffered an MI after the age of 45 years, with ages ranging from 51 to 97 years (mean 67 years). Clinical data on smoking status, blood pressure, presence of diabetes, body mass index, family history of cardiovascular disorders and total cholesterol levels were collected for the three groups. All subjects gave their written informed consent to the protocol, which was approved by the local ethics committee.
Genotype analysisGenomic DNA was extracted from peripheral blood cells collected by venipuncture in EDTA tubes using a PureLink™ Genomic DNA Mini Kit (Invitrogen). A region containing each polymorphism was amplified by polymerase chain reaction using 1 μg of DNA and 1 μM of specific primers (Table 1). Except for the VNTR polymorphism, amplicons were then digested with specific restriction enzymes (Table 1) and the digested fragments were visualized in a 2% ethidium bromide agarose gel.
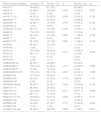
Primers and restriction enzymes used for genotype discrimination.
| Platelet receptor | Gene | Polymorphism | Primers | Restriction enzymes |
|---|---|---|---|---|
| α2bβ3 | ITGB3 | rs5918 (PlA) | F-GGACTTCTCTTTGGGCTCCT R-CTGTCTCCAGAGCCCTTGTC | MspII |
| Ib-V-IX | GP1BA | rs2243093 (Kozak) | F-AGGGGGATCCACTCAAGG R-AGGCGAGTGTAAGGCATCAG | BsuRI |
| rs6065 (HPA-2) | F-GCCAGCCACCTAGAAGTGAA R-AAAAGCAAAAGGCAGGAGGT | Lwel | ||
| VNTR | F-CTGGAGCCCACTCCAAGC R-TTGTGGCAGACACCAGGAT | - | ||
| α2β1 | ITGA2 | rs938043469 (C807T) | F-CTACCGGCCCATGTCTAAAT R-TCTTTGTCTTTTCCTTACTTTTTCA | Hpy188I |
| P2Y12 | P2RY12 | rs2046934 | F-TGCTGAAAATTGAAGCCATAC R-CAAAACAGGGCATACTTTCCA | HpyCH4IV |
| rs6801273 | F-TTGTTGAAATATCAGAAAATGTGAG R-AGTCCACCTGCTGCTATTGA | Bsh1236I | ||
| rs6798347 | F-TGATGTAAGTGGGGAAAGGAA R-CAAGTTTCAAACCCGAGGAA | BseGI |
Genotype and allelic frequencies for the control group were calculated and Hardy-Weinberg equilibrium was tested for the eight polymorphisms by means of a chi-square test using observed vs. expected genotype frequencies. Genotype frequencies in the MI >45 and MI ≤45 groups were compared with the observed genotype frequencies obtained in the control group using a chi-square test. For chi-square test p values ≤0.05, odds ratios (OR) with 95% confidence intervals (CI) were calculated as estimates of the occurrence of MI as a function of the studied polymorphisms. Binary logistic regression analysis was performed to determine the association between MI and the studied variables through calculation of ORs and their 95% CIs. Adjusted ORs were estimated for genetic and physiological profiles using multinomial logistic regression (adjusted maximum-likelihood estimation). The enter method was selected for entering independent variables into the analysis. IBM SPSS for Windows, version 24.0, was used for all statistical calculations.
ResultsTable 2 presents the clinical data of the three study groups, and shows statistically significant differences for non-genetic variables. There were significant differences in smoking status (OR 5.651; 95% CI: 2.2472-14.2041; p<0.001), hypertension (OR 16.755; 95% CI: 4.521-62.500; p<0.001), diabetes (OR 60.948; 95% CI: 3.644-1019.274; p=0.004) and obesity (OR 5.6515; 95% CI: 2.2472-14.2041; p<0.001) between the control and MI ≤45 groups. Hypertension (OR 58.823; 95% CI: 17.544-200.000; p<0.001), diabetes (OR 80.119; 95% CI: 4.812-1334.049; p=0.002), obesity (OR 3.472; 95% CI: 1.013-11.905; p=0.048), family history of thrombosis (OR 8.707; 95% CI: 2.657-28.532; p<0.001) and elevated cholesterol (OR 3.891; 95% CI: 1.292-11.765; p<0.016) were significantly different between the control and MI >45 groups. There was also significant heterogeneity in gender distribution in the MI groups, with a larger proportion of males in both groups (p<0.001 for both).
Clinical characteristics of the study population.
| Controls (n=235) | MI ≤45 (n=44) | MI >45 (n=78) | |
|---|---|---|---|
| Male gender, n (%) | 57 (24.3) | 40 (90.9)a | 67 (85.9)a |
| Active smokers, n (%) | 62 (26.3) | 30 (68.2)a | 27 (34.6) |
| Hypertension, n (%) | 9 (3.8) | 16 (36.4)a | 54 (69.2)a |
| Diabetes, n (%) | 0 (0.0) | 10 (22.7)a | 22 (28.2)a |
| Obesity (BMI >30 kg/m2), n (%) | 8 (3.4) | 11 (25.0)a | 19 (24.4)a |
| Family history of thrombosis, n (%) | 107 (45.5) | 25 (56.8) | 6 (7.7)a |
| Total cholesterol >200 mg/dl, n (%) | 59 (25.1) | 11 (25.0) | 43 (55.1)a |
The chi-square test was used to compare variables between the control and MI groups.
BMI: body mass index; MI ≤45: myocardial infarction at or before the age of 45 years; MI >45: myocardial infarction after the age of 45 years.
Genotypes were in Hardy-Weinberg equilibrium for all polymorphisms in the control group. Table 3 shows the genotype frequencies for the eight polymorphisms in the three study groups and the p values obtained when the MI groups were compared with the control group for each polymorphism. Table 3 also shows the same comparison between groups but adding the homozygotic and heterozygotic genotypes for the allele considered of higher risk. This association of genotypes was performed due to the low numbers of patients with some genotypes. As can be seen, the p values for the rs5918 polymorphism of integrin beta3 were not significant, meaning that there were no statistical differences between the control and MI groups (p=0.579 and p=0.359 for MI >45 and p=0.407 and p=0.722 for MI ≤45).
Genotype frequencies for the rs5918 (PlA), rs2243093 (Kozak), rs6065 (HPA-2), VNTR, rs938043469 (C807T), P2Y12 rs2046934, P2Y12 rs6801273 and P2Y12 rs6798347 polymorphisms.
| Polymorphism genotypes | Controls, n (%) | MI >45, n (%) | pa | MI ≤45, n (%) | pb |
|---|---|---|---|---|---|
| rs5918 TT | 144 (69.2) | 57 (74.0) | 0.579 | 28 (66.7) | 0.407 |
| rs5918 TC | 55 (26.4) | 18 (23.4) | 14 (33.3) | ||
| rs5918 CC | 9 (4.4) | 2 (2.6) | 0 (0.0) | ||
| rs5918 TC+CC | 64 (30.8) | 20 (26.0) | 0.359 | 14 (33.3) | 0.722 |
| rs2243093 TT | 168 (78.9) | 62 (80.5) | 0.778 | 29 (69.0) | 0.104 |
| rs2243093 TC | 44 (20.7) | 15 (19.5) | 13 (31.0) | ||
| rs2243093 CC | 1 (0.4) | 0 (0.0) | 0 (0.0) | ||
| rs2243093 TC+CC | 45 (21.1) | 15 (19.5) | 0.728 | 13 (31.0) | 0.118 |
| rs6065 CC | 172 (81.5) | 62 (80.5) | 0.825 | 31 (73.8) | 0.199 |
| rs6065 CT | 39 (18.5) | 15 (19.5) | 11 (26.2) | ||
| rs6065 TT | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| rs6065 CT+TT | 39 (18.5) | 15 (19.5) | 0.825 | 11 (26.2) | 0.199 |
| VNTR BC | 35 (16.1) | 12 (15.9) | 0.373 | 11 (27.0) | 0.236 |
| VNTR BD | 2 (0.9) | 1 (1.1) | 0 (0.0) | ||
| VNTR CC | 153 (70.5) | 49 (65.9) | 27 (62.2) | ||
| VNTR CD | 24 (11.1) | 14 (15.9) | 4 (10.8) | ||
| VNTR CE | 1 (0.5) | 0 (0.0) | 0 (0.0) | ||
| VNTR D/D | 2 (0.9) | 1 (1.1) | 0 (0.0) | ||
| rs938043469 CC | 82 (38.7) | 22 (28.2) | 0.011c | 20 (45.5) | 0.654 |
| rs938043469 CT | 109 (51.4) | 41 (52.6) | 20 (45.5) | ||
| rs938043469 TT | 21 (9.9) | 15 (19.2) | 4 (9.0) | ||
| rs938043469 CT+TT | 130 (61.3) | 56 (71.8) | 0.057 | 24 (54.5) | 0.358 |
| rs2046934 AA | 177 (75.3) | 44 (64.7) | 0.118 | 31 (72.1) | 0.197 |
| rs2046934 AG | 52 (22.1) | 22 (32.4) | 9 (20.9) | ||
| rs2046934 GG | 6 (2.6) | 2 (2.9) | 3 (7.0) | ||
| rs2046934 AG+GG | 58 (24.7) | 24 (35.3) | 0.043c | 12 (27.9) | 0.238 |
| rs6801273 TT | 86 (36.6) | 26 (38.2) | 0.331 | 18 (41.9) | 0.608 |
| rs6801273 TC | 123 (52.3) | 31 (45.6) | 22 (51.1) | ||
| rs6801273 CC | 26 (11.1) | 11 (16.2) | 3 (7.0) | ||
| rs6801273 TC+CC | 149 (63.4) | 42 (61.8) | 0.780 | 25 (58.1) | 0.474 |
| rs6798347 GG | 152 (64.7) | 41 (59.4) | 0.175 | 26 (60.5) | 0.828 |
| rs6798347 GA | 72 (30.6) | 27 (39.1) | 15 (34.9) | ||
| rs6798347 AA | 11 (4.7) | 1 (1.5) | 2 (4.7) | ||
| rs6798347 GA+AA | 83 (35.3) | 28 (40.6) | 0.359 | 17 (39.5) | 0.561 |
MI ≤45: myocardial infarction at or before the age of 45 years; MI >45: myocardial infarction after the age of 45 years.
There was also no statistically significant difference between the control and MI groups for GPIb alpha polymorphisms. The p value for the rs2243093 polymorphism was 0.104 when MI ≤45 was compared with the control group. It is important to note that for this polymorphism only one homozygote (CC) was found, corresponding to one control individual. For the rs6065 polymorphism, as in the previous case, although the prevalence of CT heterozygotes was higher in MI ≤45 (26.2%) compared with the control group (18.5%), the chi-square test was not statistically significant (p=0.199). No homozygotes (TT) were found for this polymorphism in all of the study population. When the control group was divided into two subpopulations by age (control ≤45 years and control >45 years) and only males were included, rs6065 was the only polymorphism that showed a statistical difference: p<0.001 for control ≤45, males only (n=44) compared with MI ≤45, males only (n=38). The phenotype distribution was 41 CC and 3 CT for control ≤45, males only and 27 CC and 11 CT for MI ≤45, males only. When ORs were calculated for CT vs. CC genotypes in MI ≤45, males only, the p value was 0.016 (OR 5.568; 95% CI: 1.421-21.822). No adjusted ORs were calculated due to the low numbers in these subgroups (Table 4).
Odds ratios of polymorphisms that showed a statistically significant chi-square test.
| Genotypes | OR (95% CI) | p | Adjusted OR (95% CI) | pa | |
|---|---|---|---|---|---|
| rs938043469 (controls vs. MI >45) | TT vs. CC | 2.662 (1.181-6.001) | 0.027b | 2.852 (1.092-7.451) | 0.032b |
| rs2046934 (controls vs. MI >45) | AG+GG vs. AA | 1.665 (0.933-2.970) | 0.090 | - | - |
| rs6065 (controls ≤45 vs. MI ≤45, males only) | CT vs. CC | 5.568 (1.421-21.822) | 0.016b | - | - |
CI: confidence interval; MI ≤45: myocardial infarction at or before the age of 45 years; MI >45: myocardial infarction after the age of 45 years; OR: odds ratio.
With respect to the other GPIb alpha polymorphism studied, VNTR, no differences were found in chi-square tests (Table 3). In our study population, no A allele for VNTR was found.
For the integrin alpha2 polymorphism rs938043469, a significant difference was found in the p value when the MI >45 group was compared with the control group (p=0.011). This difference was mainly due to a greater proportion of TT homozygotes (19.2% in MI >45 vs. 9.9% in controls) and fewer CC homozygotes (28.2% in MI >45 vs. 38.7% in controls) (Table 3). Accordingly, the OR for MI >45 was significantly higher in TT vs. CC genotypes (OR 2.662, CI: 1.181-6.001, p=0.027) (Table 4). After adjustment, the OR remained statistically significant, meaning that patients with a TT genotype have a greater risk of MI after the age of 45 years independently of other variables (OR 2.852, 95% CI: 1.092-7.451, p=0.032) (Table 4). No differences were found between the control and MI ≤45 groups for this polymorphism. When both patient groups were compared by means of binary logistic regression analysis, patients with the TT genotype were more likely to suffer MI after the age of 45 years (OR 4.528, 95% CI: 1.047-19.576, p=0.043).
For the three P2RY12 polymorphisms studied, there was a significant difference in rs2046934 when the MI >45 and control groups were compared in terms of associated genotypes, AG+GG (p=0.043). This difference was due to a greater proportion of heterozygotes in the MI >45 group compared with controls. However, when ORs were calculated comparing the AA and AG+GG genotypes, the difference was not statistically significant (OR 1.665, CI: 0.933-2.970, p=0.090) (Table 4).
DiscussionIn this study, we analyzed the effect of several platelet gene polymorphisms in patients who had suffered MI. These patients were divided into two groups according to the age at which they suffered MI: 45 years or younger and more than 45 years.
According to our results, the only polymorphism significantly associated with an increased risk for MI in the overall study population was rs938043469 in the alpha2 subunit (GPIa) of integrin α2β1. We also concluded that the presence of the T allele in homozygosity is essential for this increased risk of MI. Nevertheless, the rs938043469 polymorphism does not appear to influence early onset of MI, since the results for the MI ≤45 group showed no correlation between the presence of this polymorphism and MI (Table 3). The frequency of the TT genotype in the control group (9.9%) was within the range of previous reports.24–26 The proportion of the TT genotype in the MI >45 group (19.2%) was higher than in the control group. Since Kunicki et al. first described this polymorphism in 1997,27 several studies have assessed the relationship between the polymorphism and cardiovascular disease, mainly in younger adults, with conflicting results.25,28 In 2007, a meta-analysis of published data regarding the role of rs938043469 in coronary artery disease (CAD) concluded that there was no association between this polymorphism and CAD.29 Nevertheless, our results were very similar to those of Jian-Xia Lu et al. in Chinese patients who had suffered ischemic stroke,24 despite the differences in ethnicity and cardiovascular diseases. Moreover, a recent meta-analysis found that the T allele or TT genotype of the rs938043469 polymorphism were associated with increased risk for ischemic stroke.30
Although a silent polymorphism, rs938043469 is believed to influence the density of integrin α2β1 (GPIa-IIa) in the platelet membrane, with the T allele being responsible for the highest expression of the glycoprotein.14,15 This variation in receptor density may account for greater platelet responsiveness to collagen in the TT genotype, enhancing the thrombotic potential of platelets in pathological states. However, it should be remembered that this receptor, despite its importance for platelet adhesion to subendothelial collagen, particularly in static conditions, is not physiologically as important as GPIb-V-IX for the initiation of adhesion to subendothelial tissue in conditions of high shear stress as found in the arterial circulation.31 This may explain the difficulty in finding statistically significant evidence for the role of this polymorphism in certain cardiovascular diseases. The conflicting results found may also reflect different study designs, patients and diseases24–26,28,30 and the difficulty in establishing that a single polymorphism is in itself crucial to such a complex and multifactorial event as MI.
In many studies, including the present one, some results may fail to reach statistical significance, and as a result it may be impossible to decide definitively whether a particular association exists. Nevertheless, in our opinion, such results should not necessarily be disregarded, but should be analyzed carefully in order to determine their biological significance. Examples of such results are those concerning the rs2243093, rs6065, VNTR and rs2046934 polymorphisms in our MI groups.
Analysis of the rs2243093 polymorphism revealed a higher proportion of the TC genotype in the MI ≤45 group than in controls and the chi-square test was very close to significance (p=0.104). A similar result was found in two studies in which the rs2243093 polymorphism was associated with an increased risk of ischemic stroke.32,33 In these studies, a higher frequency of the TC genotype was reported.32,33 Another study associating the rs2243093 polymorphism and risk of CAD published in 2015 by Zhang et al. found a high frequency of the CC genotype and concluded that this genotype is a biomarker of genetic susceptibility.34 Interestingly, there was only one individual with the CC genotype in our control group, corresponding to a frequency of 0.4% in the overall population, which is within the range of other populations.13 This polymorphism lies in the 5′ UTR of the GP1BA gene, where a T or a C may be present in position -5 from the ATG start codon. The C allele has been associated with increased expression of the receptor on the cell membrane,13 which may explain the link between this polymorphism and greater likelihood of arterial thrombus formation in some vascular pathologies, due to increased platelet adhesiveness. Nevertheless, our results did not show a clear correlation between the Kozak polymorphism and MI in any of the MI groups studied.
In the rs6065 polymorphism, threonine or methionine is found at position 161 of GPIb alpha due to a C1018T nucleotide change.35 rs6065 is of clinical importance because it is implicated in neonatal alloimmune thrombocytopenic purpura, post-transfusion purpura, and refractoriness to HLA-matched platelet transfusion.36 The presence of methionine has been associated with increased risk for MI and CAD in several studies.34,37 However, in a study of the functional effects of this polymorphism in 2003, Ulrichts et al. described a stronger interaction between the GPIb alpha subunit and vWF in glycoproteins that carry threonine (C allele) than methionine (T allele).38 This finding does not agree with the above results. Nevertheless, as the authors point out in their paper, vWF binding to GPIb alpha was investigated in a stationary state and not in the shear stress conditions usually seen in a vascular stenosis.38 We found a higher percentage of CT heterozygotes in the MI ≤45 group than in the control group, while in the MI >45 group no differences were found from the control group. These results, although not statistically significant, are in agreement with the findings of Mikkelsson et al., who reported a correlation between the presence of Met161 and sudden cardiac death in men aged less than 55 years.37 Interestingly, when we reanalyzed the statistics including only men in the control ≤45 and MI ≤45 groups, there was a significant difference (p<0.001) in genotype distribution, with a higher percentage of heterozygotes in MI ≤45 compared with control ≤45. This result, despite the low numbers involved, especially in control ≤45, highlights the possible contribution of the rs6065 polymorphism to early MI.
For the VNTR polymorphism in GPIb alpha, no A allele (four repeats) and one rare E allele, which has only been reported in Caucasians, were found in our study groups.39 The A allele has been reported mainly in Japanese and Native American populations.40 The most interesting result that we observed regarding this polymorphism was the higher frequency of the BC genotype in MI ≤45 than in the control and MI >45 groups. However, the p value was not significant. This may be due to the large number of classes (six genotypes) and the small sample size, especially in the MI ≤45 group. The literature often attributes a protective role to the CC genotype,41 and it should be noted that that the CC genotype was more frequent in the control group than in the MI >45 and MI ≤45 groups. Studies assessing the physiological response of each of the different sized GPIb alpha subunits are needed. In one attempt to help clarify this issue we conducted a study in which platelets with different VNTR genotypes were subjected to PFA-100® testing using collagen/ADP and collagen/epinephrine cartridges and occlusion time was measured. Our preliminary results showed that platelets with the CC genotype take longer to occlude the cartridge than platelets bearing a B allele (BC or BD), supporting its previously reported protective role (data not shown).
The p value for the rs2046934 polymorphism was statistically significant. This difference was found when AG and GG genotypes were associated and, as in the C807T polymorphism, the difference was seen in the MI >45 group. When associated, the difference was due to a greater number of heterozygotes in the MI >45 than in the control group. rs2046934 is an intronic polymorphism that does not alter the amino acid sequence of the protein and is in linkage disequilibrium with other polymorphisms that also do not change the protein structure of the receptor. All these polymorphisms form the H2 haplotype of the P2RY12 gene.20 In aggregometry tests, the H2 haplotype was associated with higher maximal aggregation in response to ADP than the H1 haplotype.20 This result suggests a possible relationship between the H2 haplotype and atherothrombosis. However, a study by Amisten et al. found no association between the H2 haplotype and MI.42 In the present study, although there were differences in the chi-square test result, the p value of an odds ratio test calculated for AG+GG vs. AA genotypes in the MI >45 group was not statistically significant. In a recent study assessing the effects of platelet polymorphisms on antiplatelet drug responsiveness and clinical outcomes in patients with acute minor ischemic stroke that included the P2RY12 gene, the authors did not find that any of the P2RY12 polymorphisms that we also studied had a pathophysiological effect.43 Additional studies should be conducted to elucidate the pathophysiological role, if any, of the rs2046934 polymorphism. For the other P2RY12 polymorphisms studied no statistical differences were found.
The rs5918 polymorphism showed no correlation or trend with MI in any MI group. Although several studies have suggested an association between PlA2 (the C allele) and arterial events,44,45 we found no statistical correlation between this polymorphism and MI, as also reported in several other studies,46,47 and controversy remains concerning the relationship between this polymorphism and cardiovascular pathology.
The results of the present work, despite its inherent limitations, particularly a limited number of patients in our MI groups and the heterogeneity between controls and MI patient groups, provide important hints on the possible role of platelet receptor polymorphisms in thrombotic disease. The most important conclusion of our work is that the TT genotype of the rs938043469 polymorphism of integrin alpha2 (GPIa) is significantly associated with the presence of MI in patients older than 45 years. Further studies with larger and more homogenous cohorts may support our findings and help to clarify the role of the rs6065 and rs2046934 polymorphisms and their association with MI. It is important to point out that the two groups of MI patients also differ in non-genetic variables, and so further studies are required to understand the molecular pathways associated with the polymorphisms under study. In addition, the association between these polymorphisms and clinical outcomes should be studied to enable better stratification of MI patients according to their genetic profile.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study was supported by Fundação para a Ciência e Tecnologia (UID/BIO/04565/2013) and Programa Operacional Regional de Lisboa, POR Lisboa 2020 (contract LISBOA-01-0145-FEDER-007317).