Mitral annular disjunction (MAD) is an easily identifiable entity on transthoracic echocardiography, but is still poorly recognized or ignored. It is often associated with mitral valve prolapse and is itself a risk marker for ventricular arrhythmias and sudden cardiac death, but the management and risk stratification of these patients is not systematized.
Two clinical cases of MAD associated with mitral valve prolapse and ventricular arrhythmias are presented. The first case is of a patient with a history of surgical intervention on the mitral valve due to Barlow's disease. He presented to the emergency department with sustained monomorphic ventricular tachycardia requiring emergent electrical cardioversion. MAD with transmural fibrosis at the level of the inferolateral wall was documented.
The second report is of a young woman with palpitations and frequent premature ventricular contractions on Holter with documentation of valvular prolapse and MAD, and focuses on the risk stratification approach.
The present article offers a review of the literature regarding the arrhythmic risk of MAD and mitral valve prolapse, as well as a review of risk stratification in these patients.
A disjunção do anel mitral é uma entidade facilmente identificável em ecocardiografia transtorácica, mas ainda pouco reconhecida e valorizada. Encontra-se frequentemente associada ao prolapso da válvula mitral e representa por si só um marcador de risco para arritmias ventriculares e morte súbita cardíaca, contudo a orientação e a estratificação de risco destes doentes não se encontram sistematizadas.Dois casos clínicos de disjunção do anel mitral associado a prolapso da válvula mitral e arritmias ventriculares são apresentados. O primeiro caso retrata um doente com antecedentes de plastia valvular mitral por doença de Barlow, que recorre ao SU por quadro de palpitações mal tolerado com documentação de taquicardia ventricular monomórfica sustentada com necessidade de cardioversão elétrica emergente. Do estudo efetuado documenta-se disjunção do anel mitral com fibrose transmural ao nível da parede inferolateral. O segundo caso clínico relata o caso de uma jovem com palpitações e extrassistolia ventricular muito frequente no Holter com documentação de prolapso valvular e disjunção do anel mitral e centra-se na abordagem da estratificação de risco.
O presente artigo oferece uma revisão da literatura relativamente ao risco arrítmico da disjunção do anel mitral e prolapso da válvula mitral assim como uma revisão da estratificação de risco nestes doentes.
Mitral valve prolapse (MVP) is a common valve defect that affects 2-3% of the general population.1,2 Although considered a benign condition, it is the principal indication for surgical intervention in cases of mitral regurgitation in developed countries, and since the 1980s there have been reports of sudden cardiac death (SCD) in patients with MVP, even in the absence of mitral regurgitation, particularly in asymptomatic younger patients.1,3
Recent studies have shown that patients with MVP and ventricular arrhythmias frequently present concomitant mitral annular disjunction (MAD).4,5
MAD is a structural abnormality of the mitral annulus in which there is a separation between the atrial wall at the level of the valve and the left ventricular (LV) free wall, leading to hypermobility of the valve apparatus. This anatomical defect increases the mechanical stress on the mitral leaflets, resulting in myxomatous degeneration and fibrosis. Several studies have shown a greater prevalence of non-sustained ventricular tachycardia and premature ventricular contractions (PVCs) in patients with MAD.5
The present article reports two clinical cases of MVP with MAP and ventricular arrhythmias, and offers a review of the literature on this subject and of risk stratification in these patients.
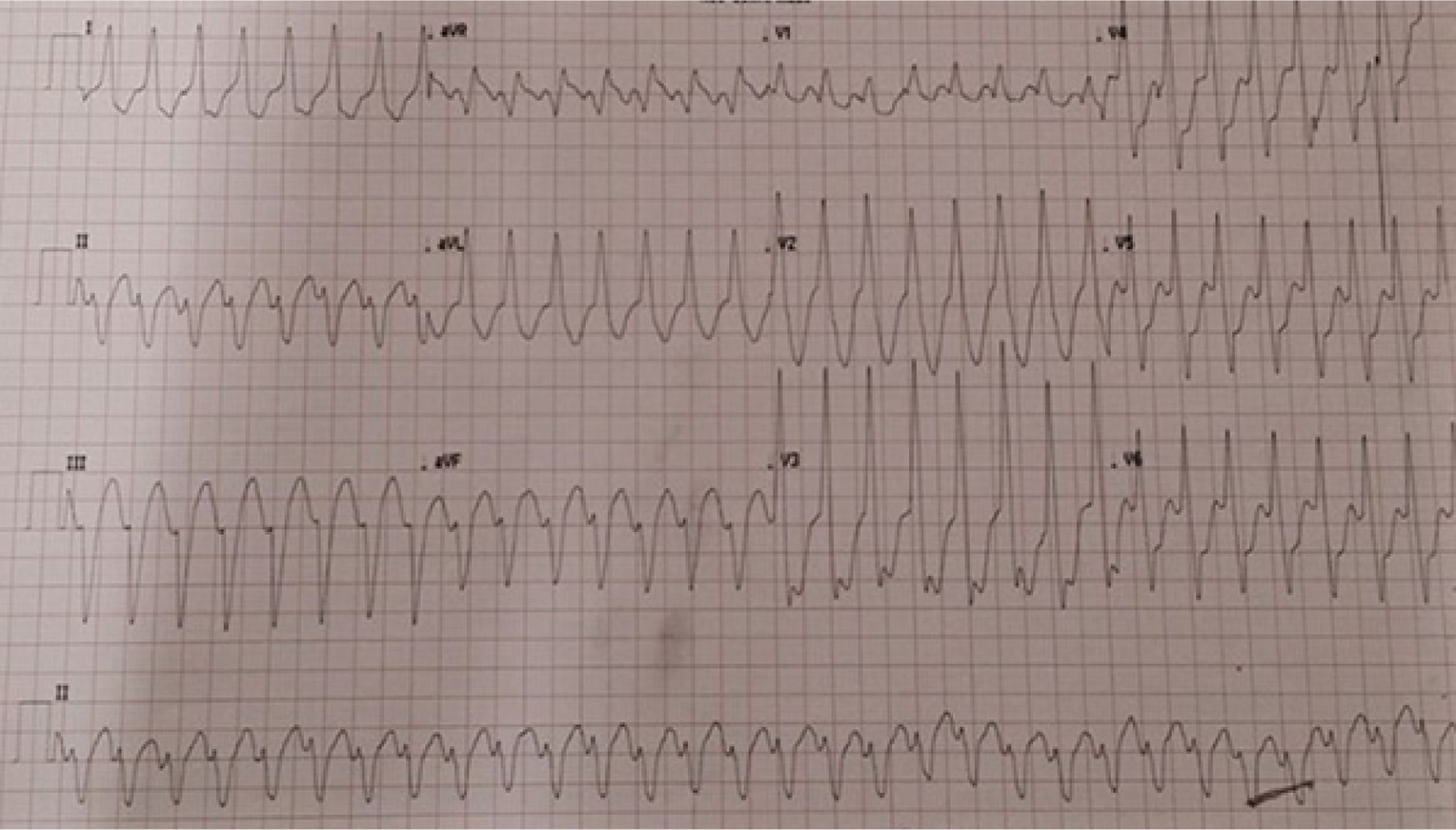
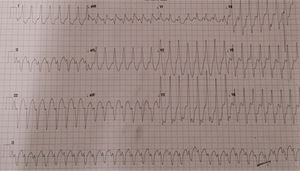
Case 1The first case is of a 76-year-old male patient with a history of cardiac valve surgery. In 2012, in France, he underwent mitral valve plasty with placement of a 36-mm Carpentier-Edwards Physio ring because of severe mitral regurgitation due to Barlow's disease. Thereafter he was generally in New York Heart Association functional class II. In October 2016 he was admitted to the emergency department with sudden-onset palpitations, dizziness, dyspnea and chest pain. The admission electrocardiogram (ECG) showed sustained monomorphic ventricular tachycardia (VT) with a pattern of left bundle branch block (LBBB) and superior axis (Figure 1).
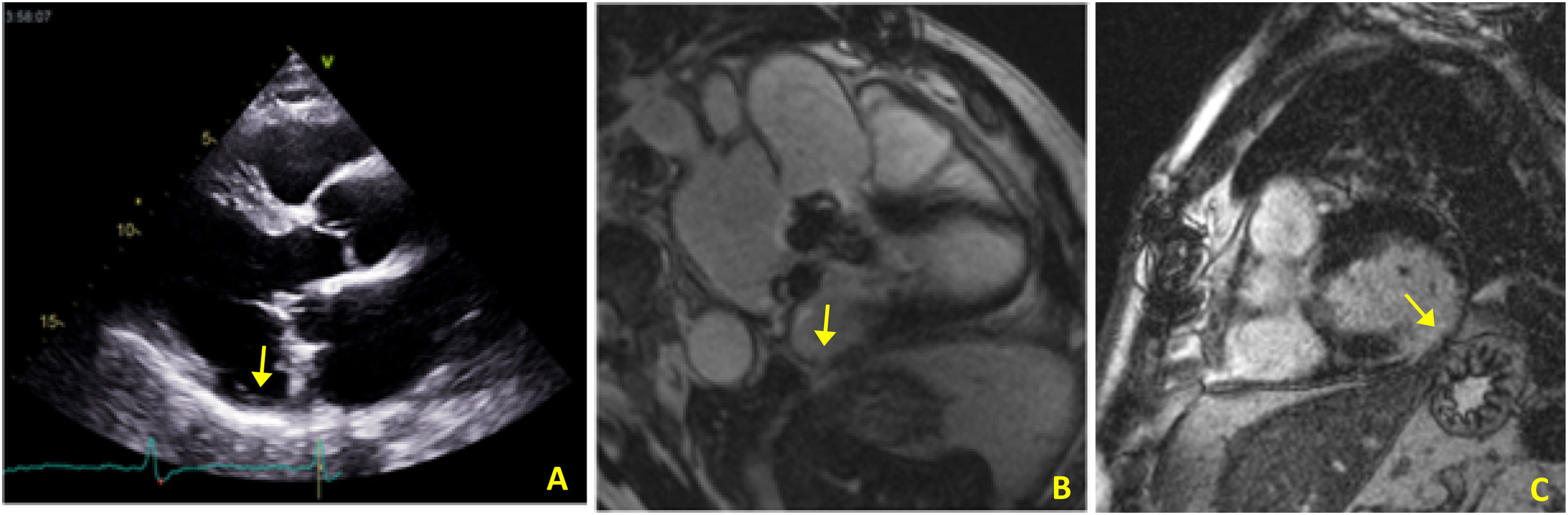
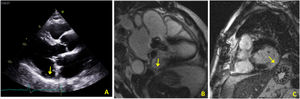
In view of the patient's hemodynamic instability, urgent electrical cardioversion was required, following which the patient was asymptomatic. The ECG showed sinus rhythm with non-specific ventricular repolarization alterations. Laboratory tests revealed no abnormalities. The transthoracic echocardiogram showed severe left atrial dilatation, prosthetic ring without signs of dysfunction, preserved LV function, and an image suggestive of MAD, with a distance of 15 mm and evidence of undulating movement of the inferolateral LV wall and stretching of the chordae tendineae (Figure 2A). Coronary angiography excluded coronary disease. The patient was referred for cardiac magnetic resonance imaging (CMRI), which revealed dyskinesia of the basal segment of the inferolateral wall with severely reduced thickness, as well as a perfusion defect and transmural late enhancement. This was interpreted as indicating possible fibrosis in the context of MAD (Figure 2B and C).
(A) Transthoracic echocardiogram in parasternal long-axis view showing mitral annular disjunction (yellow arrow); (B) cardiac magnetic resonance imaging, 3-chamber cine view, showing mitral annular disjunction (yellow arrow); (C) cardiac magnetic resonance imaging, short-axis view, showing transmural late enhancement in the basal segment of the inferolateral wall.
Considering the fragility of the relevant segment, and given the patient's clinical stability following administration of amiodarone and titration of beta-blockers, it was decided not to perform an electrophysiological study (EPS) with a view to ablation, due to the risk of rupture. A dual-chamber implantable cardioverter-defibrillator (ICD) was placed for secondary prevention and the patient was referred for arrhythmology consultations. To date he has received one appropriate shock for sustained monomorphic VT.
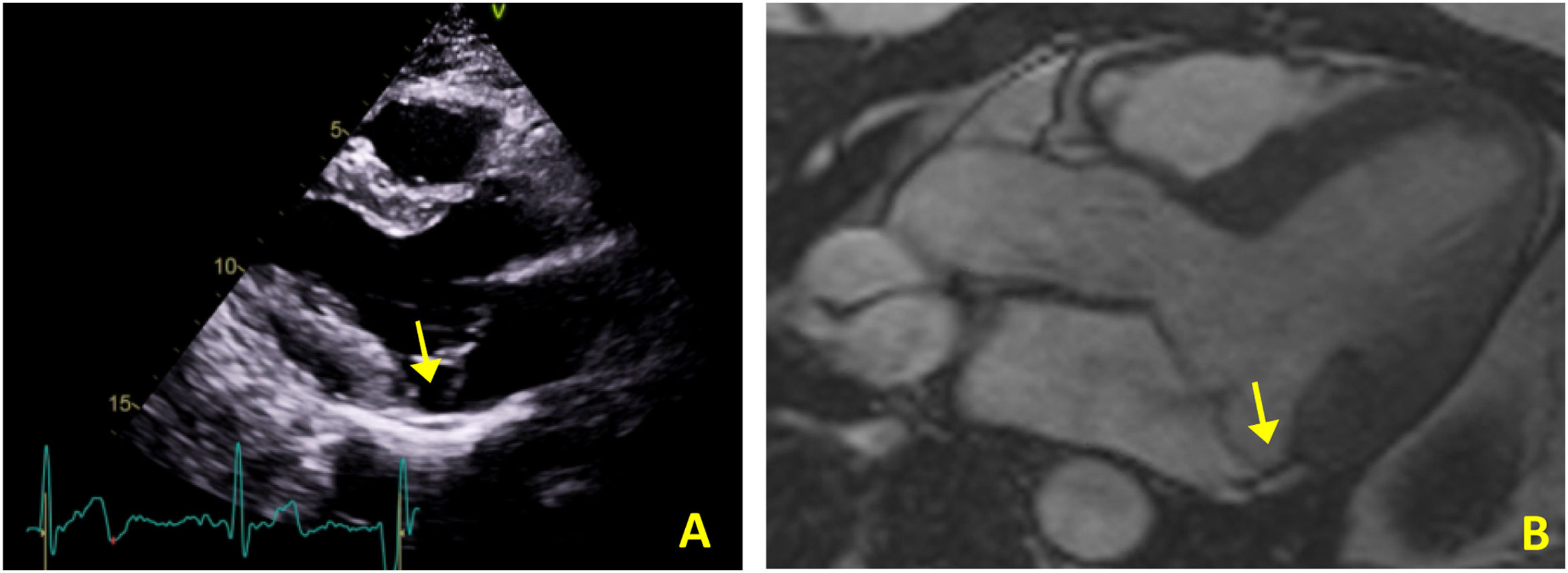
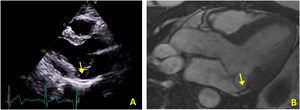
Case 2The second case is of a 34-year-old woman with no relevant personal or family history. She was referred for an arrhythmology consultation in 2014 due to non-sustained palpitations, without syncope. The 24-hour Holter revealed very frequent PVCs (16.4% of all beats), polymorphic but mainly monofocal, with LBBB morphology and superior axis, in bigeminy and trigeminy, with frequent doublets and triplets, as well as two episodes of non-sustained VT (maximum five complexes). The transthoracic echocardiogram showed the mitral valve with a myxomatous appearance and prolapse of both leaflets, causing mild mitral regurgitation, as well as MAD and preserved biventricular systolic function (Figure 3A). Laboratory tests revealed no abnormalities. CMRI revealed MVP with MAD and hypermobility of the basal segment of the inferolateral wall but no fibrosis (Figure 3B).
The patient performed an exercise test under bisoprolol which revealed non-sustained polymorphic ventricular runs that disappeared at peak exercise. In view of this result she underwent genetic testing for catecholaminergic polymorphic ventricular tachycardia, which was negative. An event recorder was implanted, which showed non-sustained PVCs. As her palpitations persisted, with very frequent ectopic ventricular activity, she underwent EPS with programmed ventricular stimulation (PVS), which failed to induce sustained arrhythmia. Voltage mapping with the CARTO ConfiDENSE® system detected low voltages in the posterior region of the mitral valve, but no PVCs were recorded, even after administration of isoprenaline, and ablation was not performed.
The patient is currently medicated with bisoprolol. Her palpitations have improved and no episodes of syncope or sustained ventricular arrhythmias have been detected by the event recorder.
DiscussionThe prevalence of ventricular arrhythmias, most frequently PVCs, in patients with MVP can reach as high as 34%.3 These patients also have an increased risk of SCD, which can affect up to 2% per year even in the absence of heart failure or significant mitral insufficiency.6
Although the arrhythmogenic mechanisms in patients with MVP are not fully understood, malignant arrhythmias appear to originate from the combination of an abnormal LV myocardium (substrate) and complex ventricular ectopy (trigger).6
Fibrosis, which makes up the arrhythmogenic substrate, is usually located in the base of the inferolateral LV wall or the papillary muscles, and appears to result from continuous mechanical stress caused by the prolapsing leaflet, which leads to myocardial hypertrophy and fibrotic replacement in the structures supporting the subvalvular apparatus. In addition, friction between the chordae and the LV endocardium can result in ‘friction lesions’, characterized by the development of endocardial fibrosis between the papillary muscle and the annulus.7
This mechanically induced fibrosis likely acts as the substrate, which increases vulnerability by either triggered ectopic activity or reentry mechanisms, leading to the development and maintenance of sustained VT.7
The subgroup of MVP patients who appear to be at greater risk of SCD is composed of young women with prolapse of both leaflets, the presence of LBBB, biphasic/inverted T waves in the inferior or inferolateral leads, fibrosis of the papillary muscle, MAD, frequent ventricular ectopic activity (with a greater load of PVCs), polymorphic PVCs with doublets, triplets and ventricular bigeminy or VT, and configurations of fascicular and papillary muscle PVCs (LBBB morphology, most with superior axis).2,3,6,8
The mitral annulus is a three-dimensional saddle-shaped structure, the configuration of which changes dynamically during the cardiac cycle. Morphologically it is a fibrous structure that is conventionally divided into anterior and posterior parts and is positioned along the atrioventricular junction, which serves as the anchoring point for the leaflets of the mitral valve. Since its composition is fibrous, the movement of the annulus is passive, being determined by the contraction and relaxation of the adjacent atrial and ventricular musculature, as well as the movement of the aortic root. Under normal conditions, the posterior part of the annulus moves anteriorly and downward during systole, in synchrony with the rest of the left ventricle.9
MAD is an anatomical abnormality of the annulus described anatomopathologically as a wide separation (≥5 mm) between the junction of the mitral valve and left atrium and its insertion in the left ventricle. This disjunction results in dilatation and systolic flattening of the annulus, moving mainly downward in systole with little or no anterior movement, which leads to an undulating movement associated with more general deformation of the leaflets. This abnormal dynamics is found even though LV systolic function is preserved, suggesting an intrinsic abnormality of the annulus and functional decoupling of the annulus from the left ventricle.1,10
The concept of MAD was originally introduced by Bharati et al. and then systematically investigated in the 1980s by Hutchins et al., who reported it as an anatomic variation of the fibrous mitral annulus.4,9,10 Hutchins performed a pathological study of 900 hearts from adult autopsies, of which 25 (3%) had MVP and 23 (92%) presented MAD.10 Because the latter patients were significantly younger than those with MVP, the authors suggested that this anatomic variation could play a role in the pathogenesis of myxomatous valve degeneration, by means of increased mechanical stress induced by the excessive mobility of the mitral valve apparatus.4,10
Nonetheless, this structural abnormality of the mitral annulus was virtually ignored, treated merely as the subject of speculation, until 2005, when Eriksson et al. observed MAD by direct surgical inspection in patients undergoing mitral valve plasty due to advanced myxomatous disease. The authors applied a standardized transesophageal echocardiographic protocol that revealed a 98% prevalence of MAD in a surgical series of patients with MVP and severe mitral insufficiency.11 They also demonstrated a positive correlation between the magnitude of disjunction and number of prolapsing mitral valve segments and the degree of mitral insufficiency, supporting the association between MAD and the severity of MVP.1,11
Carmo et al. then demonstrated for the first time that MAD is also easily detected and measured by routine transthoracic echocardiography, with the typical undulating movement of the basal LV inferolateral wall in parasternal long-axis view.5
Dejgaard et al. conducted the first large clinical trial on patients with MAD as the inclusion criterion. They assessed 116 patients, of whom 12% suffered severe arrhythmic events, defined as aborted cardiac arrest or sustained VT. An association between MAD and MVP was observed in 78% of subjects, although the presence of MVP was not associated with higher arrhythmic risk. Factors associated with severe ventricular arrhythmias were younger age, reduced ejection fraction and fibrosis of the papillary muscles. Ventricular arrhythmias, defined as severe arrhythmic events or non-sustained VT, were experienced by 34% of participants; this endpoint was associated with younger age, previous syncope, PVCs, papillary muscle fibrosis and greater longitudinal MAD distance in the posterolateral wall assessed by CMRI. The latter was the only independent risk factor for ventricular arrhythmia in multivariate analysis.2
This study also demonstrated an association between PVCs, severe arrhythmic events and MAD, independently of concomitant MVP, indicating that MAD is a clear risk marker of arrhythmic events and may itself play an important role in arrhythmogenesis. In addition, the authors found no other etiology for the 10 cases of cardiac arrest in their study, suggesting the existence of a novel clinical syndrome, that of MAD arrhythmic syndrome. They recommend that physicians should consider MAD in younger patients with no other apparent cause for frequent PVCs, particularly with findings of LBBB and superior axis or polymorphic PVCs.2
Carmo et al. found that a greater magnitude of disjunction was associated with a higher incidence of non-sustained VT; a MAD greater than 8.5 mm was a reasonable criterion to predict the risk of non-sustained VT, with a sensitivity of 67% and a specificity of 83%.8
The 2019 European Society of Cardiology guidelines on sports cardiology recognize the arrhythmic risk of MVP, proposing criteria of eligibility for participation in sports with this diagnosis.12 However, the American and European guidelines for ventricular arrhythmias and SCD do not include separate criteria for the risk of SCD secondary to MVP or MAD, nor do they contain specific recommendations for ventricular arrhythmias and SCD in patients with mitral valve disease.13,14
The risk of SCD in MVP is 1.75-2.3 times higher than in the general population, while the risk of SCD in the presence of MAD (concomitant or not) is unknown.8 It is difficult to identify patients at higher risk and to prevent SCD. It has been suggested that CMRI (to assess degree of fibrosis), exercise testing, and serial Holter monitoring could be used for risk stratification of these patients.3.6.7
Some authors propose EPS to stratify risk in patients with suspected arrhythmic syncope and multiple risk factors such as polymorphic PVCs and fibrosis. However, programmed ventricular stimulation for risk stratification of patients with MVP or MAD is not as well established as it is with other substrates that predispose to SCD.7 Miller et al. define a positive electrophysiology study as sustained monomorphic VT induced with up to three ventricular extrastimuli or polymorphic VT or ventricular fibrillation induced with up to two ventricular extrastimuli. In patients with a positive electrophysiology study, they recommend placement of an ICD. If the electrophysiology study is negative or not performed, implantation of a loop recorder for long-term rhythm monitoring should be considered, especially if other high-risk features are present.7
Beta-blockers are the first-line agents for the management of symptomatic or asymptomatic non-sustained or sustained ventricular arrhythmias. However, currently no pharmacological therapy has been shown to alter the prognosis of patients with MAD.7 Mitral valve plasty should in theory relieve the stretching of the papillary muscles and facilitate ventricular remodeling, reducing ventricular arrhythmias, but mitral valve surgery only appears to lead to such reduction in some single-center studies and in younger but not older patients. The progressive nature of the arrhythmic substrate and extent of fibrosis in the papillary muscles, chordae tendineae, and mitral annulus with age and associated comorbidities are probably the reason for this observation.3,7
Furthermore, in the presence of MAD, mitral valve plasty may not fully restore the function of the disjunctive annulus, because annular-ventricular decoupling may persist.1 Eriksson et al. observed that modification of the repair technique enables surgical correction of the annular disjunction, which seems to optimize long-term results.11
In our first case report, surgical repair had probably been performed already at an advanced stage of disease. It is unknown whether MAD was already present when mitral plasty was performed or whether the surgical technique included its correction, but at all events, despite MVP repair the disease appears to have undergone age-related evolution, in which the mechanisms underlying development of the arrhythmogenic substrate persisted.2
Regarding the same case, in view of the presence of VT with LBBB morphology and superior axis, as well as echocardiographic evidence of significant MAD (15 mm) causing ventricular-annular decoupling that was difficult to assess due to the presence of the prosthetic ring but with evidence of undulating movement of the LV inferolateral wall and stretching of the chordae tendineae (which is known to contribute to an arrhythmogenic substrate), coronary angiography to exclude coronary disease could have been omitted.
It was decided not to perform an EPS possibly followed by ablation in this patient since the reduced inferolateral wall thickness would have made this a high-risk procedure. This intervention was accordingly postponed until antiarrhythmic therapy became ineffective.
In the second case reported, the question was of risk stratification in a patient with MVP, frequent palpitations and risk markers for SCD (young age, female, prolapse of both leaflets, MAD, and frequent polymorphic ectopic ventricular activity with episodes of non-sustained VT and PVCs with LBBB pattern and superior axis.
Despite the absence of fibrosis on CRMI, the presence of polymorphic ventricular runs on exercise testing and persistence of palpitations prompted EPS with PVS, which failed to induce ventricular arrhythmia. However, the negative prognostic predictive value of PVS in this patient subgroup is unknown, and EPS results may be influenced by the stimulation site and the number and coupling interval of extrastimuli. As an additional method of risk assessment, an event recorder was implanted, as suggested by Miller et al.,7 to correlate clinical events with electrocardiographic findings; no significant events have been detected to date.
ConclusionMAD, whether or not associated with MVP, is an easily identifiable entity on transthoracic echocardiography and is itself associated with ventricular arrhythmias, giving rise to a new clinical entity – arrhythmogenic mitral annular disjunction – with non-negligible risk for SCD.
The benefit of early intervention in MVP and MAD, in order to prevent the development of fibrosis (known to act as an arrhythmogenic substrate), is currently the subject of debate. Studies are ongoing of risk predictors for ventricular arrhythmias associated with this condition and of possible ways to stratify risk in these patients.
A future carefully designed prospective trial analyzing electrocardiographic features, ventricular arrhythmia morphology, degree of fibrosis on CMRI, and electrophysiological findings in patients with MAD (with and without MVP), would help to elucidate the arrhythmogenic role of MAD and the best therapeutic approach.
Conflicts of interestThe authors have no conflicts of interest to declare.