The best outcome for coronary intervention in coronary artery bypass graft patients requires knowledge of prior coronary anatomy. This information is not always available as many cases present acutely, especially in ST-elevation myocardial infarction. We present three cases in which bypass grafts were documented as occluded but follow-up angiograms for other reasons revealed that the grafts were still patent. This presents the potential for inappropriate revascularizations.
O melhor resultado da intervenção coronária nos doentes submetidos a cirurgia de revascularização do miocárdio requer conhecimento da anatomia coronária anterior. Tal informação nem sempre está disponível, uma vez que muitos casos apresentam-se agudos, em particular nos enfartes do miocárdio com elevação do segmento ST. Apresentamos três casos em que foram referidos enxertos ocluídos, mas os angiogramas de seguimento, por outros motivos, revelaram que os enxertos ainda estavam patentes. Tal comprova o potencial de revascularizações inapropriadas.
Management of coronary artery disease (CAD) after coronary artery bypass grafting (CABG) is multifaceted. Due to the adverse risk factor profile of these patients, there is frequently significant progression of atherosclerosis in the native vessels and grafts requiring future invasive procedures.1 Coronary intervention in this population is more complex, making knowledge of prior coronary anatomy essential for achieving excellent outcomes. This information is not always available, as many cases present emergently as ST-elevation myocardial infarction (STEMI), therefore emergent intervention is performed without complete data. This has led to some inappropriate revascularizations with the attendant risk of major complications.
We highlight this highly under-reported problem and suggest management techniques to help mitigate the risk.
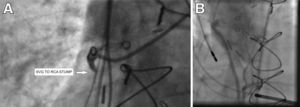
Case reportsCase AA 74-year-old male had a history of hypertension and CAD with previous CABG, including left internal mammary (LIMA) to left anterior descending (LAD), saphenous vein graft (SVG) to posterolateral (PL) branch, SVG to first diagonal and SVG to posterior descending artery (PDA). He developed chest pain and presented with an inferior wall STEMI. Per a previous angiogram performed at another hospital, his SVG to PDA graft was considered chronically occluded. Emergent cardiac catheterization revealed significant native multivessel disease. The LIMA-LAD, SVG-PL and SVG-diagonal grafts were all widely patent. The SVG to PDA graft was totally occluded proximally with fresh thrombi (Figure 1A). As this graft had an ostial stent protruding about 5 mm into the aorta, cannulation was difficult, and required a multipurpose guide catheter. Intravascular ultrasound was used to properly characterize this lesion, then aspiration thrombectomy of a fresh thrombus was successfully performed (Figure 1B) followed by placement of a drug-eluting stent. In retrospect, this graft had been considered totally occluded on a previous angiogram, probably due to the difficulty of cannulation. Proper engagement of the graft this time spared the patient significant myocardial damage that might have resulted from not intervening on this culprit artery.
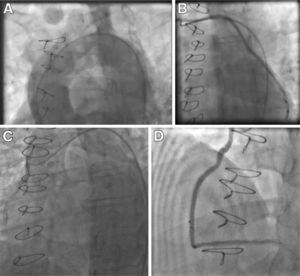
Case BA 70-year-old female with a history of CAD and previous CABG (LIMA-LAD, SVG-OM), peripheral vascular disease, diabetes, hypertension, and hyperlipidemia was admitted eight months after CABG with an acute non-ST-elevation myocardial infarction (NSTEMI), with troponin I of 8 ng/ml (normal: 0.0-0.04). Cardiac catheterization revealed significant native LAD and left circumflex (LCx) disease. The LIMA-LAD graft was patent, and since an extensive search including aortography revealed no other patent grafts (Figure 2A), it was assumed that the SVG-OM was occluded. Consequently, percutaneous transluminal balloon angioplasty and stenting of the native LCx/OM was performed.
Seven months later, she was readmitted with angina and a positive nuclear stress test suggestive of inferolateral ischemia. Repeat cardiac catheterization this time using an AL1 catheter showed a widely patent SVG-OM graft with good distal runoff (Figure 2B). Medical therapy was optimized. It was concluded that the SVG-OM was missed during the previous cardiac catheterization, possibly leading to unnecessary intervention on the native LCx. This could have potentially led to competitive flow and subsequent closure of a patent graft.
Case CA 52-year-old male had a history of CAD and previous CABG (LIMA-LAD, SVG-PDA, SVG-OM). On admission for NSTEMI, cardiac catheterization revealed a patent LIMA-LAD, an occluded SVG-OM, and 90% stenosis of the SVG-PDA, for which he received a stent.
Two years later, he presented with another NSTEMI (troponin I 8.5 ng/ml [normal: 0.0-0.04]). This time the SVG-PDA was considered totally occluded and optimized medical management was offered. On another admission for NSTEMI and worsening LV dysfunction, his ejection fraction dropped from 45% to 20%, and another cardiac catheterization showed that the SVG-PDA was not totally occluded as assumed previously, but was rather 99% stenosed ostially (Figure 2C). Selective engagement was achieved using a multipurpose catheter with gentle manipulation followed by angioplasty and successful stent placement, with restoration of TIMI 3 flow (Figure 2D). In this case, we can speculate that a timely intervention on this lesion could have avoided the drop in left ventricular function.
DiscussionThese three cases highlight the importance of accurate graft localization during coronary angiography of patients with prior CABG. The phenomenon of missing grafts and inappropriate revascularization during emergent angiography is under-reported and a meticulous literature search failed to reveal any reports commenting on its incidence or prevalence. It is a common situation that many interventional cardiologists face when treating patients with prior CABG and can lead to suboptimal management and outcome.
It is extremely important to have the prior operative and angiography report in order to prepare a plan and road map prior to intervention, but unfortunately this is not always available, especially in emergent situations. Also, even if the cardiac catheterization report is available with the patient or in their chart, this would not be as helpful as reviewing images and films that can give clues regarding anatomy beyond what is only reported.
Various methods have been used to identify bypass grafts. In the past, surgeons marked the ostia of vein grafts with radio-opaque rings, substantially aiding the angiographer to localize the vein graft ostia during angiography. However, nowadays this is not a common practice. The use of different catheters other than the usual JR4, such as Amplatz multipurpose, AL1, AL2, or AR catheters or others according to the nature of the unusual takeoff of vein grafts, is important. Often, a non-selective aortogram is the only option to either identify the ostium of the graft or to label a graft as totally occluded. In our three cases, we had the previous aortogram that did not show stumps or residual knobs, and unfortunately there was no vein markers present. Only repeated systematic search for the grafts aided in their identification in the subsequent angiogram.
Given the potential consequences of falsely labeling missing grafts as occluded, we propose taking adjunctive measures to increase the specificity of diagnosis before a graft is considered occluded. The role of non-invasive imaging in the evaluation of bypass grafts has been well studied. The American College of Cardiology endorses the use of multidetector computed tomography coronary angiography (CTCA) to assess graft patency after CABG.2 CTCA has a positive predictive value of 97-99% in assessing graft patency and stenosis.3–5 As per the 2010 appropriate use criteria for cardiac computed tomography, CCTA evaluation of graft patency for symptomatic patients after CABG is considered appropriate with a score of A (8).2 However, we did not use CCTA in any of our cases as in our institution we do not have easy access to CCTA for inpatients, and specifically there is no protocol for inpatient service presenting with acute coronary syndrome. Its use as an adjunct to conventional coronary angiogram in patients with missing grafts can be beneficial.
It might be assumed that these errors would only be found among relatively inexperienced operators, but the three operators in these cases each had an average 15 years of post-interventional cardiology training experience. There is no published study highlighting the frequency of missed grafts during cardiac catheterization, but from anecdotal reports it is not uncommon. This study is very important especially in the light of the Institute of Medicine's “To err is human” report, which emphasizes the need for physicians to undergo continuous process improvement through internal systems reviews.6
ConclusionWe present a series of cases highlighting an important yet seriously under-reported conundrum. Interventionists are performing more emergent angiograms on post-CABG patients without having adequate background information on their coronary anatomy and grafts. The consequences of falsely labeling missed grafts as occluded are dire. In order to avoid inappropriate revascularization, we propose a thorough search for grafts with different catheters, aortogram and, if needed, CTCA as a complement to routine angiography to improve diagnostic specificity when bypass grafts are initially considered occluded.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.