Cardiac magnetic resonance (CMR)-based tissue tracking (TT) enables quantification of myocardial deformation and may be used as an objective measure of myocardial involvement in myocarditis. The aims of this study were to characterize myocardial deformation alterations in myocarditis and to determine their relationship with the extent of late gadolinium enhancement (LGE), regional wall motion abnormalities (WMA) and left ventricular ejection fraction (LVEF).
MethodsA single-center, retrospective study was conducted by identifying patients with clinically suspected myocarditis who underwent CMR between 2012 and 2016. The myocardial deformation parameters were derived by TT and correlated with LVEF, LGE and WMA, through Spearman's coefficient.
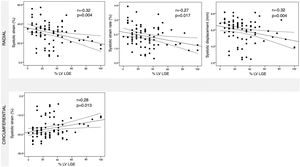
ResultsA cohort of 78 patients with myocarditis (aged 42.7±17.2 years) were included. CMR characteristics including morphologic parameters (LVEF 52.1±12.8%), extent of WMA (29.3±41.0%) and of LGE (30.5±21.8%) were assessed. Significant correlations were found between all deformation parameters (strain, strain rate, velocity and displacement) and both LVEF and extent of WMA. LGE was significantly correlated with systolic radial strain (r: -0.32, p=0.004), strain rate (r: -0.27, p=0.017) and displacement (r: -0.32, p=0.004) as well as systolic circumferential strain (r: 0.28, p=0.013).
ConclusionDeformation parameters are an objective method for quantification of myocardial function in myocarditis. They correlate with LVEF, extent of WMA and degree of myocardial damage. Further studies are needed to assess their incremental beneficial value for the diagnosis and risk stratification of myocarditis.
A avaliação de tissue tracking (TT) por ressonância magnética cardíaca (RMC) permite quantificar a deformação miocárdica e pode ser usada como uma medida objetiva em doentes com miocardite. O objetivo deste estudo foi caraterizar as alterações de deformação miocárdica na miocardite e determinar a sua relação com a quantidade de realce tardio (RT), alterações da contractilidade segmentar (ACS) e fração de ejeção ventricular esquerda (FEVE).
MétodosConduzimos um estudo unicêntrico, retrospectivo, que incluiu doentes com suspeita de miocardite, submetidos a RMC de 2012 a 2016. Os parâmetros de deformação miocárdica derivados por TT foram correlacionados com a FEVE, o RT e as ACS, através do coeficiente de Spearman.
ResultadosForam avaliadas as RMC de 78 doentes com miocardite (42,7±17,2 anos), incluindo parâmetros morfológicos (FEVE: 52,1±12,8%), quantidade de ACS (29,3±41,0%) e extensão de RT (30,5±21,8%). Encontrámos correlações significativas de todos os parâmetros de deformação (strain, strain rate, velocidade e deslocamento) com a FEVE e com a quantidade de ACS. O RT correlacionou-se de forma significativa apenas com o strain (r: -0,32, p=0,004), strain rate (r: -0,27, p=0,017) e deslocamento radial sistólico (r: -0.32, p=0.004), assim como com o strain circunferencial sistólico (r: 0,28, p=0,013).
ConclusãoOs parâmetros de deformação representam uma medida objetiva para quantificar a função nos doentes com miocardite. Relacionam-se com a FEVE, quantidade de ACS e extensão de lesão miocárdica. São necessários estudos adicionais que avaliem o benefício destes parâmetros no diagnóstico e estratificação do risco nos doentes com miocardite.
Due to the diversity of its clinical presentation, the diagnosis of myocarditis remains one of the most challenging in cardiology.1,2 The current gold standard, endomyocardial biopsy, is limited by its periprocedural risks and low diagnostic sensitivity due to sampling error.1,2 Therefore, cardiac magnetic resonance (CMR) has now become the gold standard non-invasive diagnostic modality in suspected myocarditis. Due to its unique ability to combine morphological and functional imaging with myocardial tissue characterization, CMR enables the detection of the typical features of acute inflammation, such as edema, hyperemia, and necrosis, that are usually associated with myocardial dysfunction.1–3 However, the current Lake Louise criteria for CMR-based diagnosis of myocarditis4 still lack diagnostic accuracy.3 Recently, novel quantitative CMR techniques such as tissue tracking (TT)-based strain analysis have emerged as potential novel diagnostic tools,5,6 aiming at improved diagnostic accuracy in myocarditis.7
The aims of this study were to characterize myocardial deformation alterations in patients with myocarditis by applying CMR-TT, and to correlate systolic and diastolic deformation mechanics with left ventricular ejection fraction (LVEF), extent of regional wall motion abnormalities (WMA), and extent of late gadolinium enhancement (LGE).
MethodsStudy populationThis was a single-center retrospective study in which all CMR studies performed between January 2012 and December 2016 were reviewed. During this period, a total of 120 patients were consecutively referred to our department for CMR imaging for clinically suspected myocarditis, based on the current recommendations given by the position statement of the European Society of Cardiology Working Group on myocardial and pericardial diseases2 (Table 1).
Classification of patients with suspected myocarditis according to clinical criteria.
| Myocarditis patients, % | |
|---|---|
| Clinical symptoms consistent with myocarditis | 100 |
| New-onset (days up to 3 months) or worsening of: dyspnea at rest or exercise, and/or fatigue, with or without left and/or right heart failure signs | 60.2 |
| Acute chest pain | 37.2 |
| Palpitations/arrhythmia symptoms/syncope/aborted sudden cardiac death | 2.6 |
| Diagnostic criteria consistent with myocarditis | 100 |
| Functional and structural abnormalities on cardiac imaging (echocardiography/angiography/CMR) | 52.6 |
| Elevated hsTnT | 47.4 |
| Exclusion of coronary artery disease or ischemia | 100 |
| CMR | 53.8 |
| Cardiac catheterization | 35.9 |
| Cardiac computed tomography angiography | 10.3 |
CMR: cardiac magnetic resonance; hsTnT: high-sensitivity troponin T.
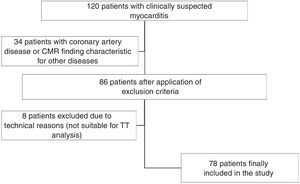
Exclusion criteria for the study were CMR findings characteristic for diseases other than myocarditis or any evidence of coronary artery disease (CAD) or ischemia from previous documented medical history, any imaging findings of CAD or ischemia, or significant epicardial coronary stenosis (>50% obstructive stenosis) by invasive coronary angiography. Of 86 available data sets, eight (9.3%) were excluded due to severe motion artifacts on cine imaging not suitable for subsequent TT analysis. Datasets from a total of 78 patients with suspected myocarditis were finally included (Figure 1). Clinical data and biomarkers such as peak C-reactive protein (CRP), peak N-terminal pro-B-type natriuretic peptide (NT-proBNP) and peak high-sensitivity cardiac troponin T (hsTnT; Elecsys Troponin T high sensitive, COBAS E601, Roche Diagnostics, Penzberg, Germany) were determined.
Cardiac magnetic resonance imaging protocol and image post-processingStudies were performed on a 1.5 T Siemens scanner (MAGNETOM Symphony TIM, Siemens Medical Solutions, Erlangen, Germany). Cine images for functional and TT analyses were acquired in 2-, 3-, and 4-chamber views, and in short-axis (SAX) views using a steady-state free precession (SSFP) sequence during breath-hold and with retrospective ECG triggering (repetition time/echo time: 2.4/1.2 ms; flip angle: 60 degrees; in-plane resolution 1.4 mm×1.4 mm; slice thickness: 8 mm). Typically, the number of reconstructed phases was 30 and the number of segments was adjusted aiming for a temporal resolution of 20-40 ms.
Myocardial edema was assessed by applying a T2-weighted triple-inversion recovery black blood sequence. Early gadolinium enhancement was assessed using fast spin-echo T1-weighted images during the first minutes after administration of a bolus of 0.2 mmol/kg of body weight of gadolinium-based contrast agent (Gadovist, Bayer Healthcare, Leverkusen, Germany). LGE images were acquired approximately 10-15 min after gadolinium administration, using a segmented gradient echo inversion recovery sequence.
Two physicians experienced in CMR analyzed the data and performed the measurements. Readers were blinded to patient information. Images were post-processed using commercially available software (cvi42, Circle Cardiovascular Imaging Inc., Calgary, Canada).
Epicardial and endocardial borders of the LV myocardium were manually traced in end-diastolic and end-systolic phases on SAX cine images in order to calculate functional parameters: LVEF, LV end-diastolic volume (LVEDV), indexed LVEDV (LVEDVi), LV end-systolic (LVESV), indexed LVESV (LVESVi), LV mass and indexed LV mass. Papillary muscles were included in the left ventricular cavity volume.
The long-axis images and SAX stack were viewed simultaneously to cross-check for the presence of WMA and LGE, which when present were assigned a location within the standard American Heart Association segment model and quantified as a percentage of the segments involved (%LV WMA and %LV LGE, respectively).8 The presence of subepicardial and/or intramyocardial LGE served as a reference standard for myocarditis. A CMR diagnosis of myocarditis was based on the presence of two or more out of three Lake Louise criteria.4
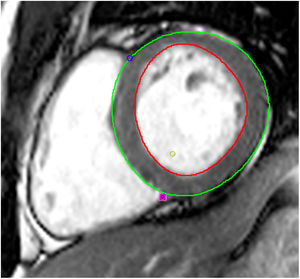
Tissue-tracking analysisTT analysis was performed using cvi42 software. For LV SAX sequences, the endocardial border was manually traced in ventricular end-diastole and the epicardial border was automatically detected and manually adjusted if deemed inadequate (Figure 2). Three levels were assessed: the atrioventricular ring (basal), the mid-ventricle where the papillary muscles were best visualized (mid), and the LV apex. The same technique was applied to 4-, 2- and 3-chamber sequences.
An average of the measurements of three SAX enabled calculation of global peak systolic radial strain, strain rate, velocity and displacement, and global peak systolic circumferential strain and strain rate. Global peak systolic longitudinal strain, strain rate, velocity and displacement were derived from the measurements of the 4-, 2- and 3-chamber cine images. As a measure of LV diastolic function, global peak radial, circumferential and longitudinal strain rate during early filling were also assessed.
Statistical analysisThe normality of continuous variables was assessed by histogram observation and the Shapiro-Wilk test. Continuous data are presented as mean ± standard deviation or median (interquartile range [IQR]) as appropriate. The Student's t test or the Mann-Whitney U test were used to compare two groups for parametric and non-parametric data, respectively. Categorical variables are presented as count (percentage) and difference between groups were analyzed by chi-square tests or Fisher's exact test. Correlation analysis was performed using Spearman's rank correlation coefficient to establish the relationship between LV systolic and diastolic myocardial mechanics and LVEF, %LV WMA and %LV LGE.
Reproducibility of TT analysis was assessed by inter- and intraobserver agreement using coefficient of variation (CoV). A two-sided p-value <0.05 was considered statistically significant. The statistical analysis was performed with IBM SPSS Statistics 22.0 (IBM Corp, Armonk, NY, USA).
ResultsClinical and cardiac magnetic resonance characteristics of myocarditis patientsA total of 78 patients with myocarditis formed the study cohort (Table 2). Their mean age was 42.7±17.2 years and 68 (87.2%) were male. The criteria for clinically suspected myocarditis were mostly the presence of dyspnea and/or signs of LV dysfunction (60.2%) or acute chest pain (37.2%). In total, 28 (39.5%) had had a recent infection in the previous three weeks (either respiratory or gastrointestinal infections). The median number of days from symptom onset to CMR was 11 (IQR: 2-138 days).
Characteristics of patients with myocarditis.
| All patients (n=78) | |
|---|---|
| Male gender, n (%) | 68 (87.2%) |
| Age, years | 42.7±17.2 |
| Body mass index, kg/m2 | 26.9±4.2 |
| Recent infection | 28 (39.5%) |
| Laboratory tests | |
| Peak hsTnT, ng/ml | 1.1±1.2 |
| Peak CRP, mg/dl | 8.5±8.4 |
| Peak NT-proBNP, pg/ml | 3429.8±787.2 |
Results are mean ± standard deviation or count (percentage).
CRP: C-reactive protein; hsTnT: high-sensitivity troponin T; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
Overall CMR morphological and functional findings are summarized in Table 3. In this cohort, 27 (34.6%) cases had LVEF <50% on CMR and 51 (65.4%) had preserved LVEF (≥50%). LV WMA was observed in 36 (46.2%) patients with myocarditis and LV LGE in 77 (98.7%) patients. The median number of segments with LGE was 4.0 (IQR: 2.8-7.0), which corresponds to 23.5% of segments involved (IQR: 16.2-41.2%).
Baseline cardiac magnetic resonance characteristics.
| All patients (n=78) | |
|---|---|
| LVEF, % | 52.1±12.8 |
| LVEDV, mlLVEDVi, ml/m2 | 190.4±54.595.4±31.9 |
| LVESV, mlLVESVi, ml/m2 | 96.2±54.147.3±26.7 |
| LV mass, gLV mass index, g/m2 | 139.8±33.869.8±24.2 |
| No. of LV segments with WMA | 1.0 (0.0-8.8) |
| % LV WMA | 5.9 (0.0-51.5) |
| No. of LV segments with edema (T2-weighted) | 4.0 (1.8-6.0) |
| % LV with edema (T2-weighted) | 23.5 (10.6-35.3) |
| No. of LV segments with LGE | 4.0 (2.8-7.0) |
| % LV LGE | 23.5 (16.2-41.2) |
| LGE pattern | |
| Epicardial, % | 49.4% |
| Mid-wall, % | 16.9% |
| Epicardial and mid-wall, % | 33.8% |
Values are displayed as mean ± standard deviation or median (interquartile range) as appropriate.
LGE: late gadolinium enhancement; LV: left ventricular; LVEDV: left ventricular end-diastolic volume; LVEDVi: indexed left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESV: left ventricular end-systolic volume; LVESVi: indexed left ventricular end-systolic volume; WMA: wall motion abnormalities.
Overall baseline characteristics, laboratory data and CMR characteristics including LGE parameters are depicted in Tables 1-3.
Assessment of myocardial deformation in myocarditis patientsTT-derived deformation parameters at global and regional levels are shown in Table 4. Figures 3-5 show the relationship between global LV systolic and diastolic deformation mechanics and extent of LV WMA, LV LGE and LVEF, respectively. Significant correlations were observed between both LVEF and LV WMA and all myocardial deformation parameters, while LV LGE was only significantly correlated with global peak systolic radial strain, strain rate and displacement, and global peak systolic circumferential strain.
Deformation results from tissue tracking.
| All patients (n=78) | ||||
|---|---|---|---|---|
| Systolic TT parameters | Strain (%) | Radial | BasalMidApicalGlobal | 32.6±12.127.9±11.135.9±15.630.8±11.2 |
| Circumferential | BasalMidApicalGlobal | -17.0±4.7-16.1±5.0-19.0±6.3-17.0±4.9 | ||
| Longitudinal | 4-chamber2-chamber3-chamberGlobal | -16.3±4.6-16.3±4.9-16.3±4.7-16.3±4.4 | ||
| Strain rate (s-1) | Radial | BasalMidApicalGlobal | 2.1±1.11.7±0.72.2±1.01.8±0.7 | |
| Circumferential | BasalMidApicalGlobal | -1.1±0.8-1.0±0.3-1.3±0.5-1.1±0.3 | ||
| Longitudinal | 4-chamber2-chamber3-chamberGlobal | -1.0±0.3-0.9±0.5-0.9±0.5-0.9±0.3 | ||
| Velocity (mm/s) | Radial | BasalMidApicalGlobal | 43.6±29.736.4±11.835.1±10.537.1±10.7 | |
| Longitudinal | 4-chamber2-chamber3-chamberGlobal | 46.9±27.733.9±82.249.4±28.339.5±11.8 | ||
| Displacement (mm) | Radial | BasalMidApicalGlobal | 6.8±1.45.7±1.45.5±1.65.9±1.4 | |
| Longitudinal | 4-chamber2-chamber3-chamberGlobal | 5.3±2.36.8±2.66.0±1.95.9±2.0 | ||
| Diastolic TT parameters | Strain rate (s-1) | Radial | BasalMidApicalGlobal | -2.9±1.3-1.9±1.0-2.8±1.3-2.1±1.0 |
| Circumferential | BasalMidApicalGlobal | 1.3±0.71.1±0.51.5±0.71.1±0.4 | ||
| Longitudinal | 4-chamber2-chamber3-chamberGlobal | 1.1±0.31.1±0.41.0±0.31.0±0.3 |
Data are presented as mean ± SD.
TT: tissue tracking.
Of the different biomarkers assessed (CRP, NT-proBNP and hsTnT), only higher NT-proBNP levels were significantly correlated with worse myocardial deformation measures, in particular, global peak systolic radial strain (r: -0.47, p=0.025) and displacement (r: -0.60, p=0.002) as well as global peak systolic longitudinal strain (r: 0.62, p=0.002), displacement (r: -0.54, p=0.008) and velocity (r: -0.43, p=0.040). Comparison between LGE patterns showed no significant differences for all myocardial deformation parameters.
In our myocarditis population, mean global peak systolic radial, circumferential and longitudinal strain was 30.8%, -17.0% and -16.3%, respectively, which are significantly lower than the reported reference values for healthy subjects (radial: 39.1%, circumferential: -23.6% and longitudinal: -19.4%) derived from a meta-analysis.9
Comparison of the current reference values9 only with the subgroup of patients with preserved LVEF (n=51 patients) shows that radial strain fell below the mean reference value in 37 (72.5%) of cases. This was also observed in 46 (90.2%) of cases for circumferential strain and in 34 (66.7%) of cases for longitudinal strain. Combining all these assessed strain values (radial, circumferential and longitudinal), in 30 (58.8%) of cases all values were worse than the reference values and in 47 (92.2%) of cases at least one of the strain values was worse than the reference value. A further examination of these patients with preserved LVEF found non-significant correlations between LGE or regional WMA and myocardial deformation parameters. Interestingly, higher values of indexed LV mass were correlated with worse values of diastolic parameters, both global peak radial (r: 0.31, p=0.028) and longitudinal (r: -0.30, p=0.031) strain rate.
Tissue tracking: inter- and intraobserver coefficient of variationInter- and intraobserver agreement for TT analysis is shown in Table 5. The CoV of interobserver variability ranged from 1.7% to 11.1%, while that for intraobserver variability was 1.9-13.0%. Global systolic longitudinal strain appears to be the most robust (interobserver CoV: 1.7%; intraobserver CoV: 1.9%) with the poorest agreement for global diastolic radial (interobserver CoV: 10.3%; intraobserver CoV: 13.0%) and circumferential strain rates (interobserver CoV: 11.1%; intraobserver CoV: 12.3%).
Tissue tracking: inter- and intraobserver coefficient of variation for systolic and diastolic deformation.
| Systolic | Diastolic | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radial | Circumferential | Longitudinal | Radial | Circumferential | Longitudinal | ||||||||
| Strain | Strain rate | Velocity | Displacement | Strain | Strain rate | Strain | Strain rate | Velocity | Displacement | Strain rate | Strain rate | Strain rate | |
| TT interobserver agreement | 6.5 | 8.2 | 5.8 | 2.8 | 5.9 | 7.4 | 1.7 | 9.2 | 9.4 | 9.1 | 10.3 | 11.1 | 4.6 |
| TT intraobserver agreement | 6.4 | 7.5 | 5.6 | 2.7 | 6.0 | 10.6 | 1.9 | 12.5 | 9.3 | 7.9 | 13.0 | 12.3 | 6.8 |
Values are %.
TT: tissue tracking.
In this study, we assessed the value of CMR-TT derived myocardial deformation parameters in patients with myocarditis. The main findings of our study were: (1) TT analysis was feasible in the majority of myocarditis patients, with good inter- and intraobserver reproducibility; (2) all myocardial deformation parameters were significantly correlated with LVEF and LV WMA; (3) myocardial deformation parameters were considerably reduced, compared to previously published normal values; (4) global peak systolic radial strain, strain rate and displacement, and global peak systolic circumferential strain were the only parameters to be closely correlated with extent of LV LGE.
Tissue-tracking technologies such as speckle-tracking echocardiography and CMR-TT have enhanced the noninvasive assessment of myocardial deformation in clinical practice. The TT technique raises the possibility of retrospective analysis of previously acquired SSFP datasets. With this post-processing approach, no additional dedicated CMR scans are required. Although good agreement has been established between speckle tracking echocardiography and CMR-TT,10,11 difficulty in obtaining adequate echocardiographic views, observer dependency, signal noise and angle dependency can hinder strain assessment by echocardiography.12 Hence, tomographic imaging modalities like CMR are a clinically valuable alternative to overcome such shortcomings. In our study, TT software delivered outputs of myocardial strain, strain rate, velocity and displacement parameters and was able to track existing cine sequences in most patients (90.7% of all myocarditis patients).
While comparative analysis shows that measures of deformation parameters are similar between CMR-TT and speckle-tracking echocardiography, it also shows that TT analysis has superior reproducibility.11 Regarding variability, we found good inter- and intraobserver agreement for all deformation parameters. Global systolic deformation parameters showed an inter- and intraobserver CoV ranging from 1.7% to 10.6%, which is comparable to previously reported values.11,13 Although slightly lower agreement was achieved in diastolic strain rates (CoV ranging from 4.6% to 13.0%), there are currently no data on the reproducibility of diastolic function by CMR-TT analysis.
In this study, it is worth noting that there was a linear relation between all strain parameters and LVEF as well as the extent of regional WMA, as shown in Figures 3 and 5. Although these relations are mainly weak, it is clear that they may be a contributing reason why TT analysis is additive to segmental changes in contractility and to global myocardial dysfunction. The absence of follow-up data precludes determination of the clinical prognostic value of CMR-TT. In addition, a clinically meaningful cut-off value remain to be assessed. This might be an objective of future trials.
Normal reference values for TT-derived systolic and diastolic deformation parameters have been published. In a contemporary systematic review and meta-analysis of 18 studies, the pooled mean of global longitudinal strain derived from three apical views was -19.4% and the global radial and circumferential strains derived from three SAX were 39.1% and -23.6%, respectively.9 In our myocarditis population, mean global longitudinal, radial and circumferential strain values were significantly lower than in the published reference values. Moreover, strain parameters were reduced even when only patients with preserved LVEF were considered. This observation is in agreement with a previous CMR-TT study7 that detected significant group differences between myocarditis patients with preserved ejection fraction and control subjects. Overall these results thus suggest that myocardial strain parameters represent new quantitative indices of cardiac deformation that are thought to be capable of detecting subtle alterations in myocardial function, and that the addition of global peak systolic strains to LVEF may improve the diagnostic accuracy of CMR for myocarditis.
Further systolic deformation parameters such as strain rate, velocity and displacement deformation parameters are rarely assessed. A study assessing a cohort of 145 healthy volunteers reported slightly lower ranges than those found in our population, however different software was used,13 which precludes direct comparisons.
Diastolic dysfunction, as assessed by echocardiography, has been reported to be prevalent in acute myocarditis.14 In a seminal paper, Eurlings et al.14 reported that inflammation induces fibrosis, cardiomyocyte stiffening and endothelial dysfunction, which could explain the impairment of myocardial diastolic properties in acute myocarditis. Assessment of diastolic function by CMR-TT has already taken its first steps in other cardiomyopathies, notably in hypertrophic cardiomyopathy (HCM). In a study of patients with HCM, Nucifora et al.15 showed that LV diastolic mechanics was influenced by the extent of replacement and interstitial fibrosis. The present study is, to the best of our knowledge, the first to investigate diastolic function parameters by CMR-TT in the setting of myocarditis. In our study, diastolic deformation parameters, as determined by strain rate, were significantly related to the extent of WMA and LVEF. In view of the otherwise limited available data, understanding of the role of diastolic function parameters, as assessed by CMR-TT, still remains elusive and our results should encourage future studies to reappraise the possible importance of diastolic function parameters in the setting of myocarditis.
We also revealed that worse global peak systolic radial strain, strain rate and displacement, and global peak systolic circumferential strain showed a significant correlation with the extent of LV LGE, regardless of the type of LGE pattern. Similarly, a case-control study found that global peak systolic circumferential strain displayed good accuracy in detecting LGE, a value worse than -20.5% detecting LGE with 73% sensitivity and 77% specificity.16 TT analysis may therefore serve as a surrogate for detection of fibrotic alterations of the myocardium. This might also have significant prognostic value in patients with suspected myocarditis, since the presence of LGE has been found to be a predictor of major adverse cardiac events.17,18 Although LGE has been shown to be related to poor prognosis and TT analysis identifies the presence of LGE, the direct prognostic implications of CMR-TT assessment in myocarditis remain to be established.
Other applications of myocardial deformation analysis could be useful in patients in whom gadolinium administration is inadvisable (for example in renal insufficiency), enabling indirect quantification of myocardial fibrosis, as administration of contrast media is unnecessary for this analysis. Furthermore, tissue characterization by CMR-TT may also overcome the technical limitations of the Lake Louise criteria, as T1 and T2 mapping parametric mapping techniques appear to do.19 In view of all these advantages, it can be expected that CMR-TT will be increasingly used in published studies.
LimitationsThe present study has several limitations. Firstly, there are the inherent drawbacks of the retrospective study design and potential selection bias, as only patients who underwent CMR were included. Future larger multicenter studies with prospective selection of patients with myocarditis should be performed in order to adequately validate the present report. Secondly, endomyocardial biopsy, the reference standard, was not systematically performed to diagnose myocarditis. Instead, the presence of myocarditis was defined by CMR-based tissue characterization, combining typical clinical features, elevated biomarkers and exclusion of coronary artery disease, which have already proved to have good diagnostic accuracy.20 Thirdly, WMA and LGE were assessed visually and quantified manually as the percentage of segments involved. Better reproducibility is usually obtained for LGE quantification using available software that delineates areas of interest, automatically traced in all slices and quantified as proportion of total LV myocardial mass,21 however there is no current consensus on the best method of LGE quantification.22 Finally, the long waiting times from symptom onset to CMR acquisition (median 11 days; IQR: 2-138 days), mainly due to low resources and high demand, may have influenced the results obtained. Supplementary material presents an analysis of the 19 patients in whom CMR was performed within 15 days of the acute event (Supplementary Tables 1 and 2).
ConclusionsCMR-TT myocardial strain analysis is an objective method for the quantification of myocardial function. Patients with myocarditis showed slightly reduced myocardial strain, and the deformation parameters correlate with LVEF, along with WMA and extent of myocardial fibrosis. Future studies are needed to determine the clinical relevance of these findings for the management of myocarditis patients.
Conflicts of interestThe authors have no conflicts of interest to declare.